Abstract
Background
MicroRNA-34a (miR-34a) has been reported to inhibit TGF-β (transforming growth factor-β)-induced epithelial-mesenchymal transition (EMT) in nasopharyngeal carcinoma (NPC). However, the underlying mechanism remain unclear. Using the bioinformatics, we found that the AXL receptor tyrosine kinase (AXL) is a predicted target of miR-34a.
Objective
we aimed to reveal the relationship between miR-34a and AXL, and investigate the effect and mechanism of miR-34a in NPC progression.
Methods
The expression patterns of miR-34a and AXL in 30 paired NPC tissues and the adjacent tissues were examined by quantitative real time PCR (qRT-PCR). The target relationship between miR-34a and AXL was evaluated by the luciferase gene reporter assay. Cell migration and invasion were assessed by wound healing and transwell chamber assays, respectively.
Results
miR-34a level was dramatically decreased in the NPC tissues compared to the adjacent tissues, while AXL expression was increased. Overexpression of miR-34a significantly reduced the luciferase activity of the luciferase vector of AXL (pGL3-AXL-WT), whereas this effect was abrogated when binding sites between miR-34a and AXL were mutated. In addition, ectopic expression of miR-34a dramatically inhibited Sune-1 cell migration and invasion abilities, decreased the levels of N-cadherin and Vimentin and increased E-cadherin and γ-catenin expressions, as well as induced significant reductions in the expressions of p-AKT and Snail. However, these effects were attenuated when the cells were treated with recombinant human AXL protein.
Conclusions
Our results demonstrate that miR-34a/AXL can inhibit NPC cell migration, invasion and EMT through inhibition of AKT/Snail signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a type of tumor derived from the epithelial cells of nasopharynx, with a high morbidity in southern China (Xu et al. 2016). Evidence shows that the pathogenesis of NPC is complex, which includes dietary, virus infection, genetic susceptibility, and carcinogen hazards (He et al. 2018). At present, radiotherapy combined with chemoradiotherapy remains the primary treatment option for NPC, and has been proved to increase patients’ survival (Chen et al. 2010; Cosmopoulos et al. 2009). However, 15 to 30% of patients develop distant metastasis, resulting in low treatment efficiency and poor prognosis (Pratt et al. 2009). Therefore, it is important to further reveal the molecular mechanisms of cancer cell migration and invasion in NPC.
MicroRNAs (miRNAs) are a class of non-coding RNA molecules with ~ 22 nucleotides. MiRNAs can post-transcriptionally modulate gene expression by directly targeting the 3′-untranslated region (3′-UTR) of target mRNAs (He and Hannon 2004). Many miRNAs are reported to present with a dysregulated expression pattern in cancers, whereas they function as suppressor or an oncogene (Pratap et al. 2018; Wang et al. 2017). MiR-34a as one of the earliest known members of the miR-34 family, has been identified to be downregulated and exert a suppressive role in several categories of cancers, including intestinal tumor (Jiang and Hermeking 2017), lung adenocarcinoma (Kasinski and Slack 2012), osteosarcoma (Yan et al. 2012), and colorectal cancer (Pang et al. 2013). In NPC, Huang et al. (2018) reported that miR-34a could efficiently inhibited TGF-β (transforming growth factor-β)-induced epithelial-mesenchymal transition (EMT), which is a requirement for cancer cell invasion and metastasis via targeting SMAD4 (Kang and Massague 2004). However, the underlying mechanisms by which miR-34a inhibits the EMT and invasion of NPC cells still need to be further elucidated.
The AXL receptor tyrosine kinase (AXL), also known as UFO and ARK, was first found in chronic myeloid leukemia as a transforming gene and is the founding member of the TAM family of receptor tyrosine kinases which contains Tyro3, AXL, and MER (Hafizi et al. 2005; Paccez et al. 2014). Jiang et al. (2016) showed that AXL expression level was markedly elevated in NPC tissues compared to those in normal nasopharyngeal epithelial tissues, and high level of AXL was correlated with the distant metastasis and advanced TNM stage in NPC, as well as the shorter overall survival. Using the TargetScan software (http://www.targetscan.org/vert_71/), we predicted that AXL was a potential target for miR-34a-5p (miR-34a) in human. Therefore, we hypothesized that AXL might be a molecular which was regulated by miR-34a and subsequently involved in NPC migration and invasion.
