Abstract
Adiponectin, as an important adipocytokine, plays a pivotal role in the regulation of insulin sensitivity and metabolism. It has been reported that circulating adiponectin levels were decreased in women with polycystic ovary syndrome (PCOS). However, the results remained inconsistent. In order to derive a more precise estimation of this relationship, a large meta-analysis was performed in this study. A comprehensive systematic electronic search was conducted in electronic databases PubMed, EMBASE, and Web of Science up to November 30, 2013. Pooled weighted mean differences (WMDs) with 95 % confidence intervals (CIs) were calculated to estimate the strength of the association. A meta-analysis technique was used to study 38 trials involving 1,944 PCOS women and 1,654 healthy controls. Overall pooled adiponectin levels in women with PCOS were significantly reduced compared with healthy controls (WMD −2.67, 95 % CI −3.22 to −2.13; P = 0.000), yet with significant heterogeneity across studies (I2 = 95.9 %, P = 0.000). In subgroup analysis by HOMA-IR ratio and total testosterone ratio, inconsistent results were presented. No single study was found to affect the overall results by sensitivity testing. Meta-regression suggested that BMI might contribute little to the heterogeneity between including studies. Cumulative meta-analysis demonstrated the reliability and stability of the meta-analysis results. No evidence of publication bias was observed. Our meta-analysis suggested that circulating adiponectin levels in women with PCOS were significantly lower than those in healthy controls, which indicated that circulating adiponectin might play a role in the development of PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic disorder with principal characteristics of hyperandrogenism, chronic oligoanovulation, and polycystic ovary in reproductive-aged women [1]. These symptoms are usually accompanied by obesity, insulin resistance, and infertility [2–4]. In addition to being an important cause of infertility and exhibiting a higher risk of type 2 diabetes, dyslipidemia, hypertension, and cardiovascular diseases, PCOS is a growing public health concern [5–8]. Although the pathogenesis of PCOS remained unknown, previous studies showed that low adiponectin level was independently associated with insulin resistance or weight in PCOS patients, which suggested that adiponectin might contribute to the development of PCOS [9–13].
Adiponectin, as an important adipocytokine, is the most abundant secreted protein expressed exclusively in adipose tissue and plays a pivotal role in the regulation of insulin sensitivity and metabolism [14]. The major action of adiponectin is to increase insulin sensitivity by stimulating glucose uptake in the liver and muscle, decreasing hepatic gluconeogenesis, and promoting fatty acid β-oxidation in the skeletal muscle [15]. Moreover, adiponectin reduced insulin-induced progesterone and androstenedione production in bovine theca cells in vitro [16]. Indeed, circulating adiponectin levels are lower in obese subjects and are negatively correlated with insulin resistance and testosterone levels [17]. In addition, it has been reported that circulating adiponectin levels were decreased in patients with insulin resistance, type 2 diabetes, cardiovascular disease, and hypertension [17–19]. Therefore, the researchers speculated that circulating adiponectin decreases might play a role in the pathogenesis of PCOS, and devoted to this research [20–23].
Until recently, a number of studies have investigated the associations between adiponectin level and PCOS risk. However, results of these studies were conflicting rather than conclusive. To better understand whether adiponectin level was associative with PCOS, Toulis et al. [24] published a meta-analysis comparing circulating adiponectin levels in women with PCOS and non-PCOS controls matched for similar body mass index (BMI). This analysis revealed that adiponectin levels are lower in women with PCOS than in controls. However, during the last 5 years, many more relevant studies have been published and represented inconsistent results [11–13, 25–46]. As the previous meta-analysis included a limited number of studies, we conducted this updated meta-analysis to investigate the association between circulating adiponectin levels and PCOS from all the relevant studies, including all the newly published studies.
Methods
Search strategy
A comprehensive systematic electronic search was conducted in electronic databases PubMed, EMBASE, and Web of Science up to November 30, 2013. All searches were limited to human subjects without language restriction. The search strategy used the following MeSH terms: “adiponectin,” “adipokines,” “polycystic ovary syndrome,” and “hyperandrogenism.” A manual search of the reference lists of additional relevant articles was performed. Two reviewers (Xiamei Huang and Shan Li) independently completed the literature search, as well as screening of titles, abstracts, full-text articles, and evaluating the relevant articles on the basis of prespecified eligibility criteria. Any discrepancy was resolved by consultation to reach consensus with the third investigator (Xue Qin). Our meta-analysis was conducted according to the Meta-analysis of Observational Studies in Epidemiology guidelines [47].
