Abstract
Glioblastoma is the most aggressive malignant primary brain tumor in humans. The activation of PI3K/Akt1 signaling pathway is involved in the proliferation of glioblastoma; however, the underlying mechanism of Akt1 activation during the development of glioblastoma remains largely unclear. Recently, the modification of molecular molecules at protein level such as acetylation has been shown to be related to the function of these molecules. Thus, in our present studies, the acetylation of Akt1 molecule and its role in the proliferation of glioblastoma cells was explored. The results showed that Akt1 was markedly acetylated in glioblastoma cells compared to normal human astrocytes. Mechanistically, PCAF-mediated Akt1 acetylation enhanced Akt1 phosphorylation at both sites of Thr308 and Ser473 and further promoted the proliferation of glioblastoma cells. Together, these data implicate that, as a post-translational regulation, PCAF-mediated Akt1 acetylation plays an important role in the proliferation of human glioblastoma, suggesting a novel target for clinical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most aggressive and the most frequent tumor, comprising approximately 50 % of the cerebral gliomas. Despite remarkable advances in surgical techniques and treatment options, including chemotherapy and radiotherapy, the prognosis of this disease is still very poor [1–3]. Patients with malignant glioblastoma have a survival rate of less than 10 % at 5 years. Therefore, new therapeutic strategies are urgently needed, and the molecular mechanisms that mediate glioblastoma proliferation need to be explored.
Malignant tumor cells are well characterized by the unfettered reproduction through cell division of their progeny and themselves. One of the important factors that consist of this phenomenon is the activation of signaling pathways. It has been proved that the signaling pathway composed of phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) plays a central role in the regulation of various events in cells including proliferation, growth, differentiation, and survival [4, 5]. PI3K is a heterodimer comprised of a regulatory and a catalytic subunit, which has the ability to catalyze the phosphorylation of phosphatidylinositol-containing lipids [6]. Akt is activated by phosphorylation, predominantly through a PI3K-dependent mechanism, which promotes Akt phosphorylation and results in its full activation [4, 7, 8]. Several studies have indicated that dysregulation of this PI3K/Akt pathway is involved in a variety of tumors, including malignant glioblastoma [5, 9, 10].
Several lines of evidence suggest that protein function is often regulated by post-translational modifications such as ubiquitination and acetylation [11–14], and the modification of ubiquitination and acetylation plays important roles in various biological events including transcriptional regulation, DNA damage repair, cell proliferation, apoptosis, and autophagy [13, 15–19]. Many studies have revealed that signaling molecule Akt1 undergoes lysine-63 chain (K63)-linked ubiquitination, which is crucial for Akt1 phosphorylation and activation in some diseases [4, 20, 21]. However, the Akt1 acetylation and its roles in regulating Akt1 activation in diseases especially human glioblastoma remain largely unclear.
P300/CBP-associated factor (PCAF), also known as lysine (K) acetyltransferase 2B, is a transcriptional coactivator. PCAF has been demonstrated to interact with Myc, β-catenin, Homeobox A10 (HOXA10), and histones [22–26]. Current evidence also reveals that PCAF can promote proliferation of cancer cells [27–29]. However, the biological roles of PCAF in regulating Akt1 activation as well as the proliferation of glioblastoma cells remain elusive.
In the present study, we reported that the acetylation of Akt1 was significantly enhanced in human glioblastoma cells together with increased phosphorylation of Akt1. Further studies revealed that the expression of PCAF was upregulated and required for Akt1 acetylation and phosphorylation in glioblastoma cells, which finally contributed to the proliferation of glioblastoma cells.
Materials and methods
Reagents and animals
Monoclonal antibodies against HA (sc-7392), His (sc-53073), and PCAF (sc-13124) were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Monoclonal antibodies against t-Akt1 (4685), p-Akt-Thr308 (13038), p-Akt-Ser473 (4060), acetylated lysine (9681), β-actin (3700), HRP-conjugated anti-rabbit (7074), and anti-mouse IgG (7076) as well as anti-rabbit IgG (Conformation Specific, 5127) and 20× LumiGLO reagent® and 20× peroxide were purchased from Cell Signaling Technology (Danvers, USA). The co-IP kit and radioimmunoprecipitation assay (RIPA) lysis buffer were purchased from Thermo (Fremont, USA). Bicinchoninic acid (BCA) protein assay reagent was from Pierce (Rockford, USA). Cell counting kit-8 (CCK-8) was purchased from Dojindo Labohumanories (Kumamoto, Japan). M-myeloblastosis virus reverse transcriptase XL was from Promega (Madison, USA). SYBR Green PCR Master Mix was purchased from Applied Biosystems (Foster City, USA). The incision enzyme KpnI, EcoRV, and T4 DNA ligase were purchased from Fermentas (Burlington, Canada). The vector of pcDNA3.1, Lipofectamine 2000, TRIzol, Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, USA). The shRNA expression plasmids of pGPU6/GFP were purchased from GenePharma (Shanghai, China).
