Abstract
The objective of this research was to saccharify cassava flour by acid-acid and acid-enzyme hydrolysis and further conversion of the resulting sugar into ethanol by fermenting with the immobilized (in Ca-alginate) cells of Saccharomyces cerevisiae. The saccharification resulted in higher total sugar recovery by acid-enzyme hydrolysis (72.88 %) than by enzyme-enzyme hydrolysis (58.1 %). Further study on ethanol production was carried out using the hydrolysate obtained from acid-enzyme hydrolysis. The growth of the yeast started in the log phage and maximum ethanol (189 ± 3.1 g ethanol/kg flour) production was achieved with 94.74 ± 2.187 % sugar conversion during the stationary phase.
Similar content being viewed by others
Introduction
Starchy substances constitute the major part of plant biomass. The examples for plants with high starch content are corn, potato, sweet potato, sorghum, wheat and cassava. Cassava (Manihot esculenta Crantz.), also called manioc, tapioca or yuca, is one of the most important food and bio-ethanol crops in the humid tropics (Ray and Swain 2011). In order to make use of the carbon and energy stored in starch, thermostable α-amylase enzymes are used to break down the polymer to smaller sugar units (dextrins), which are eventually converted to individual basic glucose units. The roots of cassava contains about 50-70 % starch on dry weight basis and can be saccharified by acid-enzyme or enzyme-enzyme hydrolysis to obtain lower molecular weight carbohydrates and finally to monomeric sugars. The resulting hydrolysate can be used as feed stocks in fermentation industries.
Currently, Saccharomyces cerevisiae is used all over the world as the major ethanol producing microorganism. S. cerevisiae remains the organism of choice, which is the same species used for making bread, wine and beer (Behera et al. 2010a). Immobilization of whole microbial cells and their application in bio-processing has been of interest for the past 30–35 years (Selvakumar et al. 1994). Immobilization of whole cells for ethanol production offers several advantages: it eases separate cell mass from bulk liquid for possible reuse, facilitates continuous operation over a prolonged period, enhances reactor productivity and ensures higher efficiency of catalysis (Behera et al. 2011). One of the most suitable carriers for cell immobilization is entrapment in calcium alginate as beads, as this is a simple and cost effective technique (Behera et al. 2010b). Sodium alginate, a precursor of calcium alginate and a non-toxic chemical, is most suitable as an immobilization matrix for entrapping bio-molecules and microorganisms. This technique has been used extensively in fermentation industries for producing amino acids (Bodalo et al. 1996), enzymes (Kar and Ray 2008), organic acids (John et al. 2007) and ethanol (Swain et al. 2007).
The present paper describes the results of: (1) saccharification of cassava flour by acid-enzyme and enzyme-enzyme hydrolysis, and (2) batch fermentation of the hydrolysates by immobilized (in Ca-alginate beads) S. cerevisiae cells.
Materials and methods
Cassava
Freshly harvested cassava roots were collected from the experimental farm of the Regional Centre of the Central Tuber Crop Research Institute, Bhubaneswar and brought to the laboratory during the year 2011 (January–March). The roots were de-skinned, sliced into pieces and then sun-dried and oven-dried for 48 h at 70 °C temperature to reduce the moisture content to about 10–12 %. The dried root slices were ground to make flour, and the flours were kept in airtight containers. The cassava flour had 56 ± 1.10 g starch per 100 g flour. The rest components are moisture, starch, crude fibre, crude protein, free reducing sugar, hydrocyanic acid and total ash (Ray 2004).
Microorganisms and culture conditions
Saccharomyces cerevisiae (CTCRI strain) was used earlier in our laboratory for ethanol fermentation (Behera et al. 2010a, b, 2011) and was maintained on malt extract-yeast extract-glucose-peptone (MYGP) medium [(g/l): malt extract, 3; yeast extract, 5; peptone, 5; glucose, 20; agar, 15; and the pH was adjusted to 5.5]. The culture was stored at 4 °C for further use.
