Abstract
Almost all fabricated polymers need high stabilization to prevent harmful effects. Adding specialized chemicals that rule as light stabilizers (or UV stabilizers) and tailor to the resin's characteristics might accomplish the desired stability. In this work, five antipyrine derivatives were synthesized by the Schiff bases using five benzaldehyde substituents (benzaldehyde, 4-bromobenzaldehyde, 4-nitrobenzaldehyde, 4-dimethylaminobenzaldehyde, and 4-hydroxybenzaldehyde) with 4-aminoantipyrene. The produced complexes are characterized using hydrogen-1 and carbon-13 nuclear magnetic resonance (1H-NMR and 13C-NMR, respectively) and Fourier-transform infrared (FTIR) spectroscopy; then they are filled with polyvinyl chloride (PVC) films. Further techniques are used to study the effects of long-term radiation exposure on these films. The IR spectra of PVC films showed side products containing polyene and carbonyl groups before, during, and after irradiation. The presence of antipyrine derivatives led to a decrease in the intensity of their associated functional groups. Furthermore, it is shown that films with antipyrine compounds performed lower weight loss when exposed to radiation compared with the virgin film.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polyvinyl chloride (PVC) is an exceptionally versatile polymer and is the third most widely produced polymer globally [1]. It is mainly used in exterior applications, such as window and door frames, wall sheathing, and drainage pipes. Nevertheless, the long-term viability of PVC products for outdoor construction purposes hinges on their capacity to withstand photodegradation caused by prolonged exposure to sunlight [2]. PVC is prone to inadequate thermal and optical stability, rapidly decomposing hydrochloric gas due to self-decomposition when exposed to heat and light during its production and use [1,2,3]. Over the past few years, there has been a significant rise in the utilization of polymeric materials. However, it is crucial to note that these materials undergo consistent photodegradation when exposed to natural weather elements [3]. To enhance the resistance of these materials to climatic conditions, it is necessary to manufacture and treat PVC resin with suitable chemical additives. This process results in the creation of a composite material that exhibits distinct behavior and properties compared to uncured PVC resin. After synthetic resins were invented and applied, it became essential to develop ways to reduce or eliminate potential decomposition from external factors like heat, light, air, and particularly sunlight. Therefore, synthetic polymers need to include a stabilizer(s) to withstand detrimental consequences and mitigate or minimize the harmful effects caused by environmental factors.
Photostabilization of polymers refers to the process of preventing or eliminating the photochemical reactions that occur in polymers and plastics when exposed to light [4, 5]. Photostabilizer mechanisms have been devised based on the efficacy of different types of stabilizers: (a) light ray protectors, (b) ultraviolet ray absorbers, (c) energy dampeners, (d) peroxide decomposers, and (e) free radical scavengers [6,7,8].
Heterojunction organic materials have garnered growing interest due to their potential for various applications. Antipyrine derivatives (APDs) are a type of heterojunction that shows great promise in fields, such as optical communications, optoelectronic materials, and biofunctional compounds. They are particularly known for their exceptional nonlinear optical (NLO) responses, as evidenced by previous studies [9,10,11,12,13,14,15]. APDs are notable for their appealing functional properties, including antioxidant [16], anti-putrefactive [17], and optical [18, 19] characteristics in the realm of chemical physics. These compounds are further used as bio-model compounds in biology and medicine [20]. The photoresponsive properties of functional materials, such as bioelectric [21, 22], photovoltaics [23,24,25], photoluminescence [26, 27], and nonlinear optics [28,29,30], have increasingly become a focal point for scientists.
4-Aminoantipyrine, a derivative of antipyrine, functions as both an anti-inflammatory and antipyretic agent. Its amino nitrogen serves as a strong coordination site, allowing it to easily form metal complexes. When 4-aminoantipyrine is exposed to aldehydes or ketones, it reacts to form Schiff bases, which have multiple applications, specifically in chemosensing. For instance, researchers have developed 4-aminoantipyrine derivatives that can effectively detect cations and anions [31]. Several research groups have shown interest in synthesizing derivatives of 4-aminoantipyrine because of their potential biological activities [32]. Hence, 4-aminoantipyrine is a promising choice for colorimetrically determining phenolic material due to its ease of use, fast results, and the use of stable reagents [33].