Cell culture
The established human malignant glioma cell line of U-87 MG was purchased from American Tissue Culture Collection (Rockville, USA). Normal human astrocytes (NHA) were obtained from ScienCell Research Labohumanories (Carlsbad, USA). Cells were cultured in DMEM supplemented with 10 % heat-inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were incubated at 37 °C in a 5 % CO2 incubator.
Plasmid construction
The plasmids of pcDNA3.1/PCAF-HA and pcDNA3.1/Akt1-His were constructed by inserting the complementary DNA (cDNA) of human PCAF gene (NM_003884.4) or Akt1 gene (NM_005163.2) into pcDNA3.1. The PCAF gene and Akt1 gene were amplified by polymerase chain reaction (PCR) from cDNA of human astrocytes. The PCR products and pcDNA3.1 vector were digested with KpnI and EcoRV and then ligated using T4 DNA ligase. Four different shRNA sequences were designed against different targeted regions of human PCAF mRNA (NM_003884.4). The different plasmids of PCAF shRNA were constructed by using pGPU6/GFP [30, 31], and the most effective shRNA expression plasmids were chosen together with the scrambled control shRNA expression plasmids for further functional experiments.
Cellular transfection
U-87 MG cells and normal human astrocytes were transfected with Lipofectamine 2000 according to the manufacturer’s instructions [32, 33]. Three micrograms of plasmids was mixed with 250 μl of serum-free DMEM, and 10 μl of Lipofectamine 2000 was mixed with 250 μl of serum-free DMEM, respectively. They were then mixed with each other and incubated for 18 min at room temperature (RT). Then, the 500 μl of mixture was transferred into each well. Finally, the medium was replaced with serum containing DMEM at 6 h after transfection.
Co-immunoprecipitation experiment
For each sample, 300 μg of extract prepared from U-87 MG cells was mixed with 50 μl protein G-Sepharose beads within the co-immunoprecipitation (co-IP) assay buffer, incubated for 2 h, and centrifuged for 2 min at 4 °C. The recovered supernatant was obtained and incubated with the corresponding antibody (2 μg for each sample, pre-immune IgG as a control reaction) at 4 °C overnight. Then, 50 μl of protein G-Sepharose beads was added into the supernatant and incubated continuously for 3 h at 4 °C. Protein G-precipitated protein complex was recovered by centrifugation and resuspended in 40 μl of 2× SDS PAGE sample buffer and then boiled for 6 min. The samples were then analyzed with specific antibodies by immunoblot assay. Meanwhile, a 40-μg aliquot of whole-cell extract (WCE) from U-87 MG cells without immunoprecipitation was detected as an input control.
RNA extraction and real-time quantitative PCR
The U-87 MG cells and normal human astrocytes were washed three times with ice-cold phosphate-buffered saline (PBS), and total RNA was extracted using TRIzol. An equal amount of total RNA (1 μg) was used for cDNA synthesis using the oligo-dT primer and M-myeloblastosis virus reverse transcriptase XL in a reaction volume of 25 μl, according to the manufacturer’s instructions. Synthesized cDNA (1 μg) was used for each PCR reaction. qPCR experiments were performed with the SYBR Green PCR Master Mix. The PCR products were subjected to melting curve analysis to exclude the synthesis of non-specific products. The Ct value was quantified using a standard curve for the specific gene and relatively quantified using β-actin as an internal reference control. The Ct value was then normalized to the average expression levels of control group, calculated according to the 2−ΔΔCt method.