Preparation of starter culture
The starter culture was prepared in 100 ml of growth medium (as mentioned above, but without agar) placed in a 250-ml Erlenmeyer flask and the pH was adjusted to 5.5 by dilute HCl or NaOH. The flask containing medium was sterilized at 121 °C for 20 min and inoculated with a loopful of the S. cerevisiae culture, and finally incubated at 30 °C for 24 h under stationary conditions. The yeast cells were immobilized in sodium alginate solution and multiplied on the gel beads as described in the following section.
Immobilization method
Sixty milliliters of yeast starter culture (prepared as above) were centrifuged at 8,000 rpm for 20 min in a refrigerated centrifuge (Model C-24, Remi Pvt., Ltd, Bombay, India), washed, and then the pellets were suspended with 60 ml of deionized water. The cell suspension was used for cell immobilization. The S. cerevisiae cell suspension (1 × 105 CFU/ml) was added to 4 % (w/v) sodium alginate solutions in 1:1 (v/v) ratio and mixed thoroughly. The cell-alginate mixture was then cast into beads by dropping from a hypodermic syringe into cold sterile 0.1 M CaCl2 solution. These beads had a diameter of approximately 3.0 mm and were hardened by keeping in the dilute (0.1 M) CaCl2 solution for 24 h at 4 °C with gentle agitation (Behera et al. 2010b). Finally, these beads were washed with sterile distilled water to remove excess Ca2+ ions and unentrapped cells, before being used for the fermentation process.
Multiplication of immobilized yeast cells
In order to obtain a high cell density, the immobilized beads/cubes containing yeast cell were immersed in MYGP broth for 24 h at 30–32 °C before being fermentation.
Saccharification process
The cassava flour was saccharified by the enzyme-enzyme and acid-enzyme processes as described below.
Saccharification by enzyme-enzyme hydrolysis
Cassava flour (100 g) placed in 1-l Erlenmeyer flasks (in triplicates, n = 3) was mixed with water (flour:water ratio 1:5, w/v) to make a slurry. Saccharification of the flour slurry was carried out by adding 0.1 ml (0.1 %) of the commercial enzyme, Termamyl (Novozymes, Denmark) and incubated for 1 h at a temperature 90 °C (Ray et al. 2008). Then the flasks were cooled to room temperature, 30 ± 2 °C. Subsequently, 2 ml of the enzyme amyloglucosidase (AMG) (Novozymes, Denmark) (2 %) was added to each flask containing the partially saccharified slurry, and the flasks were incubated for 72 h at 45 °C. At the end of 72 h incubation process, the total sugar released by this process was quantified.
Saccharification by acid-enzyme hydrolysis
Cassava flour (100 g) taken in 1 l Erlenmeyer flasks (in triplicates, n = 3) was mixed with water (flour:water ratio 1:5, w/v) to make a slurry. The slurry was treated with 1 ml of 1 N HCl and the flasks were autoclaved at 121 °C for 20 min (Kar et al. 2004). After cooling at room temperature, the pH of the slurry was adjusted to 5.5 by addition of dilute 1 N NaOH. Then, AMG (2 ml) (2 %) was added to the dextrinized slurry, followed by incubation at a temperature of 45 °C for 72 h. At the end of 72 h incubation process, the total sugar released by this process was quantified.
Fermentation process
The saccharified slurry obtained by acid-enzyme process was used further for ethanol production. The slurry was squeezed through a cheese cotton cloth and the filtrates (approximately 600 ml in each flask) were inoculated with immobilized S. cerevisiae cells, equivalent to 10 % free cells (dry weight basis). (NH4)2SO4 was added to the fermentation medium at the rate of 1 g/l as a source of nitrogen for growth of the yeast. Triplicate flasks (n = 3) were incubated for 96 h at room temperature (28 ± 2 °C) for ethanol production.
Analytical methods
At 24-h intervals, fermented broth in triplicate flasks (n = 3) were removed and the contents were analyzed for total sugar and ethanol. The ethanol content of the fermented broth was determined by measuring specific gravity of the distillate according to the procedure described by Amerine and Ough (1984). The total sugar was assayed by Anthrone method (Mahadevan and Sridhar 1999). The pH was measured by a pH meter (Systronics, Ahmadabad, India) using a glass electrode. The growth of the yeast cells inside the beads was measured by dissolving the gel beads in a 4 % (w/v) EDTA solution, and the cells were counted using a hemocytometer. Fermentation kinetics was calculated using reported Formulae described below (Bailey and Ollis 1986).
