Abstract
The exact effects of the modulation of Notch signaling pathway on cell growth have been shown to depend on tumor cell type. Recombination signal-binding protein Jκ (RBPJ) is a key transcription factor downstream of receptor activation in Notch signaling pathway. Here, we evaluated the effects of RBPJ inhibition on the growth of lung cancer cells. We found that a short hairpin interfering RNA (shRNA) for RBPJ efficiently inhibited RBPJ expression in lung cancer cells, resulting in a significant decrease in the cell growth. Further analyses showed that RBPJ inhibition altered the levels of its downstream targets, including p21, p27, CDK2, Hes1, Bcl-2, and SKP2, to prevent the cells from growing. Our data thus suggest that shRNA intervention of RBPJ expression could be a promising therapeutic approach for treating human lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is a commonly occurred malignant cancer in humans [1–3]. Most types of lung cancer are insensitive to both chemotherapy and radiation therapy and are often highly invasive [1–3]. Hence, development of novel therapeutic treatments for lung cancer is highly urgent, which may substantially improve the patients’ 5-year survival ratio [4–6].
The Notch pathway has been shown to upregulate in many cases of lung cancer, which suggests a role of this pathway in the tumorigenesis of lung cancer. Notch signaling pathway is highly conserved in evolution and plays important roles during development, in which Notch signals regulate various physiological processes, including maintenance of stem cells, cell fate decisions, proliferation, differentiation, and apoptosis [7, 8].
Recombination signal-binding protein Jκ (RBPJ) is a DNA-binding protein from CSL family of transcription factors [9]. RBPJ recognizes a consensus sequence C(T)GTGGGAA on RBPJ-binding sites from a number of factors [9]. Importantly, RBPJ also mediates signals from Notch receptors [10, 11]. In the absence of Notch signals, RBPJ is associated with some corepressors that repress Notch transcription. After ligand binding, Notch signaling is initiated by γ-secretase-mediated proteolytic cleavage and liberation of the Notch intracellular domain (NICD). NICD subsequently translocates into the nucleus to displace corepressors from RBPJ, which allows for the recruitment of coactivators to bind with RBPJ to thereby induce the activation of target genes like hairy and enhancer of split 1 (Hes-1), cyclin-dependent kinase 2 (CDK2), B-cell CLL/lymphoma 2 (Bcl-2), ubiquitin ligase complex SCFSKP2 (SKP2), p21, and p27 [10–17]. p21 is a cyclin-dependent kinase inhibitor, and it functions through binding to and inhibiting the activity of cyclin-CDK2, -CDK1, and -CDK4/6 complexes to prevent cycle progression at G1 and S phase. In addition to cell-growth arrest, p21 can mediate cellular senescence. p27 is another cyclin-dependent kinase inhibitor that in humans is encoded by the CDKN1B gene. p27 binds to and prevents the activation of cyclin E-CDK2 or cyclin D-CDK4 complexes and thus controls the cell cycle progression at G1 [18, 19].
RBPJ has been shown to play a role in the tumorigenesis of lung cancers [20–23]. A recent study reported that knockdown of RBPJ expression by RNA interference (RNAi) inhibited both the anchorage-independent growth of rhabdomyosarcoma cells and the outgrowth of xenografts in vivo [24], suggesting that transcriptional modulation of RBPJ may be a potentially effective therapy for cancers.
Here, we evaluated the regulation of lung cancer cells by RBPJ depletion. We used a short hairpin RNA (shRNA) to efficiently inhibit RBPJ expression in lung cancer cells, which significantly decreased the cancer cell growth. Further analyses showed that RBPJ inhibition altered the levels of its downstream targets, including p21, p27, CDK2, Hes1, Bcl-2, and SKP2, to prevent the cells from growing.
