Abstract
In this investigation Gloriosa superba L. was cytogenetically studied with orcein, CMA and DAPI-staining for authentic characterization. “Complex Chromocenter Type” of interphase nuclei with 3–4 bigger darkly stained heterochromatic regions was found in this species. The prophase chromosomes were “Interstitial Type” with darkly stained region at different interstitial sites of chromosomes. This species had 2n = 22 chromosomes with heterogenous centromeric formulae 14 m + 8 sm. In addition to the regular chromosomes, 1–6 small chromosomes were found in several mitotic pro-metaphase, metaphase and anaphase stage which covered 43.57% of the total cell count of this species suggesting the probable occurrence of B-chromosome. Presence of B-chromosome was probably the first report for this genus. After fluorescent banding, a pair of dot like CMA fluoresced bands were observed whereas no bright band was found in G. superba after DAPI-staining which suggested the coexistence of GC- and AT-base pairs in the genome. Thus, the compilation of the above information will be very useful for cytogenetical characterization of G. superba L. in Bangladesh.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gloriosa superba L. (Flame lily, Glory lily or Climber lily) is one of the important medicinal plants now in endangered list [31]. Flame lily belongs to Colchicaceae, is a perennial tuberous climbing herb, extensively scattered in the tropical and sub-tropical countries of the world [1]. In Bangladesh, G. superba is commonly known as Ulatchandal or Ognishikha and widely used for several ethno-medicinal purposes by tribal peoples and traditional practitioners in Bangladesh [36]. Flame lily has numerous applications as remedies to the local populations of both Africa and Asia. Gloriosa derives its name from the word ‘gloriosus’, which means handsome and superba from the word ‘superb’ means majestic. Due to the floral beauty this plant is also popular for ornamental uses [30]. In the world market flame lily considered as rich source of colchicine and gloriosine [31]. This species is not only a notorious human and livestock poison, but also widely used in several indigenous systems of medicine for the treatment of various human ailments. The flower has analgesic, antiinflammatory, antimicrobial, larvicidal, antipoxviral, antithrombotic, antitumor, enzyme inhibition potential and also used in treatment of snake bite, skin disease, respiratory disorders, etc. [1, 24, 30, 31]. In recent years, there has been a gradual increase of interest in the use of medicinal plants because it is safe without any adverse or minimal side effect especially when compared to synthetic medicines. Thus a search for new drugs with better and cheaper substitutes from plant origin is a natural choice [1, 24, 30, 31]. Increasing interest by multinational pharmaceutical companies and domestic manufacturers of herbal-based medicines is contributing significant economic growth of the global medicinal plants sector. Therefore, the conservation of such a valuable resource in the country is very important. Once, Bangladesh had a tremendous wealth of medicinal plants. Unfortunately 50% of these are extinct due to over harvest, lack of proper conservation policy, scientific use and systematic efforts to explore and exploit this valuable potential [17]. Therefore, it is an urgent need to develop conservation policy of the native medicinal plants. Before conservation authentic identification and genetical characterization is very important. For genetic characterization stable and reliable methods must be followed. Karyotype analysis is such a stable and reliable method which also provides basic information about genetic makeup. A number of earlier workers tried to characterize flame lily by chromosome study which mostly confined to chromosome counts [8, 40]. Chromosome counting is not enough to provide detail genomic information. Fluorescent chromosome banding is an excellent tool for karyotype analysis. It helps to provide information regarding the distribution of AT (Adenine–Thymine)- and GC (Guanine-Cytosine)- rich repeats in the genome [2, 14]. Staining with DNA-base specific banding with fluorochromes such as chromomycin A3 (CMA) and 4′,6-diamidino-2-phenylindole (DAPI) is relatively modern method for karyotype study. CMA binds with GC-rich repetitive sequences of the genome and gives characteristics yellow colour bands. On the other hand, DAPI binds with AT-rich repeats giving characteristic blue colour bands [4, 14, 20, 34]. Thus, it seems that fluorescent banding is quite satisfactory for detail and critical chromosome analysis such as identification of individual chromosome, determination of amount and site of AT- and GC-rich base pairs in chromosomes, etc. Study of staining properties of interphase nuclei and prophase chromosomes are other karyomorphological parameters. Tanaka [39] classified the different types of interphase nuclei and prophase chromosomes on the basis of heterochromatin condensation. Later different workers tried to characterize interphase nuclei and prophase chromosomes by differential staining with orcein, CMA and DAPI [3, 4]. The outcome of these studies showed that various taxa including varieties of many plant species could be distinguished by their staining properties of interphase nuclei and prophase chromosomes. The extreme medicinal and ornamental uses make G. superba endangered and thus under threat. If it is not managed or conserved at this stage, the consequences would be worse.