-
(1)
Final ethanol (P): Ethanol formed (ml) per liter was multiplying with 0.9.
-
(2)
Final biomass concentration (X, g/l): Cell biomass developed (g) per liter of hydrolysate.
-
(3)
Cell yield (Yx/s, g/g): Final biomass concentration (X, g/l) was divided by volumetric substrate uptake (Qs, g/l/h)
-
(4)
Volumetric product productivity (Qp, g/l/h): Product formed (g) per liter of hydrolysate per hour.
-
(5)
Volumetric substrate uptake (Qs, g/l/h): Substrate (glucose) uptake (g) per liter of hydrolysate per hour.
-
(6)
Ethanol yield (Yp/s, g/g): Mass of product (ethanol) formed per mass of substrate (glucose) consumed was multiplied with 100.
-
(7)
Conversion rate (%) into ethanol: Final ethanol (P) was divided by sugar utilization (Initial sugar-final sugar)
Statistical analysis
The data of ethanol yield using immobilized cells of S. cerevisiae were analyzed using one-way ANOVA. Where significant difference in ANOVA (p < 0.05) was detected by the Fisher’s Least Significance Difference (LSD), a multiple comparison test was applied to compare the factor level difference. The analysis was performed using MSTAT-C (version 2.0, Michigan State University, Michigan, USA).
Results and discussion
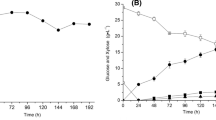
In order to compare the total sugar recovery, the cassava flour was saccharified with enzyme-enzyme and acid-enzyme hydrolysis. A high total sugar concentration (465 ± 5.076 g/kg) was obtained from the acid-enzyme hydrolysis, which represented 72.88 % of total sugar recovery from the starch available in cassava flour. But the enzyme-enzyme hydrolysis resulted in 371 ± 0.143 g/kg sugar, which represented only 58.1 % of total sugar recovery. Similar results were obtained by other researchers. For example, Thongchul et al. (2010) hydrolyzed cassava pulp and found higher sugar recovery from dried cassava pulp by acid-enzyme hydrolysis than enzyme- enzyme hydrolysis. Kongkiattikajorn and Yoonan (2006) also reported that diluted acid hydrolysis achieved higher yield of sugars than enzymatic hydrolysis from cassava bagasse. However, Woiciechowski et al. (2002) reported 94.5 and 97.3 % of reducing sugar recovery using the method of acid-acid and enzymatic hydrolysis of cassava bagasse, respectively. In our study, because of higher starch conversion by acid-enzyme hydrolysis, further study on ethanol production was carried out by taking the hydrolysate obtained from acid-enzyme hydrolysis, only. Further, the sugar utilization and ethanol production profiles are compared in Fig. 1.
In the present study, immobilized cells of S. cerevisiae started their growth in the lag phase and maximum ethanol production was achieved during stationary phase (96 h). The duration of fermentation, however, depends on the method used for starch liquefaction, saccharification, yeast type and concentration, and also the conditions of fermentation. Initially there was a fall of 52.9 % in total sugar concentration over initial sugar (465 ± 5.076 g/kg flour), with simultaneous production of 47.25 ± 1.201 g ethanol/kg flour up to 24 h of fermentation for the hydrolysate using immobilized cells of S. cerevisiae. Similar results were obtained on ethanol production from mahula (Madhuca latifolia L.) as feedstock and Ca-alginate entrapped S. cerevisiae cells as the fermenting organism (Swain et al. 2007; Behera et al. 2010a). The decrease in sugar reserve might in part be due to its utilization, for growth and metabolism of microorganism in addition to its conversion into ethanol. After 24 h, there was a gradual increase in ethanol concentration over the incubation period, with a simultaneous decrease in total sugar [Fisher’s LSD test, p < 0.05, LSD between treatments 0.7 (sugar utilization) and 1.46 (ethanol production)]. For the 48 and 72 h of fermentation, the sugar concentration was 120 ± 2.089 and 75 ± 2.142 g/kg flour, respectively, showing a 74.2 and 83.9 % decrease over the initial concentration with concomitant increase in concentration of ethanol to 84.15 ± 3.096 and 119.7 ± 4.137 g ethanol/kg flour, respectively, from cassava hydrolysate (Fig. 1). After 96 h of fermentation, there was 94.74 ± 2.187 % sugar conversion resulting in 189 ± 3.1 g ethanol/kg flour using S. cerevisiae.
