Abstract
Fermentation conditions for the production of bacterial cellulose (BC) by a newly isolated Gluconacetobacter hansenii UAC09 were optimized. The effect of various carbon and nitrogen sources on production of BC was determined. The addition of ethanol (1.5%) and acetic acid (1.0%) enhanced BC production by more than three times compared to standard Hestrin and Schramm medium. The strain was able to produce maximum BC (7.40 g l−1) with 4% glucose, 8% corn steep liquor (CSL) at 27 ± 1°C and a pH of 5.5 under stationary conditions. The BC films produced under various conditions were tested for their structural similarity using Fourier transform-infra red analysis and were found to be similar to each other irrespective of medium composition. Scanning electron microscopy images of BC revealed a porous structure with extensively interwoven microfibrils.
Similar content being viewed by others
Introduction
Cellulose is the most abundant biopolymer on Earth. Cotton and wood are the major resources for all cellulose-based products. With increasing global demand for cellulose-based products from wood and cotton, and major concerns about global warming, alternative technologies to produce cellulose from new sources are urgently needed. Bacterial cellulose (BC) is produced by many bacteria such as Gluconacetobacter, Rhizobium, Agrobacterium and Sarcina (Jonas and Farah 1998). Gluconacetobacter sp. has been applied as model microorganism for basic and applied studies on cellulose synthesis. Gluconacetobacter generally produce BC in liquid medium as a floating pellicle comprising cellulose, entrapped cells, and other medium components. BC is considered a traditional food, known as “natto”, in the Philippines. It is also popular in other Asian countries, including Indonesia, Japan and Taiwan, due to its distinctly soft texture and high fiber content (Ochaikul et al. 2006). BC is far superior to plant cellulose in terms of its high mechanical strength and highly crystalline form, which consists of an ultra fine nanofibril network structure (George et al. 2005). Further, unlike plant cellulose, which is usually associated with hemicellulose and lignin, BC contains pure cellulose. BC properties such as its hydrophilicity, and stability towards chemicals and high temperature make it applicable in biomedical science (Czaja et al. 2006). At present it is used widely as a temporary substitute for human skin in cases of burns, ulcers and other biotechnological fields. It has been investigated as a potential scaffold for tissue engineering (Svensson et al. 2005). It has a multitude of applications in the paper, textile, and food industries. It is used as a biomaterial in cosmetics and medicine (Chawla et al. 2009). Wider application of this polysaccharide is obviously dependent on the scale of production and its cost. Therefore, production process development is of great importance. In this work, an attempt has been made to optimize the nutritional and cultural conditions for enhanced production of BC from the native isolate Gluconacetobacter hansenii UAC09.
Materials and methods
Microorganism and culture conditions
The Gluconacetobacter sp. used in this study was isolated from contaminated grape wine. The isolate was identified as Gluconacetobacter species using standard microbiological methods (Sievers and Swings 1984) and confirmed by 16 S rRNA gene sequencing (Sambrook et al. 1989). The template DNA was amplified using 5′ GAGTTTGATCCTGGCTCAGG 3′ and 5′ TCATCTGTCCCACCTTCGGC 3′ primers obtained from Sigma Aldrich, India. PCR amplification was carried out with an initial denaturation of 94°C for 4 min followed by 34 cycles of denaturation at 94°C 40 s; annealing temperature of 56°C for 40 s; and elongation at 72°C for 1 min 30 s; final extension by 72°C 15 min in an automated DNA thermal cycler (Primus 96, MWG Biotech, Milton Keynes, UK). The purified PCR product was subjected to sequence analysis (Bangalore Genei, India). The resultant nucleotide sequence was subjected to BLAST programme of NCBI (http://www.ncbi.nlm.nih.gov/) to assess the percent homology with closely related strains documented in the available databases. The sequence was deposited with GenBank (National Center for Biotechnology Information Taxonomy, Bethesda, MD) under the accession no. Gluconacetobacter hansenii UAC09 (FJ655878). The organism was maintained in Hestrin and Schramm (HS) medium (Schramm and Hestrin 1954) slants at 4°C and subcultured at regular intervals of 2 weeks.
Culture medium
HS medium (g l−1): glucose 20, peptone 5, yeast extract 5, citric acid 1.115, di-sodium hydrogen phosphate 2.7, pH 4.5
Inoculum preparation
Sterile HS medium was used for the production of inoculum. One loopful of 24-h-old culture grown on an HS slant was inoculated and grown on a rotary shaker (150 rpm) at 27 ± 1°C for 24 h. The resulting suspension was used as inoculum (5% v/v) for all the experiments; inoculated flasks were incubated at 27 ± 1°C under stationary conditions for 2 weeks. Results are reported as the mean of triplicate experiments.