Eligibility criteria
Eligible studies for this study were those studies that reported serum or plasma adiponectin levels in women with PCOS compared with healthy controls, providing total adiponectin means (M), and standard deviation (SD) or sufficient information to calculate adiponectin means and SD. We excluded literature reviews, letters to the editor, studies on animals or cell lines, studies without healthy control group, as well as studies about genetic variation in adiponectin-related genes. The studies were also excluded if studies enrolled patients with a disease other than PCOS, medication treatment, or pregnant patients. We tried to contact the authors to obtain the relevant information when the data was insufficient or there was doubt about the publications. The study, which included less than 20 subjects, could not be included in this meta-analysis. All case and control subjects in the included studies were matched the BMI and age.
Data extraction
Information was carefully extracted from all eligible studies by two investigators (Xiamei Huang and Shan Li) independently, using a standardized data extraction form. Any disagreements were resolved by discussion during a consensus meeting with a third investigator (Xue Qin). The following information was extracted from each included study: first authors’ name, year of publication, region of study population, total numbers of cases and controls, methods of adiponectin measurement, the type of blood sample, total adiponectin levels (means and SD or standard error, or 95 % confidence interval (95 % CI) of means or median and interquartile range) in cases and controls, the age and the means of BMI estimation. To retrieve the missing data, we also contacted the authors of primary studies.
Quality evaluation of literatures
Quality evaluation of studies was conducted independently by two reviewers (Xiamei Huang and Shan Li) according to the Newcastle-Ottawa Scale (NOS) [48]. The NOS tool contains nine items and scores ranged 0 to 9 (Table 1). The quality assessment of all the included studies that evaluated the association between circulating adiponectin levels and PCOS is showed in Table 2.
Statistical analysis
Adiponectin levels in each study were extracted as mean ± SD. When the studies did not report the means and SD, we tried to communicate the authors for primary studies to retrieve the missing data. In case of no response or unavailability of the requested information, we transformed the reported standard error, or 95 % CI of means, medians, and ranges to means and SD using the formulas as described by Hozo et al. [49]. Homeostasis model assessment of insulin resistance (HOMA-IR) value was used as measures of insulin sensitivity. When not reported, missing HOMA-IR values were estimated according to reported mean glucose and insulin values, using the Oxford Diabetes Trials Unit calculator (http://www.dtu.ox.ac.uk).
Weighted mean differences (WMDs) together with the corresponding 95 % CIs in total adiponectin levels were initially estimated using a fixed-effects model. If there was significant heterogeneity, we turned to use a random-effects model [50]. To investigate the sources of heterogeneity between the results of different studies, we carried out the following tests: heterogeneity tests, stratified analysis, meta-regression analysis, and sensitivity analysis [51, 52]. For heterogeneity tests, we used Cochran Q test and I2 statistic to evaluate statistical heterogeneity among studies [53]. Statistically significant heterogeneity was considered when P value was less than 0.1 and I2 value was more than 50 % [54]. Stratified analysis was conducted on the basis of BMI, population region, sample size, methods of adiponectin measurement, HOMA2-IR ratio, total testosterone ratio (T-ratio), and the type of blood sample. Subsequently, restricted maximum likelihood-based random-effects meta-regression analyses was carried out to evaluate the above potential heterogeneous factors. Univariate meta-regression analysis was conducted first, after which the variables that were significant at the 0.1 level were entered into the multivariable model. To identify potentially influential studies, sensitivity analysis was also performed to examine whether the effect estimate was robust by repeating the random-effects meta-analysis after omitting one study at a time. In addition, cumulative meta-analysis was conducted to evaluate the evolution of the combined estimates over time according to the ascending date of publication. Finally, the possibility of publication bias was assessed by Begg’s funnel plots and Egger’s tests [55].
All statistical analyses were conducted using STATA version 12.0 (StataCorp LP, College Station, Texas, USA). A two-sided P value less than 0.05 was considered statistically significant.
Results
Literature search results
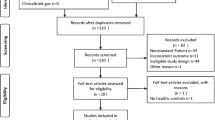
A flow chart showing the study selection is presented in Fig. 1. According to our search criteria, 62 potential eligible studies that estimated the effect of total adiponectin levels on PCOS were identified for full-text assessment. After reading the full texts, 24 studies were excluded as they did not meet the predefined selection criteria. Seven studies were excluded because they were published with insufficient information; we could not extract the data [56–62]. Seven studies were excluded because circulating adiponectin was not measured in the healthy control group [63–69]. Four studies [70–73] with different BMI between PCOS women and controls were also excluded. Three studies [74–76] that published their results in geometric mean (−2SD to +2SD) rather than mean ± SD were subsequently excluded as we were unable to obtain original data from the authors and could not extract original data from the previous meta-analysis. Another two studies [77, 78] that contained less than ten specimens in each group were also excluded. Partial subjects were overlap between two studies [40, 79] by the same authors, so the smaller size one [79] was excluded. Ultimately, we included a total of 38 studies with 3,598 subjects (1,944 PCOS women and 1,654 controls) in this meta-analysis [9–13, 20–23, 25–46, 80–86].