Consistently with hypothesis, we demonstrated that miR-34a significantly repressed NPC cell migration, invasion and EMT via targeting AXL. Our data elucidated a new mechanism by which miR-34a repressed the progression of NPC, which might be explored as a therapeutic target to inhibit the metastasis of NPC.
Materials and methods
Ethic statement
All procedures in the current study involving human samples were performed in accordance with the Helsinki declaration and approved by the Ethic Committee of The First Affiliated Hospital of Bengbu Medical College. The informed consent has been obtained from each participant.
Tissue samples
Thirty freshly frozen NPC tissues and 30 normal nasopharyngeal epithelium samples were obtained from NPC patients who accepted surgery in The First Affiliated Hospital of Bengbu Medical College prior to preoperative therapy.
Cell culture
Human NPC cell line Sune-1 was purchased from the Shanghai huiying biological technology co., LTD (Shanghai, China) and grown in RPMI-1640 medium (Thermo Fisher Scientific, MA, USA) with 10% FBS (fetal serum bovine; Thermo Fisher Scientific). Sune-1 cells were cultured in a humidified incubator at a stationary temperature of 37 °C, supplemented with 5% CO2. To increase AXL expression, Sune-1 cells were incubated with recombinant human AXL protein (100 ng/ml; No. PV6253, Thermo Fisher Scientific) for 24 h.
Cell transfection
The mimic used to upregulate miR-34a in NPC cells and the negative control mimic (NC mimic) were gained from the Shanghai GenePharma, LTD. (Shanghai, China). They were transfected into cells by using lipofectamine 2000 transfected reagent (Thermo Fisher Scientific). The sequences of the mimic and mimic control was listed as following:
miR-34a mimic: 5′-UGGCAGUGUCUUAGCUGGUUGU-3′,
NC mimic: 5′-GUAAUGUUUGGCUCGUGUGCUG-3′.
Quantitative real time PCR (qRT-PCR)
Total RNAs were extracted from cells or tissues using Trizol Reagent (Thermo Fisher Scientific) and then cDNA was abstained by reverse transcription (RT). Then, the cDNAs were submitted to the qRT-PCR using the SYBR Green Supermix (Takara, Dalian, China) to measure the expression of miR-34a and AXL. The expression level of GAPDH or RNU6B (U6) was used as the internal control for normalization of AXL or miR-34a, respectively. The 2−ΔΔCT method was applied to calculate the relative expression levels of miR-34a and AXL mRNA. The sequences of the primers used for q-PCR were listed as followings:
miR-34a−RT sequence: 5′−GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAACC−3′;
miR-34a−sense: 5′−GCTGTTGGCAGTATCTTAGC−3′, antisense: 5′−GTGCAGGGTCCGAGGT−3′;
AXL−sense: 5′− CACCCCAGAGGTGCTAATGG −3′, antisense: 5′− GAAGGTTCCTTCACTGGGCG−3′.
SIRT1 (sirtuin 1)−sense: 5′−TCCTACTGGCCTGAGGTTGAG−3′, antisense: 5′−AAGTCTACAGCAAGGCGAGC−3′;
CD44−sense: 5′−CACACCCTCCCCTCATTCAC−3′, antisense: 5′−TGGATGGCTGGTATGAGCTG−3′;
CDK6 (cyclin dependent kinase 6)−sense: 5′−CGGAGAGCCGACTGACACT−3′, antisense: 5′−CCTCGAAGCGAAGTCCTCAA−3′;
c-MET−sense: 5′−TGGGCACCGAAAGATAAACCT−3′, antisense: 5′−ATCTGGGTGTTCCAGCACAG−3′;
Cyclin D1−sense: 5′−CTGATTGGACAGGCATGGGT−3′, antisense: 5′−GTGCCTGGAAGTCAACGGTA−3′;
GAPDH−sense: 5′−CATGGTTCACACCCATGACG−3′, antisense: 5′−CCACTAGGCGCTCACTGTTCTC−3′.