Immunoblot analysis
Cells were washed with cold PBS twice, and then 150 μl RIPA buffer was added to each dish. After that, cells lysates were shaken at 4 °C for 15 min. Cell lysates were centrifuged at 12,000g at 4 °C for 10 min. The supernatant was collected, and protein content was measured using BCA protein assay reagent. Equal amounts of the protein (40 mg) from each sample were sepahumaned through 10–12 % SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membrane. And then, the membrane was blocked with 5 % (w/v) non-fat dry milk in TBS buffer (100 mM NaCl, 10 mM Tris–HCl [pH 7.6], and 0.1 % (v/v) Tween 20) for 60 min at room temperature and the primary antibodies were added overnight on a shaker at 4 °C. On the second day, PVDF membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody for 1 h at room temperature. Bound antibody was detected using enhanced chemiluminescence reagent. The semi-quantitation of proteins was surveyed with a Bio-Rad Gel Doc™ EZ System. β-Actin was used as an internal control of protein loading, and the relative protein level in each group was expressed relative to control group.
CCK8 assay
Cells were seeded into 96-well plates and cultured for different treatments. During the final 4 h of culture, CCK-8 reagent was added to the culture medium. Absorbance values were then measured at 450 nm with a microplate reader (Bio-Rad model 680, USA). The absorbance was directly proportional to the cell number [10, 34].
Statistical analysis
Results are representative of three independent experiments. Data are presented as means ± SD. One-way analysis of variance (ANOVA) followed by post hoc analysis Dunnet’s t test was used to evaluate statistical differences. P < 0.05 was considered significant.
Results
The acetylation of Akt1 is enhanced in human glioblastoma cells
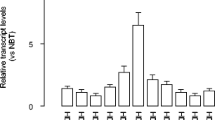
We first detected the level of Akt1 acetylation and phosphorylation in human malignant glioma cell line (U-87 MG), and normal human astrocyte (NHA) line was used as a control. As shown in Fig. 1, the levels of Akt1 acetylation (Fig. 1a) and phosphorylation at both sites of Thr308 and Ser473 (Fig. 1b–d) were synergistically enhanced in U-87 MG cells when compared with normal human astrocytes. These data indicate that Akt1 acetylation perhaps closely correlated with Akt1 phosphorylation and activation in human glioblastoma cells.
The acetylation of Akt1 in human glioblastoma cells. Human malignant glioma cell line (U-87 MG) and normal human astrocytes line (NHA) as a control were cultured in vitro, and the levels of Akt1 acetylation and phosphorylation were detected. The level of Akt1 acetylation was significantly enhanced in U-87 MG cells when compared with normal human astrocytes (a). The levels of Akt1 phosphorylation at both Thr308 (b and c) and Ser473 (b and d) were also enhanced in U-87 MG cells when compared with normal human astrocytes. **P < 0.01 vs. NHA group
PCAF expression is elevated in human glioblastoma cells
It is well known that PCAF is one of the most common acetyl transferases with the activity to acetylate various target proteins. Since the acetylation of Akt1 was found to be enhanced in U-87 MG cells (Fig. 1a), the mRNA level of PCAF was further detected in U-87 MG cells. Our results revealed that PCAF mRNA was significantly elevated in U-87 MG cells compared to normal human astrocytes (Fig. 2a). Meantime, the expression PCAF at protein level was similar to that of mRNA level (Fig. 2b).
The expression of PCAF and the interaction of Akt1 with PCAF. Real-time PCR demonstrated that PCAF mRNA level was significantly elevated in U-87 MG cells compared to normal human astrocytes (a). Immunoblot analysis showed that the expression PCAF at protein level was also increased in U-87 MG cells relative to normal human astrocytes (b). Co-IP experiment showed that U-87 MG cells could increase interaction of PCAF with Akt1 compared to normal human astrocytes (c). The interaction of PCAF with Akt1 was also observed with co-IP experiment in 293T by the overexpression of PCAF and Akt1 together (d). **P < 0.01 vs. NHA group
The interaction of Akt1 with PCAF is increased in human glioblastoma cells
Since the acetylation of Akt1 and the expression of PCAF have been found increased in U-87 MG cells (Figs. 1a and 2a, b), we set out to investigate the possible interaction between PCAF and Akt1 at protein level in U-87 MG cells. The results showed that U-87 MG cells exhibited increased interaction of PCAF with Akt1 at protein level compared to normal human astrocytes (Fig. 2c). Consistent with this, we observed the interaction of PCAF with Akt1 at protein level in 293T by the overexpression of PCAF along with Akt1 (Fig. 2d). Taken together, these findings indicate that PCAF upregulation might contribute to Akt1 acetylation in human glioblastoma cells.