Study characteristics
The main characteristics of the included studies are shown in Table 2. These studies were published between 2003 and 2013. The majority of the trials were conducted in Asia, whereas nine were done in Europe, six in America, and one in Australia. Serum specimens were used to measure total adiponectin levels in almost all studies, and only six studies used plasma specimens. Total adiponectin was measured by enzyme-linked immunosorbent assay in 24 studies and by radioimmunoassay in 14 studies. Furthermore, most studies investigated HOMA-IR and total testosterone levels between PCOS women and controls to account for a difference in adiponectin levels and a percentage of the potential variability of across-study results. There were eight studies without HOMA-IR results, and ten studies without testosterone levels. Among them, four studies [13, 21, 36, 39] reported total adiponectin level only in two subgroups of population stratified by BMI. Then we treated them as two independent studies in the following quantitative synthesis. Therefore, a total of 42 separate comparisons were included in our final meta-analysis.
Quantitative synthesis of data
A meta-analysis of 42 separate comparisons, which reported data on 3,598 subjects (1,944 patients with PCOS and 1,654 controls), was performed. The overall effect of the pooled analyses indicated that adiponectin levels in PCOS patients were significantly lower than healthy controls, with summary WMD of 2.67 μg/ml reduction (95 % CI −3.22 to −2.13, P = 0.000). However, it should be noted that significant heterogeneity across studies was present (I2 = 95.9 %, P = 0.000).
Stratified analysis
Stratified analysis was conducted to explore heterogeneity between studies and assess the robustness of our findings (Table 3). We evaluated potential sources of heterogeneity between studies including BMI, region, HOMA-IR ratio, total testosterone ratio, sample size, assay methods, and the type of blood sample. Among them, eight studies [12, 13, 21, 28, 36, 39, 44, 46] reported adiponectin levels in two subgroups of population stratified by BMI. Therefore, a total of 46 separate comparisons were included in the subgroup analysis stratified by BMI. As seen in Fig. 2, lower adiponectin levels were detected in women with PCOS in both thin (BMI <25 kg/m2, WMD −2.15, 95 % CI −2.87 to −1.43; P = 0.000) and obesity (BMI ≥25 kg/m2, WMD −2.99, 95 % CI −3.71 to −2.28; P = 0.000) women. And the overall pattern in WMD did not vary substantially by the potential sources except region, HOMA-IR ratio, and total testosterone ratio. Only one study in Australia showed that it was not significantly different in adiponectin levels between PCOS patients and healthy controls (WMD −0.10, 95 % CI −2.73 to 2.53; P = 0.941). However, as for study population in Asia, Europe, and America, adiponectin levels in PCOS patients were significantly lower than healthy controls (all P < 0.05). HOMA-IR ratio and total testosterone ratio were used to express the relative different in IR and testosterone levels between groups, respectively. No significant difference in pooled WMD of adiponectin levels was observed in studies with HOMA-IR ratio of less than 1.36 (WMD −2.40, 95 % CI −4.82 to 0.02; P = 0.052), as well as studies with HOMA-IR ratio between 1.71 and 2.12 (WMD −1.42, 95 % CI −3.41 to 0.57; P = 0.162). Similarly, summary WMD of adiponectin levels was not significantly different in studies with total testosterone ratio of more than 2.5, where adiponectin was found lower in healthy controls (WMD 1.88, 95 % CI −1.95 to 5.72; P = 0.336). Unfortunately, all subgroup analysis demonstrated large heterogeneity, and these variables did not seem to be found to contribute significantly to the heterogeneity between studies.
Meta-regression
To further investigate the impact of the above study characteristics on the study estimates of WMD in adiponectin, we conducted meta-regression analysis. SMD was used as the dependent variable, and BMI, region, HOMA-IR ratio, total testosterone ratio, sample size, assay methods, and the type of blood sample were entered as explanatory covariates. Univariate meta-regression analysis was performed first. In univariate meta-regression analysis, BMI (42 studies, P = 0.072), region (42 studies, P = 0.563), HOMA-IR ratio (33 studies, P = 0.773), total testosterone ratio (32 studies, P = 0.209), sample size (42 studies, P = 0.395), method (42 studies, P = 0.358), and blood sample (42 studies, P = 0.620) were assessed independently. Results of the univariate analysis are presented in Table 4. If the regression coefficient of the covariate was significant at the level of 0.1, then the covariate was entered into the multivariate meta-regression. Only one covariate (BMI) was found to be a significant factor in univariate analysis. Therefore, subsequent multivariate meta-regression could not be done. The results of meta-regression suggested that BMI might contribute little to the heterogeneity between included studies, and other covariates failed to account for heterogeneity in any of the preplanned comparisons.