Western blotting assay
Total protein extraction was carried out using the RIPA lysis (Beyotime) with proteinase inhibitors (Roche, Switzerland) and the protein concentration was measured with the BCA kit (Thermo Fisher Scientific, MA, USA). Then, same amount of total proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto the polyvinylidene fluoride membranes (Sigma, MA, USA). Later, the membranes were blocked with 5% BSA for 1 h at room temperature and were probed with the following primary antibodies (4 °C, 10–12 h), including E-cadherin, N-cadherin, Vimentin, β-catenin AXL, p-AKT, AKT and Snail, all purchased from Cell Signaling Technology (MA, USA) with a working concentration of 1:1000 dilution. After that, the membranes were washed with TBST for 3 times and incubated with the horse radish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology) for 1 h (room temperature). The protein expression levels were measured by using the densitometric analysis.
Luciferase gene reporter assay
3′-UTR of AXL (> hg38_ncbiRefSeq_NM_001699.6; range = chr19:41259905–41261766) including the predicted miR-34a binding sites (pGL3-AXL-WT) or the mutated binding sites (pGL3-AXL-MUT) was inserted into a pGL3-REPORT vector (Promega, Madison, USA). Then, the NPC cells were co-transfected with miR-34a mimic or NC mimic, and pGL3-AXL-WT or pGL3-AXL-MUT, together with the pGL3-REPORT β-gal control plasmid with lipofectamine 2000. After 48 h of the above transfections, the luciferase activities were detected by using the dual-Luciferase Reporter Assay System (Promega).
Wound healing assay
The NPC cells were plated in 6-well plates (Corning, USA) with adequate numbers to reach 100% confluence in the next day. Then, the wounds were scratched in each well by using a 10 µl pipette tip and the non-attached cells were removed through washing with PBS. Next, the cells were grown in FBS-free medium and incubated at 37 °C. Gap size was measured and photographed at 0, 24 and 48 h following the scratch.
Transwell chamber assay
A transwell chamber (8 µm, 24-well format; Corning, USA) precoated with a diluted Matrigel (BD Biosciences, New Jersey, USA) (50% concentration) was used to assess cell invasion. Briefly, the transfected cells (5 × 104/well) resuspended with FBS-free medium were seeded in the upper chamber, while 600 µL cell culture medium with 20% FBS were added into the lower chambers. After the cells were incubated at 37 °C for 24 h, cells in the lower of the upper chambers were stained with 1% crystal violet at room temperature for 10 min. Cell invasion and migration abilities were assessed by counting cell numbers with positive staining in six randomly selected regions.
Statistical analysis
Three independent experiments were carried out for each assay in this study. The data were expressed as the means ± standard deviation (SD). Statistical analysis between 2 groups or multiple groups was performed with Student’s t-test or One-Way Analysis of Variance (ANOVA) followed by Tukey’s test. SPSS 20.0 software was recruited for the statistical analysis. P value less than 0.05 was identified as statistically significant.
Results
miR-34a is lowly expressed while AXL is highly expressed in NPC tissues
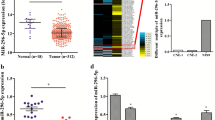
To investigate whether AXL was involved in miR-34a-mediated NPC repression, we first explored the expression leves of miR-34a and AXL in NPC tissues and the adjacent non-tumor tissues. Compared with the normal tissues, miR-34a expression was markedly decreased in the NPC tissues (Fig. 1a), while the expression of AXL was obviously increased (Fig. 1b). These results illustrated that the expression patterns of miR-34a and AXL showed inverse correlations between NPC tissues and adjacent non-tumor tissues.
miR-34a expression was decreased and AXL expression was increased in NPC tissues. a QRT-PCR analysis of the expression of AXL in 30 paired NPC tissues and the tumor adjacent tissues. b QRT-PCR analysis of the mRNA levels of AXL in 30 paired NPC tissues and the tumor adjacent tissues. Three independent experiments were carried out for each assay. (n = 30, ***P < 0.001)
AXL is a target of miR-34a in NPC cells
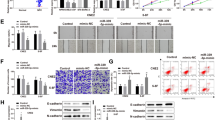
Next, we explored the relationship between miR-34a and AXL in NPC Sune-1 cells. The putative binding sites between them were shown in Fig. 2a. Transfection with miR-34a mimic dramatically increased the level of miR-34a in Sune-1 cells (Fig. 2b). And, miR-34a overexpression reduced the expression of pGL3-AXL-WT reporter, while this effect was abolished when the putative miR-34a binding site of pGL3-AXL-WT was mutated (Fig. 2c). In addition, overexpression of miR-34a significantly reduced the expression of AXL at both mRNA and protein levels (Fig. 2d, e). Moreover, we detected the mRNA levels of the known targets of miR-34a after Sune-1 cells were transfected with miR-34a mimic (Badi et al. 2018; Hong et al. 2015; Ye et al. 2016; Zuo et al. 2018). The results showed that miR-34a overexpression obviously decreased the mRNA levels of SIRT1, CD44, CDK6 and Cyclin D1, with on significant change in c-MET mRNA level (Supplementary Fig. 1). These results confirm that AXL serves as a target of miR-34a.