PCAF-mediated Akt1 acetylation is required for Akt1 phosphorylation in human glioblastoma cells
To gain further insight into the effects of PCAF on Akt1 acetylation and phosphorylation in human glioblastoma cells, U-87 MG cells were treated with PCAF shRNA to science PCAF gene expression. Immunoblot assay further showed that silencing PCAF gene by using PCAF shRNA could decrease not only Akt1 acetylation but also Akt1 phosphorylation (Thr308 and Ser473) in U-87 MG cells when compared with control shRNA (Fig. 3). Taken together, these data suggest that acetyltransferase activity of PCAF is needed for Akt1 acetylation and phosphorylation in human glioblastoma cells.
The role of PCAF-mediated Akt1 acetylation in Akt1 phosphorylation in human glioblastoma cells. U-87 MG cells were transfected with the plasmids of PCAF shRNA and control shRNA, respectively, and then cultured for 48 h. Immunoblot assay showed that silencing PCAF gene with PCAF shRNA could decrease not only Akt1 acetylation but also Akt1 phosphorylation in U-87 MG cells when compared with control shRNA treatment. **P < 0.01 vs. control shRNA group
PCAF-mediated Akt1 acetylation is necessary for human glioblastoma cell proliferation
To access the role of PCAF-mediated Akt1 acetylation in the proliferation of human glioblastoma cells, U-87 MG cells were treated with PCAF shRNA or PI3K inhibitor (Ly294002), and then the cellular proliferation was investigated. The data showed that not only inhibition of PCAF with PCAF shRNA but also inhibition of Akt1 with Ly294002 could abolish the proliferation of U-87 MG cells relative to control treatments, respectively (Fig. 4a, c). Further studies showed that the proliferation of U-87 MG cells was inhibited in a dose-dependent manner after treatment with different doses of PCAF shRNA (Fig. 4b), demonstrating the role of PCAF in promoting U-87 MG cell proliferation. Alternatively, overexpression of PCAF could induce the proliferation of not only U-87 MG cells (Fig. 4d) but also normal human astrocytes (Fig. 4e), which was also related to Akt1 acetylation (Fig. 4f) and phosphorylation (Fig. 4g). Taken together with our previous results, these data support the idea that PCAF-mediated Akt1 acetylation and phosphorylation might potentiate the proliferation of glioblastoma cells.
The role of PCAF-mediated Akt1 acetylation in glioblastoma cell proliferation. U-87 MG cells were transfected with the plasmids of PCAF shRNA and control shRNA, respectively, and then the cellular proliferation was investigated at 48 h after transfection. CCK-8 showed that PCAF shRNA could abolish the proliferation of U-87 MG cells relative to control shRNA treatment (a). **P < 0.01 vs. control shRNA group. Further studies showed that the proliferation of U-87 MG cells was inhibited in a dose-dependent manner after treatment with different doses of PCAF shRNA (b). **P < 0.01 vs. control shRNA group. Proliferation assay also showed that the inhibition of Akt1 with Ly294002 (20 μM) could abolish the proliferation of U-87 MG cells relative to DMSO treatment (c). **P < 0.01 vs. DMSO group. Overexpression of PCAF with pcDNA3.1/PCAF could induce the cell proliferation not only in U-87 MG cells (d) but also in normal human astrocytes (e) with CCK-8 analysis. **P < 0.01 vs. pcDNA3.1 empty vector treatment. Co-IP assay showed that overexpression of PCAF with pcDNA3.1/PCAF could induce Akt1 acetylation (f) and phosphorylation (g) in normal human astrocytes when compared with pcDNA3.1 empty vector treatment
Discussion
As the most aggressive malignant primary brain tumor in humans, glioblastoma proliferation is believed to be a multistep process, during which a sequence of genetic and epigenetic alterations randomly occurs to affect the genes controlling cell proliferation, cell death, and genetic stability. The proliferation of cancer cells is regulated by a complex array of signaling pathways [35–37]. Among these signaling pathways, the activation of PI3K/Akt1 signaling pathway is involved in the proliferative and anti-apoptotic effect in glioblastoma [38–40]; however, the underlying mechanism about the regulation of Akt1 activation remains elusive.