The growth and fermentation kinetics of the immobilized yeast cells using the hydrolysate obtained from acid-enzyme hydrolysis was also studied (Table 1). The ethanol concentration (P) and volumetric substrate uptake (Q s ) obtained with Ca-alginate immobilized cells of S. cerevisiae during fermentation was 31.5 ± 1.068 g/l and 0.693 ± 0.012 g/l/h, respectively. Likewise, the volumetric product productivity (Q p ) and ethanol yield (Y p/s ) was 0.473 ± 0.058 g/g and 0.328 ± 0.037 g/l/h, respectively. However the final biomass concentration (X) was 4.31 g/l. Finally, there was 94.74 ± 0.187 % final sugar to ethanol conversion (fermentation efficiency) using the immobilized cells of S. cerevisiae. The high ethanol content obtained in the course of fermentation was therefore reflected in the fermentation efficiency value. The fermentation efficiency value for cassava flour could vary depending upon the method, the enzymes and the type of yeast used in the conversion process. Ueda et al. (1981) reported alcohol yields of 82.3 and 99.6 % from their study on production of ethanol from raw cassava starch by a non-conventional method.
Cassava starch and flour have several other uses. They can be converted to maltotriose, maltose, and glucose, as well as to other modified sugars and organic acids (Ray and Swain 2011). Starch from cassava can be used to make fructose syrups (Vuilleumier 1993) and formulate gelatin capsules (Nduele et al. 1993). The use of cassava as a source of ethanol for fuel is already being exploited and is very promising (Ray and Swain 2011). But, there are only a few studies on the use of immobilized yeast cells on ethanol production from cassava. Vijayagopal and Balagopalan (1989) worked on fermentation of cassava starch hydrolysate with immobilized cells of S. cerevisiae (Strain 2177). The results showed that fermentation could be completed in 48 h, and that these cells were alive on the support even after four cycles of fermentation. Singh et al. (1995) reported that saccharified mash yielded 5.89 %, v/v ethanol in a simultaneous saccharification and fermentation of starch using a yeast strain S. cerevisiae (SC-39) at 35 °C for 4 days. Roble et al. (2003) demonstrated the production of L-Lactic acid from raw cassava starch in a bioreactor using Aspergillus awamori (fungus) and Lactococcus lactis spp. lactis (bacterium). In this context, the ethanol production from cassava flour hydrolysate using the saccharifying method (1 N HCl–AMG) combination has practical significance. However, it is important to evaluate the potential of this work for large scale operations, since the demand for ethanol, especially as fuel, is dependent on bulk production facilities and the price of petroleum.
Conclusions
The overall results obtained from this study suggested that cassava flour could be used as feed stock for ethanol production by fermenting with immobilized S. cerevisiae cells. Comparing both hydrolysis methods, the acid-enzyme method was found to release more sugars than the enzyme-enzyme method. Furthermore, the conversion efficiency of sugar to ethanol was 94.74 %.