AXL was a target of miR-34a in NPC cells. a The putative binding sites between miR-34a and AXL was displayed. b The expression of miR-34a was detected by using the qRT-PCR assay after Sune-1 cells were transfected with NC mimic or miR-34a mimic. c The luciferase intensity of the pGL3-AXL-WT/MUT was determined by the luciferase gene reporter assay after Sune-1 cells were transfected with NC mimic or miR-34a mimic. d, e The expression of AXL at both mRNA and protein levels were measured by using the RT-PCR and western blotting assay after Sune-1 cells were transfected with NC mimic or miR-34a mimic, respectively. All the experiments were repeated for at least three times. (n = 03, ***P < 0.001)
miR-34a overexpression inhibits cell migration, invasion and EMT in NPC via targeting AXL
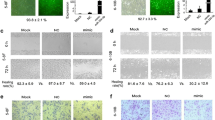
Then, we explored the effects of miR-34a/AXL axis on cell migration, invasion and EMT via carrying out the loss-of-function assay in Sune-1 cell line. Compared with the control group, cell migration and invasion abilities were all significantly reduced following the transfection with miR-34a mimic, whereas AXL protein treatment rescued these tendencies (Fig. 3a, b). In addition, miR-34a overexpression induced obvious increases in the expressions of E-cadherin and γ-catenin and decreases in the expressions of N-cadherin and Vimentin, whereas these effect induced by overexpression of miR-34a were obviously impaired following AXL treatment (Fig. 3c). Overall, these results reveal that miR-34a exerts a repressive role in regulating cell invasion, migration and EMT in NPC via targeting AXL.
miR-34a overexpression repressed NPC cell migration, invasion and EMT via targeting AXL. Sune-1 cells were treated with NC mimic, miR-34a mimic or miR-34a mimic + AXL (100 ng/ml), then the following assays were carried out. a Cell migration ability was determined by using the wound healing assay. b Cell invasion capacity was tested by using the transwell chambers. c The protein levels of E-cadherin, γ-catenin, N-cadherin and Vimentin were examined by the western blotting assay. Three independent experiments were carried out for each assay. (n = 03, **P < 0.01, ***P < 0.001)
miR-34a overexpression inhibits the activation of AKT/Snail signaling via targeting AXL
To reveal the underlying mechanism of miR-34a/AXL-induced repressions of cell migration, invasion and EMT in NPC, we explored the effect of miR-34a/AXL on the activation of AKT/Snail signaling. The results showed that the ratio of p-AKT/AKT and the expression level of Snail were obviously decreased when miR-34a was overexpressed in Sune-1 cells, whereas effects were obviously impaired following AXL treatment (Fig. 4), which indicated that miR-34a could induce a repression in the activation of the AKT/Snail signaling.
miR-34a overexpression inhibited the activation of AKT/Snail signal by targeting AXL. After cell treatment with NC mimic, miR-34a mimic or miR-34a mimic + AXL, Sune-1 cells were collected for the western blotting assay to detect the protein expression levels of p-AKT, AKT and Snail. Three independent experiments were carried out for each assay. (n = 03, ***P < 0.001)
Discussion
It has been reported that about 15–30% of patients with NPC would develop distant metastasis despite of the 80–90% 5-year local control rates (Pratt et al. 2009). With the purpose of development of potent methods to avoid or treat the metastasis and invasion in NPC, we further investigate the role of miR-34a. Our results showed that miR-34a was downregulated in NPC tissues, while its target gene AXL expression was upregulated. And, overexpression of miR-34a significantly inhibited cell migration, invasion and EMT in NPC via targeting AXL.