This paper discusses the importance of stabilizing synthetic polymers to protect them from potential harm caused by external factors. We produced five antipyrine derivatives by combining Schiff bases with different benzaldehyde substituents and 4-aminoantipyrene. The derivatives were identified through the utilization of nuclear magnetic resonance (1H-NMR and 13C-NMR) and infrared spectroscopy. Furthermore, their effectiveness as photostabilizers for PVC was investigated. This led to a decrease in weight loss and an improvement in the films' ability to withstand photodegradation.
2 Materials and methods
2.1 Chemicals and instrumentation
PVC was obtained from Petkim Petrokimya in Istanbul, Turkey; the degree of polymerization was 800 and the K value was 67. The other chemicals were provided by Sigma-Aldrich, Gillingham, UK. Melting points of complexes were determined using a Gallenkamp instrument in Calgary, Canada. Fourier-transform infrared (FTIR) spectra were acquired using a Shimadzu 8400 Spectrophotometer (Kyoto, Japan) over a range of 400–4000 cm–1 with a KBr disk. NMR spectra were registered at (1H: 500 MHz, 13C: 125 MHz). Data were recorded with a Bruker DRX-500 NMR spectrometer (Zürich, Switzerland). Scanning electron microscopy (SEM) images were obtained using a KYKY-EM3200 digital microscope by FEI Company in Prague, Czech Republic. Energy-dispersive X-ray (EDX) spectra were recorded with the Bruker XFlash 6 10 (Tokyo, Japan). Prior to EDX, the PVC sheets were gold-coated with an approximately 15 nm thick layer to enhance the conductivity and obtain clear images. The PVC films were irradiated at 25 °C using a QUV-accelerated weathering tester from Q-Panel Company (Homestead, FL, USA). The PVC sheets maintained a consistent thickness of approximately 40 µm, measured with a DIN 862 digital caliper micrometer (Vogel GmbH, Kevelaer, Germany).
2.2 Preparation of 4-antipyrine derivatives
Schiff bases were synthesized from 4-aminoantipyrine derivatives, where five derivatives of 4-aminoantipyrene. Hence, 0.203 g of 4-aminoantipyrene reacted with five derivatives of benzaldehyde (0.106 g benzaldehyde, 0.185 g 4-bromobenzaldehyde, 0.151 g 4-nitrobenzaldehyde, 0.149 g 4-dimethylaminobenzaldehyde, and 0.122 g 4-hydroxybenzaldehyde) to produce five complexes numbered 1–5 respectively. The reaction was accomplished using absolute ethanol solvent and thermal sublimation for 3–4 h, where the chemical structure is illustrated in Scheme 1. The product was precipitated through filtration and dried for 30 min under a temperature of 40–50°C. The yields of the complexes ranged between 75 and 85% for all the synthesized derivatives. The physical properties of the products are indicated in Table 1.
2.3 FTIR infrared spectroscopy
The FTIR spectra of antipyrine derivatives (3–5) demonstrate that the carbonyl group was absent in the initial compound. However, a distinct adsorption band associated with the azomethine bond (–CH=N–) appeared in the 1593–1681 cm–1 range. Table 2 presents the most common FTIR absorption bands for the five antipyrine compounds.
2.4 1H-NMR spectroscopy
Through 1H-NMR analysis of the synthesized organic compounds 1–5, the signal for the amine proton NH2 disappeared at a chemical shift of 4.5 ppm. The signal for (H–C=O) at 9.8 ppm also disappeared for all benzaldehyde derivatives. A proton signal for N=C–H appeared at a shift range of 9.34–9.71 ppm, as illustrated in Fig. 1 (a–e) and Table 3. The OH proton signal for compound 5 appeared at a chemical shift of 9.86 ppm, and the signal for the 6 protons in N–(CH3)2 for compound 4 appeared at 3.01 ppm.