It has been reported that Akt1 is able to undergo K63-linked ubiquitination, which is a critical regulator of Akt phosphorylation and subsequent activation [20, 21]. However, the roles of acetylation modification of Akt1 in regulating Akt1 activation in glioblastoma remain largely unclear. Given that protein acetylation is known to be an important post-translational modification that functions in various aspects [21, 41], Akt1 acetylation was further measured in glioblastoma cells. The data revealed that the Akt1 acetylation was significantly increased in glioblastoma cells, implying that Akt1 acetylation is probably involved in regulating Akt1 phosphorylation and activation.
PCAF is known as a kind of histone acetyltransferase (HAT) containing two functional domains including an N-terminal HAT and a C-terminal bromodomain. PCAF is believed to interact with acetyl-lysine residue which modulates concurrently multiple cell pathways via acetylating histones and non-histone proteins [42, 43]. Nevertheless, the precise role of PCAF in promoting acetylation of Akt1 in glioblastoma cells is largely unknown. Our present studies revealed that PCAF expression was significantly elevated at both mRNA and protein levels in U-87 MG cells compared to normal human astrocytes. So, the interaction between PCAF and Akt1 at protein level in glioblastoma cells was determined through co-IP analyses. The results showed that PCAF could interact with Akt1 molecule in glioblastoma cells. In our further studies, knockdown of PCAF by using shRNA not only suppressed Akt1 acetylation and phosphorylation but also inhibited the proliferation of glioblastoma cells. Meanwhile, overexpression of PCAF enhanced Akt1 acetylation and phosphorylation as well as cellular proliferation in normal human astrocytes lines. Finally, inhibition of Akt1 with Ly294002 could also abolish the proliferation of U-87 MG cells. These findings indicate that PCAF-mediated Akt1 acetylation has the ability to enhance the proliferation of human glioblastoma cells. However, it is still unclear about the mechanism of direct interaction between PCAF and Akt1. One possibility is upregulated PCAF could directly promote Akt1 acetylation. Another possibility is PCAF could promote Akt1 acetylation together with other acetyl transferases or PCAF could increase expression of other genes. On the other hand, in addition to Akt1, PCAF might also acetylate other molecules in human glioblastoma cells. Therefore, more studies need to be done to explore the above-mentioned other possible mechanisms.
In summary, the acetylation of Akt1 and its role in human glioblastoma cells were examined in the present study. Here, we presented evidence that the acetylation of Akt1 was enhanced in human glioblastoma cells. Furthermore, PCAF-mediated Akt1 acetylation was demonstrated to play an important role in mediating Akt1 phosphorylation and activation. Akt1 activation further promoted the proliferation of human glioblastoma cells. These findings might provide novel insights into the pathogenesis of human glioblastoma.
Abbreviations
- CCK-8:
-
Cell counting kit-8
- shRNA:
-
Short hairpin RNA
- WCE:
-
Whole-cell extract
- IB:
-
Immunoblot
- co-IP:
-
Co-immunoprecipitation
- OD:
-
Optical density
- PCAF:
-
P300/CBP-associated factor
- HOXA10:
-
Homeobox A10
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- PBS:
-
Phosphate-buffered saline
- HAT:
-
Histone acetyltransferases
References
Liang Q, Ma C, Zhao Y, Gao G, Ma J. Inhibition of STAT3 reduces astrocytoma cell invasion and constitutive activation of STAT3 predicts poor prognosis in human astrocytoma. PLoS One. 2013;8:e84723.
Hottinger AF, Stupp R, Homicsko K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin J Cancer. 2014;33:32–9.
Zhou J, Wang W, Gao Z, Peng X, Chen X, Chen W, et al. Microrna-155 promotes glioma cell proliferation via the regulation of MXI1. PLoS One. 2013;8:e83055.
Qiu W, Zhang Y, Liu X, Zhou J, Li Y, Zhou Y, et al. Sublytic C5b-9 complexes induce proliferative changes of glomerular mesangial cells in rat Thy-1 nephritis through TRAF6-mediated PI3K-dependent Akt1 activation. J Pathol. 2012;226:619–32.