Materials and methods
Cell culture
HEK293T cells and human lung cancer cell lines A549 (origin from carcinoma), SK-LU-1 (origin from adenocarcinoma), and NCI-H23 (origin from non-small cell lung cancer) were all purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). These three cell lines were used in the current study, since they represent different types of lung cancer. Three cell lines were all cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (Invitrogen) and 1 % penicillin and streptomycin in a 5 % CO2 humidified cell-culture incubator at 37 °C.
RT-qPCR
Total RNA was extracted from cultured cells with RNeasy kit (Qiagen, Hilden, Germany). cDNA was synthesized from 1 μg of total RNA using a reverse transcription kit (Qiagen) and purified with the QIAquick PCR Purification Kit (Qiagen). Quantitative PCR (RT-qPCR) was performed in duplicates with QuantiTect SYBR Green PCR Kit (Qiagen) with a LightCycler 1.5 Real-time PCR machine (Roche, Indianapolis, IN, USA). Primers that were designed to amplify the fragments cross exons are as follows: RBPJ (132 bp), forward primer 5′-CGCATTATTGGATGCAGATG-3′ and reverse primer 5′-CAGGAAGCGCCATCATTTAT-3′ and α-tubulin (125 bp), forward primer 5′-CCAAGCTGGAGTTCTCTA-3′, reverse primer 5′-CAGAGTGCTCCAGG-3′. Values of RBPJ were first normalized against α-tubulin and then compared with controls.
Preparation of RBPJ-shRNAs
The coding sequence of RBPJ was amplified with forward primer 5′-GGAAGATGGCGCCTGTTGTGACAG-3′ and reverse primer 5′-GTTATCTCGAGTCAAGCGTAGTCTGGGACGGTATGGGTAGGACACCACGGTTGCTGTG-3′. The underlined sequence represents a HA tag. The amplicons were digested with Xhol and BamHI and subcloned into a pcDNA3.1 expression vector (Invitrogen), resulting in a construct named pcDNA-RBPJ. Short hairpin interfering RNAs (shRNAs) targeting RBPJ mRNA (NM_005349.3) were designed according to the Dharmacon siDESIGN Center database. Five shRNAs that target the RBPJ coding region were selected based on the ranking criteria of Reynolds [25]. All the shRNAs were cloned into pLKO.1 vector (Sigma-Aldrich, St. Louis, MO, USA), with pLKO.1 itself (with a scrambled shRNA) used as the mock control.
Transfection
HEK293T cells were seeded in six-well plates in complete media 1 day prior to transfection. To generate the recombinant, the HEK293T packaging cells were transfected with 5–8 μg pVSV-G (Clontech, Mountain View, CA, USA) and 15 μg of recombinant vectors using Lipofectamine-2000 (Invitrogen), according to the manufacturer’s instruction. The transfected cells were then harvested, and whole cell lysates were extracted for Western blot using an anti-RBPJ antibody (Abcam, Cambridge, MA, USA).
Generation of RBPJ knockdown stable clones of lung cancer cells
To generate RBPJ-shRNA lentiviral particles, HEK293T cells were seeded in a 100-mm dish at 50,000 cells/cm2 and co-transfected with 10 μg of RBPJ-shRNA and 5 μg each of packaging plasmids (REV, pMDL, and VSV-G) using Lipofectamine-2000 (Invitrogen). The supernatant containing lentiviral particles was collected 48 h after transfection and filtered through a 0.45-μm syringe filter. Target lung cancer cells were seeded in 100-mm plates at 15,000 cells/cm2 1 day prior to lentiviral infection. The lentiviral particles were added along with 10 μg/ml polybrene (Sigma-Aldrich) to the cell culture for 24 h. Then, the cells were washed twice with complete media and cultured in the presence of puromycin to select the transduced cells.
