Abstract
The purpose of this study is to investigate the relationship between nestin expression and clinicopathological characteristics, immunohistochemical markers and to determine the prognostic impact of nestin expression in breast cancer so as to lay a foundation for the treatment of breast cancer. A total of 109 patients who were histologically diagnosed with breast cancer and underwent radical operations from January 2006 to September 2007 in China Medical University were enrolled in the study. Nestin protein expression was evaluated by immunohistochemistry. The relationship between nestin and other parameters was analyzed by using chi-square test and Fisher’s exact test. Nestin expression was observed in 37.6 % (41/109) of cases. There were no significant differences between the age of >40 and ≤40 years group in terms of nestin expression (39.8 vs 18.2 %; P = 0.161). The rate of nestin expression between those with and without lymph node metastasis was not significantly different (X 2 = 0.086; P = 0.769). The 5-year survival rates of the patients with nestin expression and those without were 34.1 % (14/41) and 55.9 % (38/68), respectively (P = 0.028). Overall, triple-negative breast cancers had higher expression rates than other cancers (54.1 vs 29.2 %; P = 0.011). Nestin expression rate in ER- and PR-negative tumors was found to be significantly higher than cases that were ER- and PR-positive (P = 0.011 and P = 0.036, respectively). However, it was not found that HER2 expression was related to nestin expression (P = 0.120). These results suggest that the expression of nestin might play an important role in the prognosis of breast carcinoma, especially in the triple-negative subgroups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy in women. More than 1.15 million people will be diagnosed with breast cancer every year worldwide. Breast cancer accounts for 14.1 % of deaths in all the female malignancies. Breast cancer greatly threatens the physical and mental health of women [1, 2]. Primarily derived from mammary gland epithelial cells, breast carcinoma tissue only expresses the markers of gland epithelial cells. However, some studies observed that the tumor cells also expressed markers derived from myoepithelium. Consequently, breast cancer is a heterogeneous disease, and the included phenotypes have different biological characteristics [3].
Current anti-breast cancer treatments such as endocrine therapy and molecular target therapy are suitable for those phenotypes that are ER/PR-positive and HER2-positive. However, triple-negative breast cancer accounts for approximately 10–17 % of all breast carcinomas and is associated with a poor clinical outcome [4]. Moreover, there has been no individualized treatment for triple-negative breast cancer recently.
Nestin is a type VI intermediate filament protein that is expressed in proliferating progenitor cells during the developmental stages in a variety of embryonic and fetal tissues. Studies have focused on the importance of nestin as a molecular marker for neural stem cells [5–7]. It has recently been shown that nestin is also expressed in tumor cells and proliferating microvascular endothelial cells [8, 9]. Studies demonstrated that nestin is preferentially expressed in triple-negative and basal-like breast carcinomas.
Therefore, we carried out this study to investigate the relationship between nestin expression and clinicopathological characteristics, immunohistochemical markers and determine the prognostic impact of nestin expression in breast cancer in order to lay a foundation for the treatment of breast cancer.
Materials and methods
Patients and tissues specimens
A total of 109 patients that were histologically diagnosed with breast cancer and had underwent radical operations obtained in the Surgical Oncology Department of the First Hospital of the Medical University from January 2006 to September 2007 were included. The inclusion criteria were as follows: (a) curative operations were performed; (b) resected specimens were pathologically examined; (c) more than ten lymph nodes were pathologically examined after operation; and (d) complete medical records were available.
Thin slices of tumor tissue of all cases received in our histopathology unit were fixed in 4 % formaldehyde solution (pH 7.0) for periods not exceeding 24 h. The tissues were processed routinely for paraffin embedding, and 4-μm-thick sections were cut and placed on glass slides coated with 3-aminopropyl triethoxysilane for immunohistochemistry.
Immunohistochemistry
The expression of nestin was analyzed by using the monoclonal antibodies of mice from Santa Cruz Biotechnology (Santa Cruz, CA, USA) by immunohistochemistry. Positive control included samples of normal breast, where nestin is consistently expressed in myoepithelial cells and in endothelial cells.Negative control was prepared by substituting the primary antibody over PBS.
Immunohistochemical analysis
Nestin expression was semi-quantitatively classified according to the following criteria: 0, <1 % of neoplastic cells discretely expressed nestin in their cytoplasm; 1+, 1≥, and <10 % of discrete cytoplasmic expression in morphologically unequivocal neoplastic cells; and 2+, if ≥10 % of discrete cytoplasmic expression in morphologically unequivocal neoplastic cells. Cases graded as 1+ and 2+ were considered to be positive.