Therefore, in this study, differential karyotype analysis of G. superba L. was carried out for the first time in Bangladesh with the following aims: (1) To understand the staining property of the interphase nuclei and prophase chromosomes after staining with orcein, CMA and DAPI. (2) To determine the diploid chromosome number of G. superba. (3) To make conventional orcein-stained karyotype. (4) To know the distribution of AT- and GC- rich repeats in genomes. (5) To make full strength karyotype after CMA and DAPI-staining. (6) To compile the multidimentional cytogenetical data for characterization of G. superba from Bangladesh.
Materials and methods
In this investigation, ten individuals of G. superba L. were used as materials. These plants were maintained in the Botanical Garden, Department of Botany, University of Dhaka. Healthy roots were collected and pretreated with 0.002 M 8-hydroxyquinoline for 1 h 15 min at room temperature followed by 15 min fixation in 45% acetic acid at 4 °C. These were then hydrolyzed in a mixture of 1 N HCl and 45% acetic acid (2:1) at 60 °C for 1 min 45 s. The root tips were stained and squashed in 1% aceto-orcein. For CMA- and DAPI-banding, Alam and Kondo’s [4] method was used with slight modification. After hydrolyzing the roots, the excised meristematic portion of root tips were squashed with 45% acetic acid. The cover glasses were removed quickly and allowed to air dry for at least 24 h before analysis. For CMA-staining, the air-dried slides were first incubated in McIlvaine’s buffer (pH 7.0) for 25 min, followed by a Distamycin A (0.1 mg/ml) treatment for 10 min. The slides were rinsed mildly in McIlvaine’s buffer supplemented with MgSO4 (5 mM) for 10 min. One drop of CMA (0.1 mg/ml) was added to the materials for 45 min in a humid chamber and then rinsed with McIlvaine’s buffer with Mg2+ for 15 min. Slides were mounted in 50% glycerol and kept at 4 °C overnight before observation. These were observed under Nikon (Eclipse 50i) fluorescent microscope with a blue violet (BV) filter cassette. For DAPI-staining, after 24 h of air drying, the slides were first incubated in Mcllvaine’s buffer (pH 7.0) for 25 min and treated in Actinomycin D (0.25 mg/ml) for 10 min in a humid chamber. The slides were immersed in DAPI solution (0.01 mg/ml) for 45 min. The slides treated with McIlvaine’s buffer for 15 min and mounted with 50% glycerol. These were observed under a Nikon (Eclipse 50i) fluorescent microscope with an ultraviolet (UV) filter cassette. For studying pollen viability, mature flowers with yellow anthers of G. superba L. were collected. One drop of aceto-orcein was placed on a clean slide. The mature anthers were touched to the aceto-orcein dye in such a way so that the pollen grains came in contact with aceto-orcein. A clean cover slip was placed on it and observed under microscope. A procedure proposed by Levan et al. [22] was followed for determining centromeric types of chromosomes. The measurement of relative length was done by dividing the length of a particular chromosome with the total length of the diploid complements. Centromeric index was measured by the ratio of short arm to total length of that chromosome and expressed as percent. To get an accurate measurement of lengths, chromosomes from five metaphase plates were measured. In karyotype the chromosomes were arranged gradually from bigger to smaller in length. The short arm placed on the upper side of the axis and long arm on the lower side.