Western blot analyses
Proteins were extracted in a solution of RIPA and Protease Inhibitor Cocktail (Thermo Scientific, Waltham, MA, USA) from HEK293T cells or lung cancer cells and subjected to SDS-PAGE. Quantification of total protein was carried out using BCA kit (Sigma-Aldrich). The proteins (100 μg) were subjected to 12 % SDS-PAGE. Separated proteins were electrophoretically transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) and immune-blotted with monoclonal mouse anti-human RBPJ (Abcam), polyclonal rabbit anti-human p21 (Abcam), polyclonal rabbit anti-human p27 (Abcam), polyclonal rabbit anti-human Hes1 (Abcam), polyclonal rabbit anti-human SKP2 (Cell Signaling, San Jose, CA, USA), or polyclonal rabbit anti-human α-tubulin (Cell Signaling) antibodies. α-tubulin was used as a protein loading control. The secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs, Bar Harbor, ME, USA). Images shown in the figure were representative from five repeats. Densitometry of Western blots was quantified with NIH ImageJ software.
Cell growth assay
A diphenyltetrazolium bromide (MTT) assay was performed to determine cell growth. Five thousand cells per well were seeded in a 96-well plate and grown for 24, 48, and 72 h. Then, the media were removed and washed with PBS, after which 5 g/l of thiazolyl tetrazolium (Amersco, Indianapolis, IN, USA) was added to each well. Four hours later, MTT was removed and 150 μl of dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) was added. The viability of the cells was calculated from the absorption at 570/630 nm with an enzyme-linked immunosorbent assay reader.
Cell cycle analyses
Cell pellets were resuspended in a cold solution containing 100 μg/ml propidium iodide (Sigma-Aldrich), 0.1 % trinatriumcitrate-dihydrate, and 10 % RNaseA (1 mg/ml) and then incubated at 37 °C for 30 min. Cell cycles were then analyzed by flow cytometry.
Statistics
All statistical analyses were carried out using the SPSS 19.0 statistical software package. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction. All values are depicted as mean ± standard deviation from five individuals and are considered significant if p < 0.05.
Results
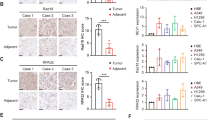
RBPJ expression was effectively inhibited by shRNA constructs
Multiple shRNA constructs were generated and then co-transfected with pcDNA-RBPJ into HEK293T cells to determine their potentials of silencing RBPJ expression. RBPJ protein levels in the transfected cells were determined by Western blot. From the five constructs, four shRNAs resulted in significant inhibition of RBPJ expression, among which shRBPJ-3 was the most efficient one and reduced RBPJ levels in HEK293T cells by more than 90 % (Fig. 1a, b). Thus, shRBPJ-3 was chosen to be used in the current study to knock down RBPJ expression in lung cancer cells.
Selection of shRNAs that inhibit RBPJ expression. a–b Western blot for RBPJ in HEK293T cells transfected with pcDNA-RBPJ plasmids, shown by representative images (a), and by quantification (b). Mock, cells transfected with pLKO.1 vector with a scrambled shRNA. shRBPJ-1 to shRBPJ-6, different shRNA constructs designed to target RBPJ based on mRNA sequence. α -tubulin was a protein loading control. *p < 0.05. n = 5. Statistics: one-way ANOVA with a Bonferroni correction
Inhibition of RBPJ by lenti-shRBPJ-3 in stably transduced lung cancer cells
Three human lung cancer cell lines A549 (origin from carcinoma), SK-LU-1 (origin from adenocarcinoma), and NCI-H23 (origin from non-small cell lung cancer) were used in the current study, since they represent different types of lung cancer. These lung cancer cells were infected with RBPJ-shRNA and pLKO.1 lentivirus to generate stable clones which were then cultured in media for 72 h before they were harvested. We found that RBPJ levels were significantly reduced in all three stably transduced lung cancer cell lines, by RT-qPCR (Fig. 2a), and by Western blot shown with representative images (Fig. 2b) and with quantification (Fig. 2c). These data suggest that shRBPJ inhibited RBPJ transcription and reduced the RBPJ protein production in lung cancer cells.