2.5 13C-NMR spectroscopy
13CNMR spectrum analysis of a pyrimidine derivative was conducted, and different aspects were concluded based on the provided information. The carbonyl group has distinct signals appearing in 162.54, 162.40, 162.49, 162.49, and 162.49 ppm, for compounds 1, 2, 3, 4, and 5, respectively, which is a common range for carbonyl groups in all antipyrine derivatives. For the C=N groups, signals at 149.41, 149.98, 149.32, 149.68, and 149.51 ppm are for compounds 1–5, respectively. Regarding the C–NO2 group, the signal appears at 148.19 ppm within the expected range (140–160 ppm) referring to C–NO2 groups belonging to compound 3. N(CH3)2 group signal appears at 151.37 ppm, which is a common signal for the N(CH3)2 group of compound 4 for 2C. However, the C–OH group’s signal appears at 156.06 ppm, within the expected range for C–OH groups belonging to compound 5. The CH3–N group appears at 35.7–36.6 ppm for the CH3–N group for all compounds. The signal appears at 13.66–13.88 ppm refers to the CH3 group, which fills in the common range of CH3 groups of all compounds. The 13CNMR spectrum of the prepared structures supports both IR and 1HNMR spectra. Hence, Table 4 shows the 13C-NMR spectral data for compounds 1–5, and Fig. 2 shows the spectra of the used components.
2.6 Preparation of PVC films
The PVC films were fabricated using the solution-casting method. An appropriate amount of antipyrine (25 mg) was combined with 5 g of PVC in 50 mL of tetrahydrofuran (THF). The mixture was stirred for 2.5 h at a temperature of 25 °C. The uniform solution was subsequently poured onto a glass plate with pores that were approximately 40 µm in thickness. The films underwent a drying process at 25 °C for a duration of 16 h, after which they were further dried in a vacuum oven at a temperature of 40 °C for 4 h.
2.7 Irradiation of PVC film
The PVC films were irradiated with a UV light (λmax = 365 nm) at an intensity of 6.2 × 10−9 ein dm−3 s −1 nm. During the irradiation procedure, the films were rotated regularly to ensure that they were exposed to the same amount of light from both sides.
3 Results and discussion
This section includes testing of organic antipyrene derivatives (1–5) against photodegradation of polymer films. Furthermore, the stabilization efficiency of PVC with and without the additives was assessed.
3.1 FTIR spectroscopy investigation of UV-irradiated films
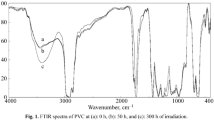
The photostability effectiveness of PVC films modified with organic antipyrine compounds in terms of stabilization was investigated by FTIR spectroscopy. Irradiating PVC films with 313 nm λmax UV light for 300 h changed the FTIR spectrum. The IR spectrum of PVC films after irradiation showed three absorption bands due to the formation of carbonyl group 1722 cm−1 (C=O), polyene 1604 cm−1 (C=C), and hydroxyl 3500 cm−1 (OH) [30,31,32,33]. As shown in Figs. 3 and 4, the growth rate of such peaks related to a reference peak (1328 cm−1) can be considered a measure of the photodegradation of PVC [34,35,36,37,38,39].
Figure 3 displays the FTIR spectra of the plain PVC film before and during undergoing 300 h of irradiation. However, the alterations in the densities of carbonyl, polyene, and hydroxyl groups in PVC films with organic compounds (1–5) were computed over 300 h of irradiation, as depicted in Fig. 4. In the grafted PVC films, the growth rates of the carbonyl, polyene, and hydroxyl groups were reduced when 0.5 wt.% of antipyrines was added, compared to the plain PVC film. Compound 5 was the best stabilizer against photodegradation and demonstrated the highest activity. In contrast, compound 1 was the least efficient among all the stabilizers. The order of increasing photostability efficiency of the organic antipyrine compounds is as follows:
5 > 4 > 3 > 2 > 1.