Results and discussion
Nature of orcein stained interphase nuclei and prophase chromosomes
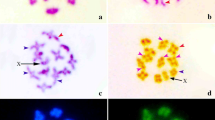
In G. superba, three to four bigger darkly stained heterochromatic bodies were observed in the interphase nuclei after orcein, CMA and DAPI staining (Fig. 1a–c). This type of staining properties of interphase nucleus was regarded as “Complex Chromocenter Type” [39]. The prophase chromosomes of G. superba L. had darker region at different interstitial sites after orcein staining (Fig. 1d). According to Tanaka [39], this type of staining properties of prophase chromosomes is known as “Interstitial Type”. Generally the localized heterochromatic bodies of the interphase nuclei scattered within the prophase chromosomes. In this regard, this species followed general features of orcein staining in the interphase nuclei and prophase chromosomes. Several less fluoresced CMA and DAPI-stained portion were also observed in prophase chromosomes (Fig. 1e–f).
Conventional karyotype
In this study, Glory lily was found to possess 2n = 22 chromosomes (Fig. 2; Tables 1, 2). The somatic chromosome number 2n = 22 for G. superba L., was also reported earlier [7, 10, 18, 25,26,27, 30, 32, 40]. Thus the present report correlates with the previous findings.
However, different 2n-chromosome number for this species were also available such as 2n = 44 [28], 2n = 88 [21] and 2n = 90 [35]. The present report regarding 2n chromosome number did not support these findings. According to chromosome number records, the genus Gloriosa is characterized by monobasic chromosome number (x = 11) such as G. superba, G. lutea and G. plantii are diploids (2n = 2x = 22), G. carsonii, G. virescens and G. richmondensis are tetraploids (2n = 4x = 44) and G. rothschildiana, G. latifolia and G. magnifica are octaploids (2n = 8x = 88) [30]. If the basic chromosome number of G. superba is considered as x = 11, then the species with 2n = 4x = 44 [28] and 2n = 8x = 88 [21] could be regarded as tetraploid and octaploid species, respectively. In contrast, 2n = 90 [35] for this species might be a case of aneuploidy.
Total length of diploid complements in this species was 98.62 ± 2.45 µm (Tables 1, 2). Gloriosa superba L. was found to possess 14 metacentric chromosomes and 8 sub-metacentric chromosomes. Relative length of individual chromosome ranged from 0.03 to 0.08 and the range of individual chromosomal length was from 3.28 ± 0.21 to 7.83 ± 0.37 µm (Tables 1, 2). The first chromosome pair of G. superba L. was comparatively larger (about 7.8 µm) than the rest 10 pair of chromosomes (Figs. 2, 3; Table 1). This pair could be placed in a distinct group on the basis of modality. In contrast, the chromosomal lengths of other 10 pairs were more or less similar (3.28–5.58 µm) i.e. difference between chromosomal length between these 10 pairs was about 2 µm. There was gradual decease of chromosomal length in these 10 chromosome pairs. These 10 pairs of chromosomes could be placed in another group on the basis of modality. In this respect, G. superba L. was found to possess slightly bimodal karyotype (Figs. 2, 3; Table 1). On the other hand, presence of both metacentric and sub-metacentric chromosomes with bimodality indicated these species had asymmetric karyotypes (Tables 1, 2). Stebbins [37] mentioned that the asymmetric karyotypes were advance character. Therefore, G. superba L. was advance in nature in respect of evolutionary point of view.