References
Amerine MA, Ough CS (1984) Wine and must analysis. Wiley, New York
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals. McGraw Hill, New York
Behera S, Mohanty RC, Ray RC (2010a) Comparative study of bio-ethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomyces cerevisiae and Zymomonas mobilis. Appl Energy 87:2352–2355
Behera S, Kar S, Mohanty RC, Ray RC (2010b) Comparative study of bio-ethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomces cerevisiae cells immobilized in agar agar and Ca-alginate matrices. Appl Energy 87:96–100
Behera S, Mohanty RC, Ray RC (2011) Ethanol production from mahula (Madhuca latifolia L.) flowers with immobilized cells of Saccharomyces cerevisiae in Luffa cylindrica L. sponge discs. Appl Energy 88:212–215
Bodalo A, Bastida J, Gomez JL, Alcarz I, Asaza ML (1996) Immobilization of Pseudomonas sp. BA2 by entrapment in calcium alginate and its application for the production of L-alanine. Enzym Microb Technol 19:176–180
John RP, Nampoothiri KM, Pandey A (2007) Production of L (+) lactic acid from cassava starch hydrolysate by immobilized Lactobacillus delbrueckii. J Basic Microbiol 47:25–30
Kar S, Ray RC (2008) Statistical optimization of α-amylase production by Streptomyces erumpens MTCC 7317 cells in calcium alginate beads using response surface methodology. Pol J Microbiol 57(1):49–57
Kar S, Chaudhury P, Ray RC (2004) Comparative study of bioethanol production from sweet potato (Ipomoea batatas L.) and cassava (Manihot esculenta Crantz.) by acid enzyme hydrolysis. In: Root and Tuber crops: nutrition, food security and sustainable environment, Proceeding of the Symposium held on 29–31 October, 2004 at Regional Centre of Central Tuber Crops Research Institute, Bhubaneswar, India, pp. 276–281
Kongkiattikajorn J, Yoonan K (2006) Conversion of cassava industry waste to fermentable sugars. In: The 2nd Int. Conf. on “Sustainable energy and environment, 21–23 November, 2006, Bangkok, Thailand
Mahadevan A, Sridhar R (1999) Methods in physiological plant pathology, 5th edn. Sivakami Publication, Madras
Nduele M, Ludwig A, Van OM (1993) The use of cassava starch in the formulation of gelatin capsules. J Pharm Belg 48:325–334
Ray RC (2004) Extracellular amylase (s) production by fungi Botryodiplodia theobromae and Rhizopus oryzae grown on cassava starch residue. J Environ Biol 25:489–495
Ray RC, Swain MR (2011) Bio-ethanol, bioplastics and other fermented industrial products from cassava starch and flour. In: Pace CM (ed) Cassava: farming, uses and economic impacts. Nova Science Publishers Inc, Hauppauge
Ray RC, Mohapatra S, Panda S, Kar S (2008) Solid substrate fermentation of cassava fibrous residue for production of α-amylase, lactic acid and ethanol. J Environ Biol 29:111–115
Roble ND, Ogbonna JC, Tanaka H (2003) L-lactic acid production from raw cassava starch in a circulating loop bioreactor with cells immobilized in loofa (Luffa cylindrica). Biotechnol Lett 25:1093–1098
Selvakumar P, Ashakumary L, Panday A (1994) Microbial fermentations with immobilized cells. J Sci Ind Res 55:443–449
Singh D, Dahiya JS, Nigam P (1995) Simultaneous raw starch hydrolysis and ethanol fermentation by glucoamylase from Rhizoctonia solani and Saccharomyces cerevisiae. J Basic Microbiol 35:117–121
Swain MR, Kar S, Sahoo AK, Ray RC (2007) Ethanol fermentation of mahula (Madhuca latifolia L.) flowers using free and immobilized yeast Saccharomyces cerevisiae. Microb Res 162:93–98
Thongchul N, Navankasattusas S, Yang ST (2010) Production of lactic acid and ethanol by Rhizopus oryzae integrated with cassava pulp hydrolysis. Bioprocess Biosyst Eng 33:407–416
Ueda S, Zenin CT, Monteiro DA, Park YK (1981) Production of ethanol from raw cassava starch by a non-conventional fermentation method. In: John Wiley and Sons Inc. Biotechnol Bioeng 23:291–299
Vijayagopal K, Balagopalan C (1989) Fermentation of cassava starch hydrolysate with immobilised cells of Saccharomyces cerevisiae. Starch-Starke 41:271–275
Vuilleumier S (1993) Worldwide production of highfructose syrup and crystalline fructose. Am J Clin Nutr 58:733S–736S
Woiciechowski AL, Nitsche S, Pandey A, Soccol CR (2002) Acid and enzymatic hydrolysis to recover reducing sugars from cassava bagasse: an economic study. Braz Arch Biol Technol 45:393–400
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Behera, S., Ray, R.C. Batch ethanol production from cassava (Manihot esculenta Crantz.) flour using Saccharomyces cerevisiae cells immobilized in calcium alginate. Ann Microbiol 65, 779–783 (2015). https://doi.org/10.1007/s13213-014-0918-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-0918-8