Sensitivity analysis
We also conducted a sensitivity analysis using random-effects estimates by omitting one study at a time and calculating the summary SMD for the remaining studies. We found that there was no change in the direction of effect when any one study was excluded, which indicated that the results of our meta-analysis was reliable and stable (data not shown).
Cumulative meta-analysis
In overall included studies, the random-effects pooled WMD was always insignificantly larger or smaller than zero from the year 2003 to the first trial in 2008 by Aroda et al. [84], representing no statistically significant difference in adiponectin level between PCOS patients and healthy controls. But a statistically significant effect was consistently observed from the study by Glucelik et al. in 2008 [10], and it changed little after that study, indicating the stability of the association (data not shown).
Publication bias
Publication bias of literatures was evaluated by Begg’s funnel plots and Egger’s tests. As shown in Fig. 3, the Begg’s funnel plots are slightly asymmetrical in distribution, which could raise the possibility of publication bias, although the Egger’s regression test suggests no statistically significant asymmetry of the funnel plot (t = −1.82, P = 0.076). We further undertook analysis using the trim-and-fill method [87]. The results from the trim-and-fill analysis did not change the summary estimate of effect, suggesting that publication bias is unlikely to affect the results of the meta-analysis.
Discussion
PCOS is considered to be the most common cause of anovulatory infertility, which affects 5–10 % in reproductive-aged women [88]. Moreover, PCOS is frequently associated with the metabolic syndrome such as insulin resistance, dyslipidemia, obesity, and an increased risk of type 2 diabetes, dyslipidemia, hypertension, and cardiovascular diseases [17–19]. Although increasing number of studies are in an effort to the research of PCOS, the pathogenesis of PCOS is still unknown. As adiponectin presents the effects of insulin sensitivity, antidiabetic and anti-inflammatory, and decreasing circulating adiponectin levels are present in women with obesity and type 2 diabetes, it has been suggested that adiponectin might play a role in the pathogenesis of PCOS. This study aimed to systematically estimate the association between adiponectin levels and the incidence of PCOS by combining the primary data from all relevant studies. The overall results of our meta-analysis with 38 including studies demonstrated that total adiponectin levels were significantly lower in women with PCOS than in healthy controls (random-effects WMD, −2.67, 95 % CI −3.22 to −2.13; I2 = 95.9 %), which were consistent with the results of the previous meta-analysis (16 studies, random-effects WMD, −1.71, 95 % CI −2.82 to −0.6; I2 = 80.7 %) [24].
Mechanisms of adiponectin and PCOS
Adiponectin decreases the triglyceride content of muscles, regulates insulin signalization, activates peroxisome proliferator-activated receptor, increases the use of fatty acids and energy, increases muscle fat oxidation and transport via activation of adenosine monophosphate-activated protein kinase, inhibits gluconeogenic enzyme expression, and decreases hepatic glucose production [89]. Because of these properties, adiponectin has an increasing effect on insulin sensitivity as well as anti-atherogenic, anti-inflammatory, and anti-diabetic effects.
Evidence in previous animal studies has shown that adiponectin may exert advantageous effects on increasing insulin sensitivity and antidiabetic effects. Yamauchi et al. [90] found that decreased expression of adiponectin correlated with insulin resistance in mouse models of altered insulin sensitivity, and adiponectin decreased insulin resistance by decreasing triglyceride content in muscle and liver. The phenotypes of adiponectin-deficient and transgenic adiponectin-overproducing animal models underscore the role of adiponectin in the maintenance of glucose and lipid homeostasis [91]. Moreover, pharmacologic adiponectin treatments in rodents increased insulin sensitivity, which indicates the replenishment of adiponectin that might provide a noble treatment modality for insulin resistance and type 2 diabetes [91].
It is clear that most women with PCOS have marked insulin resistance, as well as dyslipidemia and obesity. In humans, many studies reported that adiponectin levels were statistically significantly lower in women in PCOS, which were negatively correlated with insulin resistance and positively correlated with obesity in PCOS [9, 13, 28, 37, 84]. Some studies, however, have not found an association between adiponectin and PCOS [21, 22, 25, 26, 31]. Several studies reported that adiponectin levels reduced in women with PCOS independent of BMI and severity of insulin resistance [11, 30]. In addition, adiponectin circulates in different multimer complexes, and the high-molecular weight (HMW) multimer is the most biologically potent form, which is decreased in women with PCOS compared with normal controls [11, 13, 38, 84]. Tao et al. [13] revealed that HMW adiponectin was a stronger predictor of insulin resistance than total adiponectin in both women with PCOS and normal women.