Probable reasons for the origin of sub-metacentric chromosomes
In this study, at least 50 mitotic metaphase cells of ten different individuals of G. superba L. were observed for each staining. Glory lily was found to possess eight sub-metacentric and fourteen metacentric chromosomes (14 m + 8sm) in most of the mitotic metaphase cells (Figs. 2, 3; Tables 1, 2). Two large metacentric chromosomes were observed in chromosome pair I in about 84% mitotic metaphase cells of G. superba (Fig. 2a, c, d, f–h, i–l, p–q, s–x). However, in some metaphase cell (about 16%), there was one large metacentric chromosome and another large sub-metacentric chromosome (Fig. 2b, e, m, r, * indicates sub-metacentric chromosome). This sub-metacentric chromosome in pair I might be originated from metacentric chromosomes by pericentric inversion.
Fluorescent banding
In G. superba, a pair of dot like CMA fluoresced bands were observed on the short arms of both homologues of chromosome pair XI (Figs. 2i–p, 3b). The total length of CMA banded region was 2.00 µm which covered about 2.03% of the total chromatin length. The CMA banded karyotype formula of this species was 2α + 20β (Table 2). No significant brightly fluoresced DAPI-band was observed in G. superba. The DAPI banded karyotype formula of this species was 22β (Figs. 2q–x, 3c; Table 2). However, all AT-rich or GC-rich heterochromatic regions do not react equally to these fluorochromes i.e. they fluoresce with the same brightness as euchromatin [6, 9, 11, 33]. Heterochromatins could react with different base-specific fluorochromes in a preferential manner and could be categorized as AT-rich, GC-rich or neutral [12]. In this study, presence of only a pair of bright CMA-band and absence of bright DAPI-band in this species suggested the neutral distribution or coexistence of GC- and AT-base pairs in the genome.
B-chromosome?
In the present investigation, a total of 482 mitotic cells from ten individuals of Glory lily were assessed. In addition to regular 2n = 22 chromosomes, 1–6 small chromosomes were found in several mitotic metaphase cells of these ten individuals. Numbers of these chromosomes were variable in different mitotic cells. Moreover, these chromosomes were remarkably smaller (about 1.0–1.5 µm) than the smallest regular chromosomes (3.28 µm) in length and thus could not be arranged with the normal complements in the respective karyotype (Figs. 2, 3; Table 3). In mitotic pro-metaphase these small chromosomes were stayed separated (Fig. 4e). During mitotic anaphase these chromosomes showed preferential segregation (Fig. 4f–i). The above features suggested these small chromosomes as B-chromosomes (Bs). However, in some mitotic anaphase cells B-chromosomes were found to express laggard nature (Fig. 4f–i). This kind of laggard nature of B-chromosomes at anaphase stage was also reported by Ali et al. [5] in Rye. There is no previous report on the presence of B-chromosome in the genus Gloriosa [16, 23]. Thus, existence of B-chromosome might be the new report for this species. However, B-chromosomes were reported earlier in three species of the genus Androcymbium such as A. gramineum, A. rechingeri and A. wyssianum under the family Colchicaceae [16]. According to previous reports, B chromosomes tend to be neutral in their phenotypic effects in low numbers and harmful in high numbers [13, 15, 29, 41]. In other word, high number of B-chromosomes showed deleterious effect on the vigor and fertility of a plant population such as rye, wheat, maize, etc. [13, 15, 41]. However, according to the present observation, 56.43% of the total counted cells did not exhibit B-chromosomes whereas 43.57% displayed Bs, among which 20.54% had 1 B, 15.35% had 2 Bs, 4.77% had 3 Bs, 1.87% had 4 Bs, 5 and 6 Bs were rare in those plants (Fig. 2, Table 3). Therefore, it might be suggested that, analyzed B-chromosome caring plants in this experiment showed about 96% pollen fertility and regular fruit formation with viable seed due to possessing low number of Bs (Fig. 4a–d). Generally B-chromosomes are heterochromatic in nature [13, 15, 38]. In this study, B-chromosomes were brightly fluoresced after CMA-staining and less fluoresced with DAPI-staining (Fig. 2i–x). The above observation suggested that the heterochromatins of B-chromosomes of G. superba were mostly made up of GC nucleotides. Komissarov et al. [19] reported GC-rich B-chromosomes in Lates calcarifer which correlates with the present findings.