Inhibition of RBPJ by lenti-shRBPJ-3 in stably transduced lung cancer cells (a–c). Three lung cancer cells were infected with RBPJ-shRNA and pLKO.1 lentivirus to generate stable clones which were then cultured in media for 72 h before they were harvested. RBPJ levels were significantly reduced in all three stably transduced lung cancer cell lines, by RT-qPCR (a) and by Western blot shown with representative images (b) and with quantification (c). α-tubulin was a protein loading control. *p < 0.05. n = 5. Statistics: one-way ANOVA with a Bonferroni correction
Inhibition of RBPJ suppressed growth of lung cancer cells
In order to figure out whether inhibition of RBPJ may affect the growth of lung cancer cells, we analyzed cell growth in a MTT assay. Significant reduced cell growth by RBPJ inhibition was detected as early as 48 h after seeding, compared with controls, in all three lines (Fig. 3a). We then analyzed these cancer cells in a cell cycle phase assay at 72 h after seeding, and we found that the percentage of S-phase proliferating cells in RBPJ-depleted lung cancer cells significantly decreased, while the percentage of G0/G1-phase cells in RBPJ-depleted lung cancer cells significantly increased, compared to mock cells (Fig. 3b). These results suggest that inhibition of RBPJ suppressed growth of lung cancer cells, possibly through inhibiting cell proliferation.
Inhibition of RBPJ suppressed growth of lung cancer cells. a Cell growth was examined in a MTT assay. Significant reduced cell growth by RBPJ inhibition was detected as early as 48 h after seeding, compared with controls, in all three lines. b Cancer cells were analyzed in a cell cycle phase assay at 72 h after seeding, showing that the percentage of S phase proliferating cells in RBPJ-depleted lung cancer cells significantly reduced, while the percentage of G0/G1-phase cells in RBPJ-depleted lung cancer cells significantly increased, compared to mock cells. *p < 0.05. n = 5. Statistics: one-way ANOVA with a Bonferroni correction
RBPJ-depletion inhibited cell proliferation by suppressing S-phase transition
To understand the mechanism underlying the reduced cell growth by RBP depletion, we examined the expression of downstream targets of RBPJ by Western blot. CDK2 is a catalytic subunit of the cyclin-dependent kinase complex, and its activity is restricted to the G1–S phase of the cell cycle for inducing G1–S transition. p21 is an inhibitor for cyclin-CDK2 or -CDK1 complexes. p27 directly inhibits CDK4, resulting in cell arrest in the G1 phase of the cell cycle. The ubiquitin-ligase complex SKP2 is required for the degradation of p21 at both G1–S transition and S phase in the cell cycle [10–17, 26–29]. We found that RBPJ-depleted lung cancer cells exhibited a reduction in the levels of CDK2, Hes1, Bcl-2, and SKP2 but an increase in the levels of p21 and p27, compared to control mock cells (Fig. 4). These results further suggest that RBPJ-depletion inhibited cell proliferation by suppressing S-phase transition.
RBPJ-depletion inhibited cell proliferation by suppressing S-phase transition The levels of downstream targets of RBPJ were examined by Western blot, showing that RBPJ-depleted lung cancer cells exhibited a reduction in the levels of CDK2, Hes1, Bcl-2, and SKP2 but an increase in the levels of p21 and p27, compared to control mock cells. α-tubulin was a protein loading control
Discussion
Inhibition of Notch pathway has been shown to suppress tumorigenesis of lung cancers [30–39]. Moreover, RBPJ acts downstream of the Notch pathway and has been shown to be essential for a functional activated Notch signaling [10–17]. Therefore, here, we studied the specific role of RBPJ in the Notch pathway, and evaluated its effect as a potential therapeutic target for lung cancer.