EMT is of great importance for tumor invasion and metastasis in many cancers, which was characterized by the decreased expressions of E-cadherin and γ-catenin and the increased expressions of N-cadherin and Vimentin (Kang and Massague 2004; Sui et al. 2014). miR-34a has been reported to play a role in EMT in variety of cancers and other diseases. For example, Yamamoto et al. (2019) demonstrated that the introduction of miR-34a mimic into lung cancer A549/ABCA3 cells significantly increased the expression ofα-smooth muscle actin (α-SMA), another EMT marker. Repression of miR-34a by the long non-coding RNA (lncRNA) small nucleolar RNA host gene 7 (SNHG7) apparently accelerated EMT in breast cancer cells. Han et al. (2019) reported that miR-34a significantly repressed the EMT in lens epithelial cells, a crucial process in the pathogenesis of posterior capsule opacification by targeting Notch1. In NPC, Long et al. (2014) illustrated that increased expression of miR-34a induced by hypofractionated radiotherapy obviously increased NPC CNE1 and CNE2 cell apoptosis. Suppression of lncRNA NEAT1 was identified to inhibit several cell functions, such as proliferation, invasion, migration, and EMT via downregulating miR-34a-5p expression in 5-8F NPC cells (Ji et al. 2019). In addition, miR-34a was expressed at a low level in the cancer tissues and suppressed NPC cell metastasis in vivo, as well as inhibit TGF-β-induced migration, invasion and EMT via targeting SMAD4 in NPC CNE1 cells (Huang et al. 2018). All of the above finings demonstrated that miR-34a could repress the migration, invasion and EMT in NPC cells. Consistently, this study also showed that upregulation of miR-34a in NPC Sune-1 cells with mimic transfection leaded to apparent repressions in cell migration, invasion and EMT, as detected by the wound healing, transwell chambers and western blotting assays.
Mechanistically, the results from the current study confirmed that miR-34a could target AXL and repress AXL expression at both mRNA and protein levels. AXL has been confirmed to play an essential role in modulating cancer cell growth, migration, invasion and chemosensitivity, and was identified to be a promising anti-cancer target in several types of human cancers (Zhu et al. 2019), including non-small cancer (Linger et al. 2013), gastric cancer (Wu et al. 2002), ovarian cancer (Sun et al. 2004) and multiple myeloma (Yan et al. 2019). Jiang et al. (2016) reported that the AXL expression level in NPC tissues was obviously elevated compared with those in normal nasopharyngeal epithelial tissues, which was closely correlated with the distant metastasis, advanced TNM stage and poor prognosis in NPC patients, revealing that AXL served as a potential marker for the migration and invasion of NPC. In the current study, we found that AXL was overexpressed in NPC tissues and was a target of miR-34a in NPC Sune-1 cells, and overexpression of AXL dramatically impaired the role of miR-34a in the repressions of cell migration, invasion and EMT in NPC, suggesting that miR-34a inhibited NPC migration and invasion via targeting AXL.
Moreover, we observed that the expressions of Snail and the phosphorylation level of AKT were significantly reduced when Sune-1 cells were transfected with the miR-34a mimic to upregulate miR-34a expression, suggesting that the inhibition of AKT/Snail signaling might play a role in miR-34a-mediated repressions in NPC cell migration, invasion and EMT. The activation of PI3K/AKT/Snail signaling pathway has been verified to exert a positive role in EMT (Zhou et al. 2019). Moreover, AXL knockdown was demonstrated to significantly repress the expressions of PI3K, p-AKT and Snail, resulting in the repressions of EMT and invasion of breast cancer cells (Li et al. 2014). Furthermore, AXL has been identified to be an activator of the PI3K/AKT signaling in cancers (Li et al. 2015; Ma et al. 2018). Therefore, we conjecture that miR-34a represses NPC cell migration, invasion and EMT through repressing the PI3K/AKT signaling via targeting AXL, which will be verified in our next study.
In conclusion, the findings in this study indicated that miR-34a is downregulated in NPC tissues, and can suppress NPC cell migration, invasion and EMT via targeting AXL. This study further points out the important value of miR-34a in the diagnosis and treatment of NPC.