3.2 Functional groups indexes
The functional group indexes (IPO, IC=O, IOH, and IC=C) are applied in this work as quantitative measures to evaluate the concentration or intensity of particular functional groups within a substance, PVC in our work. The indexes are calculated based on the absorbance measurements of the functional groups (As: AC=O, APO, AOH, and AC=C) and a stable reference peak (Ar: AC–H). In general, as the exposure time increases, the value of the indexes increases [3, 5]. Higher indexes indicate a stronger presence of the functional groups, indicating more significant degradation or the creation of new chemical species. The efficacy of antipyrine derivatives as photostabilizers is assessed based on their influence on these parameters [8]. However, the indexes of all groups showed a reduction in their values as the PVC was filled with these additives, which shows a higher stability for the modified PVC. This showcases their effectiveness in attenuating UV-induced harm and minimizing the production of degradation byproducts [17]. Figure 5 shows the effect of the student indexes as a function of irradiation time to UV light.
3.3 Effects of UV irradiation on weight loss
The process of PVC photo-oxidation results in the formation of free radicals, leading to cross-linking and the removal of HCl. The PVC dehydrochlorination process reduces the mass of the polymer. The efficacy of antipyrine compounds 1–5 as PVC photostabilizers was assessed by quantifying the weight reduction caused by photo-oxidation and photodegradation. The percentage of weight loss was determined using Eq. 1, where W1 and W2 represent the initial and final weights of the PVC, respectively, after irradiation.
The rate of PVC weight loss was initially high during the first 100 h of the irradiation process. Subsequently, the weight loss continued to increase in the absence of antipyrines 1–5 as depicted in Fig. 6. Undoubtedly, the additives exerted a substantial influence on the stabilization of the PVC. After 100 h of irradiation, the blank PVC film experienced a weight loss of 0.54%. This weight loss increased to 1.28% after 300 h of irradiation. The weight loss for the PVC films containing antipyrine components (1–5) was 0.69%, 0.59%, 0.51%, 0.48%, and 0.42%, respectively, at the end of the irradiation operation. From the figure, antipyrine 5 had the greatest effect on PVC stabilization of all of the compounds tested. The blank film lost 307% more weight than the one with additive 5.
3.4 Scanning electrons microscopy (SEM) analysis
The surface morphology of PVC films was investigated using SEM to determine the impact of ultraviolet radiation [41]. Figures 7 and 8 display the SEM images of PVC films. Before irradiation, the surface of the PVC film was glossy and even. Nevertheless, following 300 h of radiation exposure, the surface suffered considerable harm, exhibiting more noticeable damage in comparison to the films that included antipyrine polymer additives. The cracks in the irradiated film were more extensive and had greater depth compared to the non-irradiated film. This is likely because of the formation of cross-links and the degradation of volatile molecules in the hydrochloride chain [42]. The PVC surface devoid of any substance displayed more pronounced deterioration compared to the PVC films containing compounds 1–5. Out of all the samples, the SEM image of the PVC + 5 film that was exposed to radiation displayed the least amount of surface damage.
3.5 Energy-dispersive X-ray
The atomic carbon and chlorine displayed prominent absorption bands in the PVC film EDX spectra (Fig. 9a–g). Additionally, bands corresponding to oxygen and nitrogen atoms were observed in the PVC containing antipyrines 1–5. Among the blends, the PVC + 4 had a notably abundant band corresponding to atomic nitrogen. Moreover, atomic bromine was detected in the PVC + 2 EDX spectrum. The assignment of EDX peaks aligns with previously published data [43]. As radiation increased, the atomic fraction of oxygen rose due to the removal of HCl, which led to higher rates of PVC photo-oxidation and a decrease in the amount of atomic chlorine. Additionally, cross-linking and the creation of short-chain fragments resulted in a reduction in atomic carbon. The EDX results indicated that the percentages of atomic carbon and chlorine dropped when the blank PVC was exposed to radiation. In contrast, the decrease in atomic concentrations of carbon and chlorine for PVC/antipyrine blends was less dramatic due to fewer HCl fumes generated during combustion [40]. This demonstrates that antipyrine derivatives function effectively as PVC photostabilizers [41].