Sources of heterogeneity
Heterogeneity is a potential problem that may affect the interpretation of the results. The present meta-analysis showed that there was large heterogeneity between studies. Subsequent subgroup analysis stratified by seven potential sources was performed. Unfortunately, as seen in Table 3, large heterogeneity was identified as well. We then conducted a meta-regression and found BIM might contribute little to the overall heterogeneity (P = 0.072). In addition, sensitivity analysis suggested that no single study influenced the pooled SMD qualitatively. In subgroup analysis, we found no significant differences in circulating adiponectin levels between PCOS women and healthy controls only in Australia population. Inconsistent results were also presented in the subgroup analysis stratified by HOMA-IR ratio and T-ratio (Table 3). These conflicting results and the heterogeneity between studies were likely due to geographical differences and variability in PCOS diagnostic criteria. Other factors such as study designs and limited sample size might also contribute to the heterogeneity. A potential confounding factor in the present study was that some of the cases have higher HOMA-IR compared with the controls. This might be one of the reasons for the between-group differences in adiponectin levels.
Study strengths
To our knowledge, this is the most comprehensive meta-analysis to date to evaluate the association between adiponectin levels and PCOS. Substantial number of cases and controls were pooled from all publications concerned with circulating adiponectin levels and PCOS, which greatly increased statistical power of the analysis and provided enough evidence for us to draw a safe conclusion. Although obvious heterogeneity was observed across the studies, sensitivity analysis suggested that no single study influenced the pooled SMD qualitatively. And cumulative meta-analysis showed that no substantive change had occurred in pooled WMD after the study was published in 2008, indicating the stability of the association between low adiponectin levels and PCOS women. In addition, no publication bias was detected in this meta-analysis, which indicated that the pooled results of our study should be reliable. Taken together, these data further confirm the reliability and stability of the meta-analysis results.
Limitations of this meta-analysis
Some limitations of the present meta-analysis should be considered. Firstly, substantial heterogeneity across the studies for the pooled estimates was recorded, which was unsatisfactorily explained although we conducted subgroup analysis, meta-regression analysis, and sensitivity analysis to find the sources. The heterogeneity might reflect clinical heterogeneity related to variability in PCOS diagnostic criteria, ethnicity, or diet and physical activity. Secondly, our results were based on unadjusted estimates, whereas a more precise evaluation should consider the confounding factors such as smoking status, alcoholic consumption, environmental factors, and other diet lifestyle. Thirdly, dyslipidemia is also relative with adiponectin and PCOS, but we were not able to conduct further analysis because of data limitation. Last but not the least, some of the included studies contained small numbers of cases and the backgrounds of patients varied, which would result in low statistical power and the inconsistent results between studies. With these limitations in mind, caution should be applied in extrapolating the results mentioned above for wider application.
Conclusions
In conclusion, the current evidence suggested that circulating adiponectin levels in women with PCOS were significantly lower than those in healthy controls, which indicated that circulating adiponectin might play a role in the development of PCOS. However, for the subgroup of HOMA-IR ratio and total testosterone ratio, inconsistent results were presented. The well-designed studies with larger sample size, adjusted with confounding factors are needed to answer the question of whether low circulating adiponectin levels are relative with IR and testosterone.
References
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis, and diagnosis. Nat Rev Endocrinol. 2011;7:219–31.
Carmina E, Azziz R. Diagnosis, phenotype, and prevalence of polycystic ovary syndrome. Fertil Steril. 2006;86 Suppl 1:S7–8.
Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83:1717–23.
Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030.
Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes, and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–63.
Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18:280–5.
Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53.
Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update. 2011;17:741–60.
Gulcelik NE, Aral Y, Serter R, Demir Y, Culha C. Adiponectin is an independent determinant of insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2006;22:511–5.
Gulcelik NE, Aral Y, Serter R, Koc G. Association of hypoadiponectinemia with metabolic syndrome in patients with polycystic ovary syndrome. J Natl Med Assoc. 2008;100:64–8.
O’Connor A, Phelan N, Tun TK, Boran G, Gibney J, Roche HM. High-molecular-weight adiponectin is selectively reduced in women with polycystic ovary syndrome independent of body mass index and severity of insulin resistance. J Clin Endocrinol Metab. 2010;95:1378–85.
Sharifi F, Hajihosseini R, Mazloomi S, Amirmogaddami H, Nazem H. Decreased adiponectin levels in polycystic ovary syndrome, independent of body mass index. Metab Syndr Relat Disord. 2010;8:47–52.
Tao T, Wickham 3rd EP, Fan W, Yang J, Liu W. Distribution of adiponectin multimeric forms in Chinese women with polycystic ovary syndrome and their relation to insulin resistance. Eur J Endocrinol. 2010;163:399–406.
Bohler Jr H, Mokshagundam S, Winters SJ. Adipose tissue and reproduction in women. Fertil Steril. 2010;94:795–825.
Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril. 2010;94:1949–57.
Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008;284:38–45.
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–88.
Zhang BC, Liu WJ, Che WL, Xu YW. Serum total adiponectin level and risk of cardiovascular disease in Han Chinese populations: a meta-analysis of 17 case–control studies. Clin Endocrinol (Oxf). 2012;77:370–8.
Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension. 2013;62:27–32.
Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchon R, Luque-Ramirez M, et al. Adiponectin and resistin in PCOS: a clinical, biochemical, and molecular genetic study. Hum Reprod. 2006;21:2257–65.
Orio Jr F, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2619–23.
Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1790–6.
Carmina E, Orio F, Palomba S, Cascella T, Longo RA, Colao AM, et al. Evidence for altered adipocyte function in polycystic ovary syndrome. Eur J Endocrinol. 2005;152:389–94.
Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, et al. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15:297–307.
Li X, Huang HY, Ma D, Zhu MW. Lin JF: [correlations between adipocytokines and insulin resistance in women with polycystic ovary syndrome]. Zhonghua Yi Xue Za Zhi. 2009;89:2607–10.
Pinhas-Hamiel O, Singer S, Pilpel N, Koren I, Boyko V, Hemi R, et al. Adiponectin levels in adolescent girls with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2009;71:823–7.
Wang Y. Yu P: [clinical significance and changes of serum visfatin, adiponectin, and leptin levels in patients with polycystic ovarian syndrome]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:72–5.
Yilmaz M, Bukan N, Demirci H, Ozturk C, Kan E, Ayvaz G, et al. Serum resistin and adiponectin levels in women with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:246–52.
Arikan S, Bahceci M, Tuzcu A, Kale E, Gokalp D. Serum resistin and adiponectin levels in young non-obese women with polycystic ovary syndrome. Gynecol Endocrinol. 2010;26:161–6.
Demirci H, Yilmaz M, Ergun MA, Yurtcu E, Bukan N, Ayvaz G. Frequency of adiponectin gene polymorphisms in polycystic ovary syndrome and the association with serum adiponectin, androgen levels, insulin resistance, and clinical parameters. Gynecol Endocrinol. 2010;26:348–55.
Tarkun I, Dikmen E, Cetinarslan B, Canturk Z. Impact of treatment with metformin on adipokines in patients with polycystic ovary syndrome. Eur Cytokine Netw. 2010;21:272–7.
Cekmez F, Cekmez Y, Pirgon O, Canpolat FE, Aydinoz S, Metin Ipcioglu O, et al. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur Cytokine Netw. 2011;22:32–7.
Choi JH, Rhee EJ, Kim KH, Woo HY, Lee WY, Sung KC. Plasma omentin-1 levels are reduced in non-obese women with normal glucose tolerance and polycystic ovary syndrome. Eur J Endocrinol. 2011;165:789–96.
Olszanecka-Glinianowicz M, Kuglin D, Dabkowska-Huc A, Skalba P. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;154:51–6.
Pangaribuan B, Yusuf I, Mansyur M, Wijaya A. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2011;2:235–45.
Savastano S, Valentino R, Di Somma C, Orio F, Pivonello C, Passaretti F, et al. Serum 25-hydroxyvitamin D levels, phosphoprotein enriched in diabetes gene product (ped/pea-15), and leptin-to-adiponectin ratio in women with PCOS. Nutr Metab (Lond). 2011;8:84.
Shin HY, Lee DC, Lee JW. Adiponectin in women with polycystic ovary syndrome. Korean J Fam Med. 2011;32:243–8.
Wickham 3rd EP, Cheang KI, Clore JN, Baillargeon JP, Nestler JE. Total and high-molecular weight adiponectin in women with the polycystic ovary syndrome. Metabolism. 2011;60:366–72.
Yasar L, Ekin M, Gedikbasi A, Erturk AD, Savan K, Ozdemir A, et al. Serum adiponectin levels in high school girls with polycystic ovary syndrome and hyperandrogenism. J Pediatr Adolesc Gynecol. 2011;24:90–3.
Aydogdu A, Uckaya G, Tasci I, Baysan O, Tapan S, Bugan B, et al. The relationship of epicardial adipose tissue thickness to clinical and biochemical features in women with polycystic ovary syndrome. Endocr J. 2012;59:509–16.
Golbahar J, Das NM, Al-Ayadhi MA, Gumaa K. Leptin-to-adiponectin, adiponectin-to-leptin ratios, and insulin are specific and sensitive markers associated with polycystic ovary syndrome: a case–control study from Bahrain. Metab Syndr Relat Disord. 2012;10:98–102.