Statistical analysis
Statistical analysis was performed with SPSS statistics software (version 17.0). Correlations between nestin and other parameters were performed by using chi-square test and Fisher’s extract test. The correlation among ER, PR, HER-2 and nestin protein expression was assessed by linear regression. Regression (r) values were regarded as indicating no correlation (0.0–0.2), a low degree of correlation (0.2–0.4), a moderate degree of correlation (0.4–0.6), a marked degree of correlation (0.6–0.8), or a high degree of correlation (0.8–1.0). Breast cancer-specific survival was expressed as the number of months from surgery to the occurrence of an event (distant metastasis or disease-related death). Cumulative survival probabilities were analyzed using the Kaplan–Meier method. Differences of survival rates were tested by the log-rank test. Multivariate analysis was carried out by using logistic regression and the Cox proportional hazards model. All tests were two-tailed, with a confidence interval of 95 %. A P value of less than 0.05 was considered as statistically significant.
Results
Patient characteristics
A hundred and nine cases had invasive ductal carcinoma. The mean age was 51.03 years (range, 32–79 years). There were 37 patients with lymph node metastasis and 72 without lymph node metastasis. Negative expression of ER, PR, and HER2 was found in 60, 66, and 88 cases, respectively. There were 41 cases with triple-negative breast cancers accounting for 34 % of all included patients.
Relationship between nestin expression and clinicopathological features
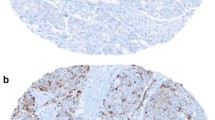
Immunohistochemical examination showed that nestin was located in the cytoplasm of the tumor cells (Fig. 1a) and the myoepithelial cells displayed strong nestin expression as positive control (Fig. 1b). There was no nestin expression in the negative control (Fig. 1c). Nestin expression was observed in 38.53 % (42/109) cases. There was no association between nestin expression and age, histological type, tumor stage, and lymph node metastasis were observed. Overall, triple-negative breast cancers had higher expression rates of nestin than other cancers (52.63 vs 30.98 %, P = 0.027; Table 1).
Nestin expression and ER, PR, and HER-2
After univariate analysis, nestin expression rate was found to be significantly higher in the ER- and PR-negative group than in ER- and PR-positive group (P = 0.011 and P = 0.036, respectively; Table 2). HER2-positive cases had higher nestin expression rates compared with HER2-negative cases, but this was not significantly different (52.4 vs 34.1 %; P = 0.120). We performed logistic analysis on the above factors in order to exclude the effects of confounding factors. Multivariate analysis revealed that ER, PR, and HER2 expression were not found to be related to nestin expression (P = 0.078, P = 0.237, and P = 0.255, respectively; Table 3).
Correlation among ER, PR, HER-2, and nestin expression in breast carcinoma
After linear regression, a significant positive correlation between ER and PR expression was observed (r = 1.000, P = 0.000; Table 4). At the same time, we found inverse correlations between nestin and ER and between nestin and PR (r = −0.245, P = 0.011; r = −0.201, P = 0.037, respectively; Table 4). Nevertheless, no association was observed between HER-2 and ER or nestin PR (r = 0.161, P = 0.095; r = 0.149, P = 0.122, respectively; Table 4).
Survival outcome
Survival analysis revealed that nestin was associated with breast cancer-specific survival in 109 cases (P = 0.014 log rank test; Fig. 2a). Subgroup analysis showed that nestin expression was associated with a shorter breast cancer-specific survival in the lymph node negative group (P = 0.014, log-rank test; Fig. 2b), and there was no association between nestin expression and shorter breast cancer-specific survival in the lymph positive group (P = 0.381, log-rank test; Fig. 2c). However, application of COX regression model analysis showed that nestin was not an independent prognostic factor of breast cancer.
a Univariate analysis of the prognostic impact of nestin expression on breast cancer-specific survival (P = 0.014, log-rank test). b Lymph node negative on the breast cancer-specific survival according to the nestin expression (P = 0.014, log-rank test). c Lymph node negative on the breast cancer-specific survival according to the nestin expression (P = 0.381, log-rank test)
Discussion
Comprehensive therapy for breast cancer has improved the clinical curative effect, but there are no effective treatments for some phenotypes such as triple-negative breast cancer because of its heterogeneity. Moreover, more than 40 % of breast cancer patients still recur. Relapse and metastasis are still the main cause of treatment failure and death from breast cancer [2].
Since Makino discovered some subset of tumor cells that were defined as cancer stem cells (CSCs) or tumor-initiating cells (TICs) in tumor cell groups in 1959 [10], the study on CSCs has gained great attention. Studies have shown that TICs are the basal cause of the occurrence, progression, invasion, metastasis, resistance to radiation and chemotherapy, and the recurrence of the tumor [11, 12]. So far, tumor stem cells have been detected in various tumors, including breast, lung, colon cancer, malignant melanomas, gliomas, and so on [13].