3.6 X-ray mapping
X-Ray mapping, in conjunction with SEM, was utilized to generate images that display the spatial arrangement and proportional representation of elements in distinct hues within a specimen [44]. The arrangement of elements and the prevalence of antipyrine compounds on the PVC surface contributes to the mitigation of photodegradation as depicted in Fig. 10. After exposure of PVC to ultraviolet radiation, chlorine is removed through the release of hydrogen chloride gas, resulting in a decreased abundance of chlorine. In the presence of antipyrines, the decrease in chlorine is less pronounced. Antipyrine derivatives as polymeric additives help prevent the growth of peroxide radicals and reduce their abundance after irradiation compared to PVC without additives. Hence, compound 5 demonstrates the greatest photostabilizing effect compared to the other additives, which is further presented in Fig. 11.
3.7 Photostabilization suggested mechanisms
Photostabilizers shield PVC from ultraviolet radiation and inhibit the rupture of chemical bonds within the polymer chains. Antipyrine compounds 1–5 can protect PVC from degradation due to light exposure by employing various mechanisms. The aromatic moieties (phenyl and aryl groups) within the antipyrine skeleton function as UV absorbers, effectively absorbing UV irradiation. These groups emit the absorbed energy as heat at a rate that is not detrimental to PVC, thus effectively protecting light [45]. The presence of the hydroxyl group (which strongly donates electrons), the nitro group (which strongly withdraws electrons), and the bromine group (which withdraws electrons through induction) attached to the aryl groups results in varying performance results for antipyrines, as depicted in Scheme 2.
The hydroxyl group exerts a substantial stabilizing effect on PVC by amplifying the resonance of the aryl groups. Additionally, the –CH=N bond directly absorbs ultraviolet (UV) light, creating an excited and stable charge-separated species as shown in Scheme 3. This group can emit the absorbed light energy as heat through rotational and vibrational movements, which prevents any damage to the PVC material.
Antipyrine compounds 1–5 can scavenge radicals when a chromophore (POO•) is presented [46]. Antipyrines form stable complexes with chromophores (polymeric proxy radicals; POO•) [5], resulting in the production of stable complexes that are illustrated in Scheme 4. The absorbed energy can be counteracted and stable through the resonance of aryl groups, which subsequently propagate this energy throughout a significant number of atoms.
Antipyrine compounds improve the ability of PVC to withstand degradation because of light by forming a coordinated bond between the polarized atoms of the –CH=N groups in the Schiff bases and the C–Cl bonds in the polymer chains. Moreover, the alignment of the polarized oxygen atoms in antipyrines with the carbon atoms of the C–Cl bonds can enhance the stability of the polymer. The coordination bonds enable the efficient transfer of excited state energy from PVC to antipyrine derivatives without causing any damage to the polymer. Nevertheless, the probability of establishing robust coordination bonds within macromolecules is low.
The mechanism of photostability is not yet fully understood. However, our research and review of previous literature suggest that functional groups, which are atoms or groups of atoms attached to the benzene ring in aromatic compounds, significantly influence the chemical and physical properties of these compounds.
Electron-donating groups, such as –OH and –N(CH3)2, increase the electron density in the aromatic ring, enhancing its stability. These groups stabilize free radicals by delocalizing electrons through resonance within the aromatic ring and the antipyrene part via the imine bond. This stabilization increases the activation energy of photo-oxidation reactions, making them slower. Consequently, the compound's resistance to photodegradation, especially in the ultraviolet region, is significantly enhanced.
On the other hand, electron-accepting groups, such as –Br and –NO2, withdraw electrons from the aromatic ring, making it less stable compared to electron-donating groups. Consequently, these groups are less effective in providing resistance against photo-oxidation.
4 Conclusions
Antipyrine compounds improve the ability of PVC to withstand degradation caused by light by forming a coordinated bond between the polarized atoms of the –CH=N groups in the Schiff bases and the C–Cl bonds in the polymer chains. Moreover, the alignment of the polarized oxygen atoms in antipyrines with the carbon atoms of the C–Cl bonds can enhance the stability of the polymer. The coordination bonds enable the efficient transfer of excited state energy from PVC to antipyrine derivatives without causing any damage to the polymer. Nevertheless, the probability of establishing robust coordination bonds within macromolecules is low.