We used a lentivirus-mediated shRNA delivery method to stably suppress RBPJ gene expression. Both short interfering RNAs (siRNAs) and shRNAs are the most frequently applied methods to specifically and efficiently silence genes at the mRNA level [40, 41]. Delivery of siRNAs or shRNAs by plasmid transfection to target cells suffers from low and variable transfection efficiency and only suppresses gene expression transiently [40, 41]. On the contrary, lentiviral vectors are more efficient and conduct stable gene delivery [40, 41]. The lentiviral vector pLKO.1 is able to integrate into the host genome that allows for the stable expression of shRNA.
Our results showed that the downregulation of RBPJ expression resulted in a significant reduction of cell growth in transduced lung cancer cells, confirmed by cell cycle analyses showing that knockdown of RBPJ decreased S-phase proliferating cells. Moreover, we found that knockdown of RBPJ suppressed the expression of its target genes CDK2, Hes1, Bcl-2, and SKP2 but increased others like p21 and p27. Given the roles of these genes in cell cycle progression, our results suggest that the knockdown of RBPJ inhibits lung cancer cell proliferation by inducing cell-cycle arrest at G1 phase through inhibiting the G1–S transition.
Since we examined several lung cancer cell lines with different tissue origin, our results should not be limited to an individual cell line. In other words, our results are unlikely to be cell-line specific and may be applicable to most lung cancer types. Future studies may be designed to compare the effects of RBPJ knockdown on normal lung cells to lung cancer cells to evaluate the toxicity of the treatment.
Since Notch signaling pathway has been shown to play essential roles in many cancers other than lung cancer, here, the inhibitory effect of RBPJ-depletion on growth of lung cancer cells may also be not limited to an individual cancer type. Future studies may be designed to test this model in other cancers in humans.
References
Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, et al. Treatment of non-small cell lung cancer (NSCLC). J Thorac Dis. 2013;5:S389–96.
Mitsudomi T, Suda K, Yatabe Y. Surgery for nsclc in the era of personalized medicine. Nat Rev Clin Oncol. 2013;10:235–44.
Pallis AG, Syrigos KN. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of NSCLC. Lung Cancer. 2013;80:120–30.
Jian H, Zhao Y, Liu B, Lu S. SEMA4b inhibits MMP9 to prevent metastasis of non-small cell lung cancer. Tumour Biol. 2014;35:11051–6.
Pei J, Lou Y, Zhong R, Han B. MMP9 activation triggered by epidermal growth factor induced FoxO1 nuclear exclusion in non-small cell lung cancer. Tumour Biol. 2014;35:6673–8.
6 Wang W, Wu X, Tian Y: Crosstalk of AP4 and TGFbeta receptor signaling in NSCLC. Tumour Biol 2014.
Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol. 2012;727:186–98.
Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28:3621–30.
Hsieh JJ, Hayward SD. Masking of the CBF1/RBPJ kappa transcriptional repression domain by Epstein-Barr virus EBNA2. Science. 1995;268:560–3.
Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by notch signaling status. Genes Dev. 2013;27:1059–71.
Hori K, Cholewa-Waclaw J, Nakada Y, Glasgow SM, Masui T, Henke RM, et al. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of notch signaling. Genes Dev. 2008;22:166–78.
Guo D, Ye J, Dai J, Li L, Chen F, Ma D, et al. Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk Res. 2009;33:678–85.
Gao F, Yao M, Shi Y, Hao J, Ren Y, Liu Q, et al. Notch pathway is involved in high glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways. J Cell Biochem. 2013;114:1029–38.
Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, et al. Delta-1 activation of notch-1 signaling results in HES-1 transactivation. Mol Cell Biol. 1998;18:7423–31.
Dohda T, Maljukova A, Liu L, Heyman M, Grander D, Brodin D, et al. Notch signaling induces SKP2 expression and promotes reduction of p27Kip1 in t-cell acute lymphoblastic leukemia cell lines. Exp Cell Res. 2007;313:3141–52.
Nakamura T, Miyagawa S, Katsu Y, Mizutani T, Sato T, Takeuchi T, et al. P21 and notch signalings in the persistently altered vagina induced by neonatal diethylstilbestrol exposure in mice. J Vet Med Sci. 2012;74:1589–95.