This study consisted of cytogenetical analysis of G. superba and yielded a new report of B chromosome for the first time in this species as well as within the genus Gloriosa. Interestingly, the analysis of the mitotic behavior of these supernumerary chromosomes showed some particular features confirming its variability and also its laggard nature during anaphase.
References
Ade R, Rai M. Review: current advances in Gloriosa superba L. Biodiversity. 2009;10:210–4.
Akter L, Mahbub M, Habib MA, Alam SS. Characterization of three varieties of Lathyrus sativus L. by fluorescent karyotype and RAPD analysis. Cytologia. 2015;80:457–65.
Alam SS, Khatun M, Sultana SS. Differential chromosome banding and isozyme assay in Corchorus aestuans. Bangladesh J Bot. 2011;40:47–52.
Alam SS, Kondo K. Differential staining with Orcein, Giemsa, CMA and DAPI for comparative chromosome study of 12 species of Australian Drosera (Droseraceae). Am J Bot. 1995;82:1278–86.
Ali M, Moghaddam B, Schubert V, Kumke K, Weiβ O, Klemme S, Nagaki K, Macas J, González-Sánchez M, Heredia V, Gómez-Revilla D, González-García M, Vega JM, Puertas MJ, Houben A. Nondisjunction in favor of a chromosome: the mechanism of rye B chromosome drive during pollen mitosis. Plant Cell. 2012;24:4124–34.
Bennett ST, Leitch IJ, Bennett MD. Chromosome identification and mapping in the grass Zingeria biebersteiniana (2n = 4) using fluorochromes. Chromosome Res. 1995;3:101–8.
Biswas A, Muntaha SN, Rahman MM. Comparative karyotype analysis in two life-forms of Gloriosa superba L. J Pharma Biol. 2014;4:77–80.
Bose TK, Yadab LP. Commercial flowers. Naya Prakash. 1989.
Cuellar T, Belhassen E, Fernández-Calvín B, Orellana J, Bella JL. Chromosomal differentiation in Helianthus annuus var. macrocarpus: heterochromatin characterization and rDNA location. Heredity. 1996;76:586–91.
Delay C. Recherches sur la structure des noyaux quiescents chez les Phanerogames. Rev. Cytol. et Cytophysiol Veg. 1947;9:169–222.
Galasso I, Saponetti LS, Pignone D. Cytotaxonomic studies in Vigna. III. Chromosomal distribution and reacting properties of the heterochromatin in five wild species of the section Vigna. Caryologia. 1996;49:311–9.
Guerra M. Patterns of heterochromatin distribution in plant chromosomes. Genet. Mol. Biol. 2000;23:1029–41.
Houben A. B chromosomes-a matter of chromosome drive. Front Plant Sci. 2017;8:1–6.
Islam M, Alam SS. Karyotype characterization with fluorescent banding in one released and two wild germplasms of Hibiscus cannabinus L. Cytologia. 2011;76:223–7.
Jones N, Houben A. B chromosomes in plants: escapees from the A chromosome genome? Trends Plant Sci. 2003;8:417–23.
Jones N. New species with B chromosomes discovered since 1980. Nucleus. 2017;60:263–81.
Khan MS. Towards sustainable development: conservation of genetic resources of Bangladesh, a background paper for national conservation strategies-Bangladesh. The World Conservation Union and Bangladesh Agricultural Research Council; 1995.
Khoshoo TN. Cytology of Gloriosa. Curr Sci. 1956;25:165–6.
Komissarov A, Vij S, Yurchenko A, Trifonov V, Thevasagayam N, Saju J, Sridatta PSR, Purushothaman K, Graphodatsky A, Orbán L, Kuznetsova I. B chromosomes of the Asian seabass (Lates calcarifer) contribute to genome variations at the level of individuals and populations. Genes. 2018;9:464.
Kondo T, Hizume M. Banding for the chromosomes of Cryptomeria japonica D. Don Jpn J For Soc. 1982;64:356–8.