Hu P, Li B, Zhang W, Li Y, Li G, Jiang X, et al. Acsdkp regulates cell proliferation through the PI3KCA/Akt signaling pathway. PLoS One. 2013;8:e79321.
Brader S, Eccles SA. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori. 2004;90:2–8.
Mahajan K, Coppola D, Challa S, Fang B, Chen YA, Zhu W, et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS One. 2010;19:e9646.
Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006;20:3364–75.
Dudley A, Sater M, Le PU, Trinh G, Sadr MS, Bergeron J, Deleavey GF, Bedell B, Damha MJ, Petrecca K. DRR regulates AKT activation to drive brain cancer invasion. Oncogene 2013.
Wu Z, Wang G, Xu S, Li Y, Tian Y, Niu H, et al. Effects of tetrandrine on glioma cell malignant phenotype via inhibition of ADAM17. Tumour Biol. 2014;35:2205–10.
Beltrao P, Albanese V, Kenner LR, Swaney DL, Burlingame A, Villen J, et al. Systematic functional prioritization of protein posttranslational modifications. Cell. 2012;150:413–25.
Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–96.
Qiu W, Zhou J, Zhu G, Zhao D, He F, Zhang J, et al. Sublytic C5b-9 triggers glomerular mesangial cell apoptosis via XAF1 gene activation mediated by p300-dependent IRF-1 acetylation. Cell Death Dis. 2014;5:e1176.
Yan J, Li Q, Mao AP, Hu MM, Shu HB. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J Mol Cell Biol. 2014;6:154–63.
Pejanovic N, Hochrainer K, Liu T, Aerne BL, Soares MP, Anrather J. Regulation of nuclear factor kappab (NF-kappab) transcriptional activity via p65 acetylation by the chaperonin containing TCP1 (CCT). PLoS One. 2012;7:e42020.
Li T, Diner BA, Chen J, Cristea IM. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc Natl Acad Sci U S A. 2012;109:10558–63.
Xie J, Peng M, Guillemette S, Quan S, Maniatis S, Wu Y, et al. FANCJ/BACH1 acetylation at lysine 1249 regulates the DNA damage response. PLoS Genet. 2012;8:e1002786.
Eckner R. p53-dependent growth arrest and induction of p21: a critical role for PCAF-mediated histone acetylation. Cell Cycle. 2012;11:2591.
Webster BR, Scott I, Han K, Li JH, Lu Z, Stevens MV, et al. Restricted mitochondrial protein acetylation initiates mitochondrial autophagy. J Cell Sci. 2013;126:4843–9.
Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–8.
Liu J, Netherland C, Pickle T, Sinensky MS, Thewke DP. Stimulation of Akt poly-ubiquitination and proteasomal degradation in P388D1 cells by 7-ketocholesterol and 25-hydroxycholesterol. Arch Biochem Biophys. 2009;487:54–8.
Zhao J, Gong AY, Zhou R, Liu J, Eischeid AN, Chen XM. Downregulation of PCAF by miR-181a/b provides feedback regulation to TNF-alpha-induced transcription of proinflammatory genes in liver epithelial cells. J Immunol. 2012;188:1266–74.
Love IM, Sekaric P, Shi D, Grossman SR, Androphy EJ. The histone acetyltransferase PCAF regulates p21 transcription through stress-induced acetylation of histone H3. Cell Cycle. 2012;11:2458–66.
Ge X, Jin Q, Zhang F, Yan T, Zhai Q. PCAF acetylates {beta}-catenin and improves its stability. Mol Biol Cell. 2009;20:419–27.
Patel JH, Du Y, Ard PG, Phillips C, Carella B, Chen CJ, et al. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24:10826–34.
Zhu LH, Sun LH, Hu YL, Jiang Y, Liu HY, Shen XY, et al. PCAF impairs endometrial receptivity and embryo implantation by down-regulating beta3-integrin expression via HOXA10 acetylation. J Clin Endocrinol Metab. 2013;98:4417–28.
Malatesta M, Steinhauer C, Mohammad F, Pandey DP, Squatrito M, Helin K. Histone acetyltransferase PCAF is required for Hedgehog-Gli-dependent transcription and cancer cell proliferation. Cancer Res. 2013;73:6323–33.
Lin R, Tao R, Gao X, Li T, Zhou X, Guan KL, et al. Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol Cell. 2013;51:506–18.