References
Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, Milano G, Brambilla F, Saccu C, Bianchi ME et al (2018) miR-34a Promotes Vascular Smooth Muscle Cell Calcification by Downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase). Arterioscler Thromb Vasc Biol 38:2079–2090
Chen SJ, Chen GH, Chen YH, Liu CY, Chang KP, Chang YS, Chen HC (2010) Characterization of Epstein-Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PLoS One 5:e12745
Cosmopoulos K, Pegtel M, Hawkins J, Moffett H, Novina C, Middeldorp J, Thorley-Lawson DA (2009) Comprehensive profiling of Epstein-Barr virus microRNAs in nasopharyngeal carcinoma. J Virol 83:2357–2367
Hafizi S, Gustafsson A, Stenhoff J, Dahlback B (2005) The Ran binding protein RanBPM interacts with Axl and Sky receptor tyrosine kinases. Int J Biochem Cell Biol 37:2344–2356
Han R, Hao P, Wang L, Li J, Shui S, Wang Y, Ying M, Liu J, Tang X, Li X (2019) MicroRNA-34a inhibits epithelial-mesenchymal transition of lens epithelial cells by targeting Notch1. Exp Eye Res 185:107684
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
He Y, Guo T, Guan H, Wang J, Sun Y, Peng X (2018) Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: a meta-analysis. Cancer Manag Res 10:1419–1428
Hong JH, Roh KS, Suh SS, Lee S, Sung SW, Park JK, Byun JH, Kang JH (2015) The expression of microRNA-34a is inversely correlated with c-MET and CDK6 and has a prognostic significance in lung adenocarcinoma patients. Tumour Biol 36:9327–9337
Huang G, Du MY, Zhu H, Zhang N, Lu ZW, Qian LX, Zhang W, Tian X, He X, Yin L (2018) MiRNA-34a reversed TGF-beta-induced epithelial-mesenchymal transition via suppression of SMAD4 in NPC cells. Biomed Pharmacother 106:217–224
Ji Y, Wang M, Li X, Cui F (2019) The long noncoding RNA NEAT1 targets miR-34a-5p and drives nasopharyngeal carcinoma progression via Wnt/beta-Catenin signaling. Yonsei Med J 60:336–345
Jiang C, Zhou L, Wang H, Zhang Q, Xu Y (2016) Axl is a potential cancer prognostic marker for the migration and invasion of nasopharyngeal carcinoma. Adv Clin Exp Med 25:531–537
Jiang L, Hermeking H (2017) miR-34a and miR-34b/c suppress intestinal tumorigenesis. Cancer Res 77:2746–2758
Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118:277–279
Kasinski AL, Slack FJ (2012) miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res 72:5576–5587
Li Y, Jia L, Ren D, Liu C, Gong Y, Wang N, Zhang X, Zhao Y (2014) Axl mediates tumor invasion and chemosensitivity through PI3K/Akt signaling pathway and is transcriptionally regulated by slug in breast carcinoma. IUBMB Life 66:507–518
Li Y, Jia L, Liu C, Gong Y, Ren D, Wang N, Zhang X, Zhao Y (2015) Axl as a downstream effector of TGF-beta1 via PI3K/Akt-PAK1 signaling pathway promotes tumor invasion and chemoresistance in breast carcinoma. Tumour Biol 36:1115–1127
Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, Lu X, Baron AE, Franklin WA, Merrick DT et al (2013) Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene 32:3420–3431
Long Z, Wang B, Tao D, Huang Y, Tao Z (2014) Hypofractionated radiotherapy induces miR-34a expression and enhances apoptosis in human nasopharyngeal carcinoma cells. Int J Mol Med 34:1388–1394
Ma Y, Zhou G, Li M, Hu D, Zhang L, Liu P, Lin K (2018) Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-kappaB signaling pathway. Neurochem Int 118:233–241
Paccez JD, Vogelsang M, Parker MI, Zerbini LF (2014) The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer 134:1024–1033
Pang RT, Leung CO, Lee CL, Lam KK, Ye TM, Chiu PC, Yeung WS (2013) MicroRNA-34a is a tumor suppressor in choriocarcinoma via regulation of Delta-like1. BMC Cancer 13:25
Pratap P, Raza ST, Abbas S, Mahdi F (2018) MicroRNA-associated carcinogenesis in lung carcinoma. J Cancer Res Ther 14:249–254