La-Cour LF. Heterochromatin and the organization of nucleoli in plants. Heredity. 1951;5:37–50.
Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52:201–20.
Levin DA, Palestis BG, Jones RN, Trivers R. Phyletic hot spots for B chromosomes in angiosperms. Evolution. 2005;59:962–9.
Maroyi A, Maesen LJGVD. Gloriosa superba L. (family Colchicaceae): Remedy or poison? J. Med Pl Res. 2011;5:6112–21.
Mehra PN, Sachdeva SK. Cytological observations on some W. Himalayan Monocots: II. Smilacaceae, Liliaceae and Trilliaceae. Cytologia. 1976;41:5–22.
Miller EW. A preliminary note on the cytology of the Melanthioideae section of the Liliaceae. Proc Univ Durham Philos Soc. 1930;8:267–71.
Mitra K, Datta N. IOPB chromosome number reports VIII. Taxon. 1967;16:445–61.
Narain P. Chromosomal mosaicism in the microsporocytes of Gloriosa. Cytologia. 1980;45:271–9.
Ohta S, Saruhashi Y. Geographical distribution of B chromosomes in Aegilops mutica Boiss., a wild relative of wheat. Hereditas. 1999;130:177–83.
Padmapriya S, Rajamani K, Sathiyamurthy VA. Glory Lily (Gloriosa superba L.)—a review. Int J Curr Pharm Rev Res. 2015;7:43–9.
Ragupathi G. Phytochemical and antioxidant screening of Gloriosa superba L. from different geographical positions of South India. Int J Bot Stud. 2016;1:13–9.
Sato D. Karyotype alteration and phylogeny in Liliaceae and allied families. Jpn J Bot. 1942;12:57–161.
Schwarzacher T, Schweizer D. Karyotype analysis and heterochromatin differentiation with Giemsa C-banding and fluorescent counterstaining in Cephalanthera (Orchidaceae). Plant Syst Evol. 1982;141:91–113.
Schweizer D. Reverse fluorescent chromosome banding with Chromomycin and DAPI. Chromosoma. 1976;58:307–24.
Sharma AK, Sharma A. An investigation of the cytology of some species of Liliaceae. Genet Iber. 1961;13:25–42.
Siddiqui KU, Islam MA, Ahmed ZU, Begum ZNT, Hassan MA, Khondker M, Rahman MM, Kabir SMH, Ahmad M, Ahmed ATM, Rahman AKA, Haque EU. Encyclopedia of flora and fauna of Bangladesh. Angiosperms: Monocotyledons (Agavaceae-Najadaceae), Asiat Soc Bangladesh; 2007.
Stebbins GL. Chromosomal evolution in higher plants. Boston: Addison-Wesley publishing company; 1971.
Su H, Liu Y, Liu Y, Birchler JA, Han F. The behavior of the maize B chromosome and centromere. Genes. 2018;9:476.
Tanaka R. Type of resting nuclei in Orchidaceae. Bot Mag Tokyo. 1971;84:118–22.
Tjio JH. The somatic chromosomes of some tropical plants. Hereditas. 1948;34:135–46.
Williams E, Barclay PC. The effects of B-chromosomes on vigour and fertility in Dactylis hybrids. N Z J Bot. 1968;6:405–16.
Acknowledgements
This research work was funded by Bangabandhu Science and Technology Fellowship Trust, Ministry of Science and Technology, Government of the Peoples’ Republic of Bangladesh.
Author information
Authors and Affiliations
Contributions
SSS—Field investigation, performing the experiment, data analysis, drafting manuscript; CKD—Material collection, performing the experiment, data analysis; SSA—Study design, originator of the research, interpretation of results; MAH—Study design, interpretation of results, critical revision and finalization of the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Sultana, S.S., Dash, C.K., Alam, S.S. et al. Karyotype analysis and report on B-chromosome in Gloriosa superba L. by differential staining. Nucleus 62, 31–38 (2019). https://doi.org/10.1007/s13237-018-0259-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-018-0259-2