Gong AY, Eischeid AN, Xiao J, Zhao J, Chen D, Wang ZY, et al. miR-17-5p targets the p300/CBP-associated factor and modulates androgen receptor transcriptional activity in cultured prostate cancer cells. BMC Cancer. 2012;12:492.
Wang J, Zu J, Xu G, Zhao W, Jinglong Y. Inhibition of focal adhesion kinase induces apoptosis in human osteosarcoma SAOS-2 cells. Tumour Biol. 2014;35:1551–6.
Yang T, Qiu H, Bao W, Li B, Lu C, Du G, et al. Epigenetic inactivation of EFEMP1 is associated with tumor suppressive function in endometrial carcinoma. PLoS One. 2013;8:e67458.
Liang X, Li X, Chang J, Duan Y, Li Z. Properties and evaluation of quaternized chitosan/lipid cation polymeric liposomes for cancer-targeted gene delivery. Langmuir. 2013;29:8683–93.
Perrone S, Usai M, Lazzari P, Tucker SJ, Wallace HM, Zanda M. Efficient cell transfection with melamine-based gemini surfactants. Bioconjug Chem. 2013;24:176–87.
Kim HY, Hwang JY, Kim SW, Lee HJ, Yun HJ, Kim S, et al. The CXCR4 antagonist AMD3100 has dual effects on survival and proliferation of myeloma cells in vitro. Cancer Res Treat. 2010;42:225–34.
Magi S, Saeki Y, Kasamatsu M, Tashiro E, Imoto M. Chemical genomic-based pathway analyses for epidermal growth factor-mediated signaling in migrating cancer cells. PLoS One. 2014;9:e96776.
Xiao W, Chen X, He M. Inhibition of the jagged/notch pathway inhibits retinoblastoma cell proliferation via suppressing the PI3K/Akt, Src, p38MAPK and Wnt/betacatenin signaling pathways. Mol Med Rep 2014.
Hsu FT, Liu YC, Chiang IT, Liu RS, Wang HE, Lin WJ, Hwang JJ: Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-kappab signaling. Int J Oncol 2014.
Peng R, Jiang B, Ma J, Ma Z, Wan X, Liu H, et al. Forced downregulation of RACK1 inhibits glioma development by suppressing Src/Akt signaling activity. Oncol Rep. 2013;30:2195–202.
Zhang Z, Wu L, Wang J, Li G, Feng D, Zhang B, et al. Opposing effects of PI3K/Akt and Smad-dependent signaling pathways in NAG-1-induced glioblastoma cell apoptosis. PLoS One. 2014;9:e96283.
Xue X, Wang X, Liu Y, Teng G, Wang Y, Zang X, Wang K, Zhang J, Xu Y, Wang J, Pan L. SchA-p85-FAK complex dictates isoform-specific activation of Akt2 and subsequent PCBP1-mediated post-transcriptional regulation of TGFbeta-mediated epithelial to mesenchymal transition in human lung cancer cell line A549. Tumour Biol 2014.
Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, et al. Helicobacter pylori CagA activates NF-kappab by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–9.
Puttagunta R, Tedeschi A, Soria MG, Hervera A, Lindner R, Rathore KI, et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat Commun. 2014;5:3527.
Shi S, Lin J, Cai Y, Yu J, Hong H, Ji K, et al. Dimeric structure of p300/CBP associated factor. BMC Struct Biol. 2014;14:2.
Acknowledgments
This work was supported by the China Natural Science Foundation (81000963, 81370062, and 81302196), Jiangsu Province’s 333 Talent Program (BRA2011046), Jiangsu Province “six personnel peak” funded projects (2013-WSN-028), Jiangsu Province’s Natural Science Foundation (BK2012670), Medical Research Foundation by Jiangsu Province Health Department (YG201301 and Z201318), the Clinical Technology Development of Jiangsu University (JLY20120053), the Kunshan Social Development Foundation (KS1006, KS1009), and the Suzhou Social Development Foundation (SYS201063).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shuguang Zhang, Guan Sun, Zhimin Wang, and Yi Wan are co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, S., Sun, G., Wang, Z. et al. PCAF-mediated Akt1 acetylation enhances the proliferation of human glioblastoma cells. Tumor Biol. 36, 1455–1462 (2015). https://doi.org/10.1007/s13277-014-2522-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2522-8