Marcelo KL, Sills TM, Coskun S, Vasavada H, Sanglikar S, Goldie LC, et al. Hemogenic endothelial cell specification requires c-Kit, notch signaling, and p27-mediated cell-cycle control. Dev Cell. 2013;27:504–15.
Harashima H, Dissmeyer N, Schnittger A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol. 2013;23:345–56.
Teixeira LK, Reed SI. Ubiquitin ligases and cell cycle control. Annu Rev Biochem. 2013;82:387–414.
Maraver A, Fernandez-Marcos PJ, Herranz D, Canamero M, Munoz-Martin M, Gomez-Lopez G, et al. Therapeutic effect of gamma-secretase inhibition in KrasG12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell. 2012;22:222–34.
Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–9.
Hu XB, Feng F, Wang YC, Wang L, He F, Dou GR, et al. Blockade of notch signaling in tumor-bearing mice may lead to tumor regression, progression, or metastasis, depending on tumor cell types. Neoplasia. 2009;11:32–8.
Dou GR, Wang YC, Hu XB, Hou LH, Wang CM, Xu JF, et al. RBP-J, the transcription factor downstream of notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. FASEB J. 2008;22:1606–17.
Nagao H, Setoguchi T, Kitamoto S, Ishidou Y, Nagano S, Yokouchi M, et al. RBPJ is a novel target for rhabdomyosarcoma therapy. PLoS One. 2012;7:e39268.
Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational sirna design for rna interference. Nat Biotechnol. 2004;22:326–30.
Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci U S A. 2014;111:E1211–1220.
Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–99.
Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29.
Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70.
Tang Y, Hu C, Yang H, Cao L, Li Y, Deng P, et al. Rnd3 regulates lung cancer cell proliferation through notch signaling. PLoS One. 2014;9:e111897.
Theys J, Yahyanejad S, Habets R, Span P, Dubois L, Paesmans K, et al. High notch activity induces radiation resistance in non small cell lung cancer. Radiother Oncol. 2013;108:440–5.
Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, et al. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res. 2013;19:1972–80.
Xie M, Liu M, He CS. Sirt1 regulates endothelial notch signaling in lung cancer. PLoS One. 2012;7:e45331.
Xie M, Zhang L, He CS, Xu F, Liu JL, Hu ZH, et al. Activation of Notch-1 enhances epithelial-mesenchymal transition in gefitinib-acquired resistant lung cancer cells. J Cell Biochem. 2012;113:1501–13.
Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem. 2011;112:2773–83.
Galluzzo P, Bocchetta M. Notch signaling in lung cancer. Expert Rev Anticancer Ther. 2011;11:533–40.
Garcia Campelo MR, Alonso Curbera G, Aparicio Gallego G, Grande Pulido E, Anton Aparicio LM. Stem cell and lung cancer development: blaming the Wnt, Hh and Notch signalling pathway. Clin Transl Oncol. 2011;13:77–83.
Westhoff B, Colaluca IN, D’Ario G, Donzelli M, Tosoni D, Volorio S, et al. Alterations of the notch pathway in lung cancer. Proc Natl Acad Sci U S A. 2009;106:22293–8.
Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–5.
Tilesi F, Fradiani P, Socci V, Willems D, Ascenzioni F. Design and validation of siRNAs and shRNAs. Curr Opin Mol Ther. 2009;11:156–64.
Paddison PJ, Hannon GJ. siRNAs and shRNAs: skeleton keys to the human genome. Curr Opin Mol Ther. 2003;5:217–24.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qun Lv and Ronglin Shen contribute equally.
Rights and permissions
About this article
Cite this article
Lv, Q., Shen, R. & Wang, J. RBPJ inhibition impairs the growth of lung cancer. Tumor Biol. 36, 3751–3756 (2015). https://doi.org/10.1007/s13277-014-3015-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-3015-5