References
M.E. Pekdemir, M. Kök, A. Cherkezova, Poly (vinyl chloride) and poly (ethylene glycol) binary blend films: a study of thermal and shape memory properties. Macromol. Res. 31, 511–518 (2023)
E. Yousif, J. Salimon, N. Salih, New photostabilizers for PVC based on some diorganotin (IV) complexes. J. Saudi Chem. Soc. 19(2), 133–141 (2015)
C. Fan, Y. Cui, Fluorescence behaviors of metal complexes/PVC composites. Macromol. Res. 25, 357–364 (2017)
P. Coghlan, A Discussion of Some of the Scientific Issues Concerning the Use of PVC (CSIRO, 2001), pp.1–45
E. Yousif, R. Haddad, Photodegradation and photostabilization of polymers, especially polystyrene. Springerplus 2, 1–32 (2013)
E.J. Park, S.Y. Lee, A. Canlier, T.S. Hwang, Controlled dehydrochlorination of poly(vinyl chloride) for fabrication of membranes with polyacetylene-like structure: XPS analysis and ion exchange membrane discussion. Macromol. Res. 27, 33–47 (2019)
H.N. Aliyu, H.J. Abdullahi, Synthesis and characterisation of Manganese (II), Cobalt (II), Nickel (II) and Copper (II) N, N’-bis (Benzoin) ethylenediiminato complexes. Bayero J. Pure Appl. Sci. 2(2), 110–112 (2009)
E.J. Park, B.C. Park, Y.J. Kim, A. Canlier, T.S. Hwang, Elimination and substitution compete during amination of poly(vinyl chloride) with ethylenediamine: XPS analysis and approach of active site index. Macromol. Res. 26, 913–923 (2018)
S.H. Ahn, J.T. Park, J.H. Kim, Y. Ko, S.U. Hong, Nanocomposite membranes consisting of poly(vinyl chloride) graft copolymer and surface-modified silica nanoparticles. Macromol. Res. 19, 1195–1201 (2011)
A. Karakas, H. Ünver, Third-order nonlinear optical properties and structures of (E)-N-(4-nitrobenzylidene)-2, 6-dimethylaniline and (E)-N-(4-nitrobenzylidene)-2, 3-dimethylaniline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 75(5), 1492–1496 (2010)
Y.X. Sun, Q.L. Hao, W.X. Wei, Z.X. Yu, L.D. Lu, X. Wang, Y.S. Wang, Experimental and density functional studies on 4-(3, 4-dihydroxybenzylideneamino) antipyrine, and 4-(2, 3, 4-trihydroxybenzylideneamino) antipyrine. J. Mol. Struct. (Thoechem) 904(1–3), 74–82 (2009)
Y.X. Sun, Q.L. Hao, Z.X. Yu, W.X. Wei, L.D. Lu, X. Wang, Experimental and density functional studies on 4-(4-cyanobenzylideneamino) antipyrine. Mol. Phys. 107(3), 223–235 (2009)
Y.X. Sun, Q.L. Hao, W.X. Wei, Z.X. Yu, L.D. Lu, X. Wang, Y.S. Wang, Experimental and density functional studies on 4-(2, 3-dichlorobenzylideneamino) antipyrine and 4-(2, 5-dichlorobenzylideneamino) antipyrine. J. Mol. Struct. 929(1–3), 10–21 (2009)
Y.X. Sun, W.X. Wei, Q.L. Hao, L.D. Lu, X. Wang, Experimental and theoretical studies on 4-(2, 4-dichlorobenzylideneamino) antipyrine and 4-(2, 6-dichlorobenzylideneamino) antipyrine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 73(4), 772–781 (2009)
H. Ünver, A. Karakaş, A. Elmali, T.N. Durlu, The investigation of nonlinear optical properties of N-(3-fluorophenyl) naphthaldimine. J. Mol. Struct. 737(2–3), 131–137 (2005)
N.V. Bashkatova, E.I. Korotkova, Y.A. Karbainov, A.Y. Yagovkin, A.A. Bakibaev, Electrochemical, quantum-chemical and antioxidant properties of antipyrine and its derivatives. J. Pharm. Biomed. Anal. 37(5), 1143–1147 (2005)