Mazloomi S, Sharifi F, Hajihosseini R, Kalantari S, Mazloomzadeh S. Association between hypoadiponectinemia and low serum concentrations of calcium and vitamin d in women with polycystic ovary syndrome. ISRN Endocrinol. 2012;2012:949427.
Spanos N, Tziomalos K, Macut D, Koiou E, Kandaraki EA, Delkos D, et al. Adipokines, insulin resistance and hyperandrogenemia in obese patients with polycystic ovary syndrome: cross-sectional correlations and the effects of weight loss. Obes Facts. 2012;5:495–504.
Woo HY, Kim KH, Rhee EJ, Park H, Lee MK. Differences of the association of anti-mullerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59:781–90.
Kale-Gurbuz T, Akhan SE, Bastu E, Telci A, Iyibozkurt AC, Topuz S. Adiponectin, leptin and ghrelin levels in obese adolescent girls with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2013;26:27–30.
Lee H, Oh JY, Sung YA. Adipokines, insulin-like growth factor binding protein-3 levels, and insulin sensitivity in women with polycystic ovary syndrome. Korean J Intern Med. 2013;28:456–63.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–12.
GA Wells BS, D O’Connell, J Peterson, V Welch, M Losos, P Tugwell: The newcastle-ottawa scale (nos) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 9, 2013
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–62.
Li S, Liu Y, Peng Q, Xie L, Wang J, Qin X. Chewing gum reduces postoperative ileus following abdominal surgery: a meta-analysis of 17 randomized controlled trials. J Gastroenterol Hepatol. 2013;28:1122–32.
Shen W, Li T, Hu Y, Liu H, Song M. Calpain-10 genetic polymorphisms and polycystic ovary syndrome risk: a meta-analysis and meta-regression. Gene. 2013;531:426–34.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. 2002;31:88–95.
Panidis D, Farmakiotis D, Rousso D, Koliakos G, Kaltsas T, Krassas G. Decrease in adiponectin levels in women with polycystic ovary syndrome after an oral glucose tolerance test. Fertil Steril. 2005;83:232–4.
Lecke SB, Mattei F, Morsch DM, Spritzer PM. Abdominal subcutaneous fat gene expression and circulating levels of leptin and adiponectin in polycystic ovary syndrome. Fertil Steril. 2011;95:2044–9.
Safar FH, Mojiminiyi OA, Al-Rumaih HM, Diejomaoh MF. Computational methods are significant determinants of the associations and definitions of insulin resistance using the homeostasis model assessment in women of reproductive age. Clin Chem. 2011;57:279–85.
Pepene CE. Evidence for visfatin as an independent predictor of endothelial dysfunction in polycystic ovary syndrome. Clin Endocrinol (Oxf). 2012;76:119–25.
Singh S, Akhtar N, Ahmad J. Plasma adiponectin levels in women with polycystic ovary syndrome: impact of metformin treatment in a case–control study. Diabetes Metab Syndr. 2012;6:207–11.
Lecke SB, Morsch DM, Spritzer PM: association between adipose tissue expression and serum levels of leptin and adiponectin in women with polycystic ovary syndrome. Genet Mol Res 2013; 12.
Lecke SB, Morsch DM, Spritzer PM. Association between adipose tissue expression and serum levels of leptin and adiponectin in women with polycystic ovary syndrome. Genet Mol Res. 2013;12:4292–6.
Jensterle M, Weber M, Pfeifer M, Prezelj J, Pfutzner A, Janez A. Assessment of insulin resistance in young women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2008;102:137–40.
Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:2670–8.
Majuri A, Santaniemi M, Rautio K, Kunnari A, Vartiainen J, Ruokonen A, et al. Rosiglitazone treatment increases plasma levels of adiponectin and decreases levels of resistin in overweight women with PCOS: a randomized placebo-controlled study. Eur J Endocrinol. 2007;156:263–9.
Lewandowski KC, Szosland K, O’Callaghan C, Tan BK, Randeva HS, Lewinski A. Adiponectin and resistin serum levels in women with polycystic ovary syndrome during oral glucose tolerance test: a significant reciprocal correlation between adiponectin and resistin independent of insulin resistance indices. Mol Genet Metab. 2005;85:61–9.
Aigner E, Bachofner N, Klein K, De Geyter C, Hohla F, Patsch W, et al. Retinol-binding protein 4 in polycystic ovary syndrome–association with steroid hormones and response to pioglitazone treatment. J Clin Endocrinol Metab. 2009;94:1229–35.
Chang CY, Chen MJ, Yang WS, Yeh CY, Ho HN, Chen SU, et al. Hypoadiponectinemia: a useful marker of dyslipidemia in women with polycystic ovary syndrome. Taiwan J Obstet Gynecol. 2012;51:583–90.