Researchers have found that the expression of nestin may determine the high tumorigenicity of breast cancer stem cells by establishing the tumor mouse model. Nestin is expressed in dividing cells during the early stages of development in the central nervous system, peripheral nervous system, myogenic, and other tissues [14]. Nestin has been utilized as a marker of proliferation and migration, and it has recently been suggested that nestin can be used as a myoepithelial marker [15]. Moreover, studies demonstrated that it was associated with the degree of malignancy and the poor clinical prognosis of tumors [16, 17]. Based on these observations, we deduced that nestin could be an indicator of tumor malignancy.
Recent research on western patients demonstrated that nestin is preferentially expressed in basal-like breast carcinomas [18]. A study based on Asian patients also confirmed this [17]. At the same time, the former found that high levels of nestin are expressed in a small subgroup of luminal cancers and the nestin was not associated with breast cancer-specific survival.
In our study, nestin was preferentially expressed in triple-negative breast cancers, which coincides with previous results. And nestin was observed to be associated with 5-year breast cancer-specific survival, corroborating a study based on Asian patients [17]. While compared to past study results, these are somewhat different. Parry et al. found that there was no correlation between nestin expression, metastasis-free survival, or breast cancer-specific survival [7]. The difference of these may be caused by the selected cases and the nations. Linear regression analysis revealed that nestin expression correlated to the negative expression of ER and PR, and there was no obvious correlation between the expression of nestin and HER2, consistent with previous reports [17, 19]. Based on our results, it is evident that nestin may have an important effect on the development and metastasis in breast carcinoma.
Conclusion
This study detected nestin expression in 109 cases with breast carcinoma by immunohistochemical method. Our study showed that triple-negative breast cancer has a high level of nestin expression. Nestin expression was associated with 5-year breast cancer-specific survival. Nestin might be utilized as a new potential marker for clinical diagnosis and therapy for breast carcinoma. However, the clinical significance and the underlying mechanisms of nestin expression are not completely clear, and further study in larger cohorts of breast carcinoma is needed to explore the relationship between nestin expression and its clinical implications.
References
Desantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–18.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Briest S, Stearns V. Tamoxifen metabolism and its effect on endocrine treatment of breast cancer. Clin Adv Hematol Oncol. 2009;7(3):185–92.
Weigelt B, Mackay A, A'hern R, Natrajan R, Tan DS, Dowsett M, et al. Breast cancer molecular profiling with single sample predictors: a retrospective analysis. Lancet Oncol. 2010;11(4):339–49.
Tropepe V, Alton K, Sachewsky N, Cheng V, Kuo C, Morshead CM. Neurogenic potential of isolated precursor cells from early post-gastrula somitic tissue. Stem Cells Dev. 2009;18(10):1533–42.
Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25(20):4808–19.
Parry S, Savage K, Marchiò C, Reis-Filho JS. Nestin is expressed in basal-like and triple negative breast cancers. J Clin Pathol. 2008;61(9):1548–50.
Piras F, Ionta MT, Lai S, Perra MT, Atzori F, Minerba L, et al. Nestin expression associates with poor prognosis and triple negative phenotype in locally advanced (T4) breast cancer. Eur J Histochem. 2011;55(39):215–20.
Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17(4):409–18.
Makino S. The role of tumor stem-cells in regrowth of the tumor following drastic applications. Acta Unio Int Contra Cancrum. 1959;15 Suppl 1:196–98.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Different. 2006;13(7):1238–41.
Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99.
Hoffman RM. The potential of nestin-expressing hair follicle stem cells in regenerative medicine. Expert Opin Biol Ther. 2007;7:289–91.
Kolar Z, Ehrmann Jr J, Turashvili G, Bouchal J, Mokry J. A novel myoepithelial/progenitor cell marker in the breast? Virchows Arch. 2007;450:607–9.
Yang XH, Wu QL, Yu XB, Xu CX, Ma BF, Zhang XM, et al. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;6(1):467–73.
Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin F, et al. Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 2010;101(3):815–19.
Li H, Cherukuri P, Li N, Cowling V, Spinella M, Cole M, et al. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501–10.
Zhu J. The relationship between nestin expression and clinicopathological factors. Liaoning:China Medical University, 2010.
Acknowledgments
This study was supported by the national natural science funds (no. 81172047 and no. 81102029).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ningning Gao and Hong Xu were co-first authors
Rights and permissions
About this article
Cite this article
Gao, N., Xu, H., Liu, C. et al. Nestin: predicting specific survival factors for breast cancer. Tumor Biol. 35, 1751–1755 (2014). https://doi.org/10.1007/s13277-013-1548-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1548-7