S.S. Abd El Rehim, M.A. Ibrahim, K.F. Khalid, The inhibition of 4-(2′-amino-5′-methylphenylazo) antipyrine on corrosion of mild steel in HCl solution. Mater. Chem. Phys. 70(3), 268–273 (2001)
M.S. Collado, V.E. Mantovani, H.C. Goicoechea, A.C. Olivieri, Simultaneous spectrophotometric-multivariate calibration determination of several components of ophthalmic solutions: phenylephrine, chloramphenicol, antipyrine, methylparaben and thimerosal. Talanta 52(5), 909–920 (2000)
S.A. Coolen, T. Ligor, M. van Lieshout, F.A. Huf, Determination of phenolic derivatives of antipyrine in plasma with solid-phase extraction and high-performance liquid chromatography–atmospheric-pressure chemical ionization mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 732(1), 103–113 (1999)
T. Bansal, M. Singh, G. Mishra, S. Talegaonkar, R.K. Khar, M. Jaggi, R. Mukherjee, Concurrent determination of topotecan and model permeability markers (atenolol, antipyrine, propranolol and furosemide) by reversed phase liquid chromatography: utility in Caco-2 intestinal absorption studies. J. Chromatogr. B 859(2), 261–266 (2007)
M.R. Wasielewski, Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 92(3), 435–461 (1992)
T. Elston, H. Wang, G. Oster, Energy transduction in ATP synthase. Nature 391(6666), 510–513 (1998)
A.W. Hains, Z. Liang, M.A. Woodhouse, B.A. Gregg, Molecular semiconductors in organic photovoltaic cells. Chem. Rev. 110(11), 6689–6735 (2010)
D. Venkataraman, S. Yurt, B.H. Venkatraman, N. Gavvalapalli, Role of molecular architecture in organic photovoltaic cells. J. Phys. Chem. Lett. 1(6), 947–958 (2010)
H. Dinçalp, S. Yavuz, Ö. Haklı, C. Zafer, C. Özsoy, I. Durucasu, S. İçli, Optical and photovoltaic properties of salicylaldimine-based azo ligands. J. Photochem. Photobiol., A 210(1), 8–16 (2010)
T. Yu, K. Zhang, Y. Zhao, C. Yang, H. Zhang, L. Qian, Y. Qiu, Synthesis, crystal structure and photoluminescent properties of an aromatic bridged Schiff base ligand and its zinc complex. Inorg. Chim. Acta 361(1), 233–240 (2008)
D. Ke, C. Zhan, S. Xu, X. Ding, A. Peng, J. Sun, J. Yao, Self-assembled hollow nanospheres strongly enhance photoluminescence. J. Am. Chem. Soc. 133(29), 11022–11025 (2011)
C. Ravikumar, I.H. Joe, Electronic absorption and vibrational spectra and nonlinear optical properties of 4-methoxy-2-nitroaniline. Phys. Chem. Chem. Phys. 12(32), 9452–9460 (2010)
Y. Wang, Z. Yu, Y. Sun, Y. Wang, L. Lu, Synthesis, vibrational spectral and nonlinear optical studies of N-(4-hydroxy-phenyl)-2-hydroxybenzaldehyde-imine: a combined experimental and theoretical investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 79(5), 1475–1482 (2011)
Y. Sun, Q. Hao, W. Tang, Y. Wang, X. Yang, L. Lu, X. Wang, Two-photon absorption, nonlinear optical and UV–vis spectral properties of 2-furanylmethyleneaminoantipyrine, benzylideneaminoantipyrine and cinnamilideneaminoantipyrine. Mater. Chem. Phys. 129(1–2), 217–222 (2011)
C. Varadaraju, G. Tamilselvan, I.V.M.V. Enoch, P.M. Selvakumar, Phenol sensing studies by 4-aminoantipyrine Method–a review. Organ. Med. Chem. Int. J. 5(2), 46–52 (2018)
S. Cunha, S.M. Oliveira, M.T. Rodrigues Jr., R.M. Bastos, J. Ferrari, C.M. de Oliveira, C. Lariucci, Structural studies of 4-aminoantipyrine derivatives. J. Mol. Struct. 752(1–3), 32–39 (2005)