Tao T, Xu B, Liu W. Ovarian HMW adiponectin is associated with folliculogenesis in women with polycystic ovary syndrome. Reprod Biol Endocrinol. 2013;11:99.
Panidis D, Kourtis A, Kukuvitis A, Farmakiotis D, Xita N, Georgiou I, et al. Association of the t45g polymorphism in exon 2 of the adiponectin gene with polycystic ovary syndrome: role of delta4-androstenedione. Hum Reprod. 2004;19:1728–33.
Haap M, Machicao F, Stefan N, Thamer C, Tschritter O, Schnuck F, et al. Genetic determinants of insulin action in polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2005;113:275–81.
Zhang N, Shi YH, Hao CF, Gu HF, Li Y, Zhao YR, et al. Association of +45g15g(t/g) and +276(g/t) polymorphisms in the adipoq gene with polycystic ovary syndrome among Han Chinese women. Eur J Endocrinol. 2008;158:255–60.
Lauria PB, Del Puerto HL, Reis AM, Candido AL, Reis FM, Tao T, et al. Low plasma atrial natriuretic peptide: a new piece in the puzzle of polycystic ovary syndrome ovarian HMW adiponectin is associated with folliculogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:4882–9.
Glintborg D, Frystyk J, Hojlund K, Andersen KK, Henriksen JE, Hermann AP, et al. Total and high molecular weight (HMW) adiponectin levels and measures of glucose and lipid metabolism following pioglitazone treatment in a randomized placebo-controlled study in polycystic ovary syndrome. Clin Endocrinol (Oxf). 2008;68:165–74.
Barber TM, Hazell M, Christodoulides C, Golding SJ, Alvey C, Burling K, et al. Serum levels of retinol-binding protein 4 and adiponectin in women with polycystic ovary syndrome: associations with visceral fat but no evidence for fat mass-independent effects on pathogenesis in this condition. J Clin Endocrinol Metab. 2008;93:2859–65.
Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrom V, Flyvbjerg A, et al. Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin, ghrelin, leptin, and body composition in hirsute PCOS patients and controls. Eur J Endocrinol. 2006;155:337–45.
Ducluzeau PH, Cousin P, Malvoisin E, Bornet H, Vidal H, Laville M, et al. Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in nonobese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:3626–31.
Tan BK, Chen J, Digby JE, Keay SD, Kennedy CR, Randeva HS. Upregulation of adiponectin receptor 1 and 2 MRNA and protein in adipose tissue and adipocytes in insulin-resistant women with polycystic ovary syndrome. Diabetologia. 2006;49:2723–8.
Aydogdu A, Tasci I, Tapan S, Sonmez A, Aydogan U, Akbulut H, et al. Women with polycystic ovary syndrome have increased plasma chitotriosidase activity: a pathophysiological link between inflammation and impaired insulin sensitivity? Exp Clin Endocrinol Diabetes. 2012;120:261–5.
Orio Jr F, Palomba S, Cascella T, Di Biase S, Labella D, Russo T, et al. Lack of an association between peroxisome proliferator-activated receptor-gamma gene pro12ala polymorphism and adiponectin levels in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5110–5.
Beckman JA, Goldfine AB, Dunaif A, Gerhard-Herman M, Creager MA. Endothelial function varies according to insulin resistance disease type. Diabetes Care. 2007;30:1226–32.
Moran LJ, Noakes M, Clifton PM, Wittert GA, Belobrajdic DP, Norman RJ. C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2944–51.
Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007;92:4609–14.
Aroda V, Ciaraldi TP, Chang SA, Dahan MH, Chang RJ, Henry RR. Circulating and cellular adiponectin in polycystic ovary syndrome: relationship to glucose tolerance and insulin action. Fertil Steril. 2008;89:1200–8.
Thomann R, Rossinelli N, Keller U, Tirri BF, De Geyter C, Ruiz J, et al. Differences in low-grade chronic inflammation and insulin resistance in women with previous gestational diabetes mellitus and women with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:199–206.
Aroda VR, Ciaraldi TP, Burke P, Mudaliar S, Clopton P, Phillips S, et al. Metabolic and hormonal changes induced by pioglitazone in polycystic ovary syndrome: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2009;94:469–76.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Brassard M, AinMelk Y, Baillargeon JP. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008;92:1163–92. xi.
Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51:1884–8.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6.
Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–13.
Acknowledgments
This work was not supported by any kind of fund.
Conflict of interest
The authors declared that they have no conflict of interest in relation to this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shan Li, Xiamei Huang, and Huizhi Zhong contributed equally to this study and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Li, S., Huang, X., Zhong, H. et al. Low circulating adiponectin levels in women with polycystic ovary syndrome: an updated meta-analysis. Tumor Biol. 35, 3961–3973 (2014). https://doi.org/10.1007/s13277-013-1595-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1595-0