Pratt ZL, Kuzembayeva M, Sengupta S, Sugden B (2009) The microRNAs of Epstein-Barr Virus are expressed at dramatically differing levels among cell lines. Virology 386:387–397
Sui H, Zhu L, Deng W, Li Q (2014) Epithelial-mesenchymal transition and drug resistance: role, molecular mechanisms, and therapeutic strategies. Oncol Res Treat 37:584–589
Sun W, Fujimoto J, Tamaya T (2004) Coexpression of Gas6/Axl in human ovarian cancers. Oncology 66:450–457
Wang L, Mou Y, Meng D, Sun Y, Chen X, Yang X, Jia C, Song X, Li X (2017) MicroRNA-203 inhibits tumour growth and metastasis through PDPN. Clin Otolaryngol 42:620–628
Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS, Lui WY, Lin WC (2002) Clinical significance of AXL kinase family in gastric cancer. Anticancer Res 22:1071–1078
Xu C, Chen YP, Ma J (2016) Clinical trials in nasopharyngeal carcinoma-past, present and future. Chin Clin Oncol 5:20
Yamamoto A, Kawami M, Konaka T, Takenaka S, Yumoto R, Takano M (2019) Anticancer Drug-Induced Epithelial-Mesenchymal Transition via p53/miR-34a axis in A549/ABCA3 Cells. J Pharm Pharm Sci 22:516–524
Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, Ma B (2012) MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One 7:e33778
Yan S, Vandewalle N, De Beule N, Faict S, Maes K, De Bruyne E, Menu E, Vanderkerken K, De Veirman K (2019) AXL receptor tyrosine kinase as a therapeutic target in hematological malignancies: focus on multiple myeloma. Cancers (Basel) 11:1727
Ye J, Li L, Feng P, Wan J, Li J (2016) Downregulation of miR-34a contributes to the proliferation and migration of laryngeal carcinoma cells by targeting cyclin D1. Oncol Rep 36:390–398
Zhou F, Geng J, Xu S, Meng Q, Chen K, Liu F, Yang F, Pan B, Yu Y (2019) FAM83A signaling induces epithelial-mesenchymal transition by the PI3K/AKT/Snail pathway in NSCLC. Aging 11:6069–6088
Zhu C, Wei Y, Wei X (2019) AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer 18:153
Zuo J, Zhu K, Wang Y, Yu Z (2018) MicroRNA-34a suppresses invasion and metastatic in esophageal squamous cell carcinoma by regulating CD44. Mol Cell Biochem 443:139–149
Acknowledgements
Not applicable.
Funding
This work was supported by Anhui Provincial Key Project of Natural Science Research in Institutions of Higher Learning in 2018 (Grant No. KJ2018A0996) and 2017 Bengbu Medical College General Program (Grant No. BYKY1772).
Author information
Authors and Affiliations
Contributions
CYJ and ZQC conceived and designed the experiments,TJ and YJX analyzed and interpreted the results of the experiments, BW performed the experiments.
Corresponding author
Ethics declarations
Conflict of Interest
Chengyi Jiang, Zhongqiang Cheng, Tao Jiang, Yajia Xu and Bin Wang state that there are no conflicts of interest to disclose.
Ethics approval and consent to participate
All procedures in the current study involving human samples were performed in accordance with the Helsinki declaration and approved by the Ethic Committee of The First Affiliated Hospital of Bengbu Medical College. The informed consent has been obtained from each participant.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Patient consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Evaluation of the expression of known targets of miR-34a. QRT-PCR was performed to detect the mRNA levels of (A) SIRT1, (B) CD44, (C) c-MET, (D) CDK6 and (E) cyclin D1 after Sune-1 cells were transfected with NC mimic or miR-34a mimic. Three independent experiments were carried out. (n = 03, *P<0.05, **P<0.01). 1 (TIF 272.0 kb)
Rights and permissions
About this article
Cite this article
Jiang, C., Cheng, Z., Jiang, T. et al. MicroRNA-34a inhibits cell invasion and epithelial-mesenchymal transition via targeting AXL/PI3K/AKT/Snail signaling in nasopharyngeal carcinoma. Genes Genom 42, 971–978 (2020). https://doi.org/10.1007/s13258-020-00963-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-020-00963-3