M. Ettinger, C. Ruchhoft, R. Lishka, Sensitive 4-aminoantipyrine method for phenolic compounds. Anal. Chem. 23(12), 1783–1788 (1951)
F. Awaja, S. Zhang, M. Tripathi, A. Nikiforov, N. Pugno, Cracks, microcracks and fracture in polymer structures: formation, detection, autonomic repair. Prog. Mater. Sci. 83, 536–573 (2016)
L. Valko, E. Klein, P. Kovařı́k, T. Bleha, P. Šimon, Kinetic study of thermal dehydrochlorination of poly (vinyl chloride) in the presence of oxygen: III—statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 37(6), 1123–1132 (2001)
E. Yousif, E. Bakir, J. Salimon, N. Salih, Evaluation of Schiff bases of 2, 5-dimercapto-1, 3, 4-thiadiazole as photostabilizer for poly (methyl methacrylate). J. Saudi Chem. Soc. 16(3), 279–285 (2012)
W.H. Starnes Jr., B. Du, S. Kim, V.G. Zaikov, X. Ge, E.K. Culyba, Thermal stabilization and plasticization of poly (vinyl chloride) by ester thiols: update and current status. Thermochim. Acta 442(1–2), 78–80 (2006)
R. Rasheed, H. Mansoor, E. Yousif, A. Hameed, Y. Farina, A. Graisa, Photostabilizing of PVC films by 2-(aryl)-5-[4-(aryloxy)-phenyl]-1, 3, 4-oxadiazole compounds. Eur. J. Sci. Res. 30(3), 464–477 (2009)
E. Yousif, J. Salimon, N. Salih, E. Yousif, Improvement of the photostabilization of PVC films in the presence of thioacetic acid benzothiazole complexes. Malays. J. Anal. Sci. 15(1), 81–92 (2011)
A.J. Jafari, J.D. Donaldson, Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper (II) oxide and copper (II) chloride. E-J. Chem. 6(3), 685–692 (2009)
S.H. Mohamed, A.S. Hameed, G.A. El-Hiti, D.S. Ahmed, M. Kadhom, M.A. Baashen, E. Yousif, A process for the synthesis and use of highly aromatic organosilanes as additives for poly (vinyl chloride) films. Processes 9(1), 91 (2021)
M.A. Tooma, T.S. Najim, Q.F. Alsalhy, T. Marino, A. Criscuoli, L. Giorno, A. Figoli, Modification of polyvinyl chloride (PVC) membrane for vacuum membrane distillation (VMD) application. Desalination 373, 58–70 (2015)
D.E. Newbury, The new X-ray mapping: X-ray spectrum imaging above 100 kHz output count rate with the silicon drift detector. Microsc. Microanal. 12(1), 26–35 (2006)
M.W. Sabaa, E.H. Oraby, A.S.A. Naby, R.R. Mohamed, Anthraquinone derivatives as organic stabilizers for rigid poly (vinyl chloride) against photo-degradation. Eur. Polym. J. 41(11), 2530–2543 (2005)
A.A. Balakit, A. Ahmed, G.A. El-Hiti, K. Smith, E. Yousif, Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly (vinyl chloride). Int. J. Polym. Sci. 2015(1), 510390 (2015)
J. Pospíšil, P.P. Klemchuk, Oxidation Inhibition in Organic Materials, vol. 1 (CRC Press, Boca Raton, FL, USA, 1989), pp.48–49
Acknowledgements
The authors appreciate the effort of the Department of Chemistry at Al-Nahrain University in providing partial support for this work.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no known conflict of interest for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohamed, S., Yousif, E., Kadhom, M. et al. Enhanced photostability of polyvinyl chloride films through antipyrine derivatives: a comprehensive study on UV resistance and degradation inhibition. Macromol. Res. (2024). https://doi.org/10.1007/s13233-024-00310-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13233-024-00310-5