Effect of various culture conditions
Initial screening with various carbon sources (2%) including monosaccharides, disaccharides and polysaccharides was carried out using HS medium as the base. Organic and inorganic nitrogen sources were also studied to identify the preferred choice. The most suitable carbon (1–20%) and nitrogen (2–10%) sources were tried in various concentrations. Further, the effect of various nutritional and cultural conditions on BC production was investigated using HS medium (100 ml volume in a 500-ml flask), testing pH (2.5–9.5), inoculum ratio (2–10%), and incubation temperature (18–40°C). The effect of addition of ethyl alcohol (at 1% and 1.5%), in combination with acetic acid (0.25–1.5%) was also tested; solutions were prepared individually, sterilized, inoculated and incubated as mentioned above.
Purification of pellicle
Pellicle grown on the liquid surface was harvested once a week under sterile conditions. The pellicle was washed thoroughly with water, immersed in 1 N NaOH for 1 day at room temperature to remove cells and other impurities embedded in the pellicle, and rinsed thoroughly with water until a neutral pH was attained in the drained water. The pellicle was press dried between filter papers at 60°C until the film weight became constant (Yoshino et al. 1996; Ramana et al. 2000).
Scanning electron microscopy
The NaOH-treated BC pellicle from optimized conditions was freeze-dried, placed on a plate (anode) and coated with gold dust (cathode). The coated sample was exposed to a beam of electrons and examined for its surface morphology using a scanning electron microscope (LEO 435 VP Electron Microscope, Cambridge, UK).
Fourier-transform infra-red spectral studies
Thin films with a uniform thickness of 25 μm were used to obtain the IR spectra of BC films using Fourier-transform infra-red (FT-IR)-RAMAN Nicolet 5700. All measurements were carried out at 20°C in anhydrous conditions with air as the background. For each sample, 32 scans at a 2 cm−1 resolution were collected in the range of 4,000–400 cm−1.
Results and discussion
Gluconacetobacter is known to produce BC but conditions for production vary with the strain. The organism being a native strain, nutritional parameters such as the source and concentrations of sugar, nitrogen and additives, along with physical parameters such as pH and temperature, were included in the study. The BC films produced under various conditions were tested for their structural similarity using FT-IR analysis.
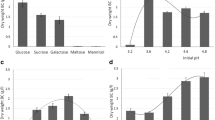
The effect of various temperatures (18–40°C), pH (2.5–9.5) and inoculum concentrations (2–10%, v/v) were examined using standard HS medium (Fig. 1). The optimum temperature for BC production was found to be 27°C. No growth of G. hansenii UAC09 was observed at 40°C (Fig. 1a). A broad pH range of 4.5–8.5 was found to produce a high level of BC, and maximum yield was at pH 5.5 (Fig. 1b). As the inoculum ratio had no major influence on BC production (Fig. 1c), 5% inoculum was used for all further studies.
To demonstrate the ability of G. hansenii UAC09 to metabolize a range of carbon sources, various carbon sources were used at 2% (w/v) concentrations in HS medium. A high amount of BC was produced from glucose, followed by glycerol, sucrose and fructose (Table 1). The results indicated glucose as the best carbon source (1.5 g l−1). Efficient cellulose production by Gluconacetobacter lies in its ability to synthesize glucose from various carbon substrates, followed by glucose polymerization to BC (Mikkelsen et al. 2009). BC production was enhanced with increased glucose concentration up to 4% (w/v), but decreased above 4% along with the decrease in the pH of the medium to below 2.58 (Fig. 1d). A similar result, i.e., that BC production decreased with an increase in the initial glucose concentration, has been reported earlier (Masaoka et al. 1993), since initial high glucose concentration leads to accumulation of gluconic acid, which lowers the pH of the medium below the optimum level thus inhibiting growth and BC production (Son et al. 2001).
Various nitrogen sources were added to the HS medium at 0.5, 1 and 2% (w/v) individually to investigate their effects on BC production. As shown in Table 1, medium containing peptone and corn steep liquor (CSL) as nitrogen sources produced high amounts of BC (2.3 and 1.83 g l−1 respectively). Of all the N sources tested, 2% peptone supported maximum cellulose production by G. hansenii UAC09. In general, the addition of peptone to culture media is economically not viable. Therefore, for the economic production of BC, CSL, which produced the next highest BC yield, was substituted for peptone at 2% concentration. Increasing the CSL concentration up to 8% enhanced BC yield (Fig. 1e). Matsuoka et al. (1996) observed that the presence of lactate and methionine in CSL are effective components for enhanced cellulose production, and they reported yields of 4.04 g l−1 BC from Acetobacter xylinum subsp. sucrofermentans BPR2001. Lactate stimulates cell growth by promoting the tricarboxylic acid (TCA) cycle and also by generating energy by the oxidation reaction if lactate to pyruvate resulting in increased cellulose production. Earlier, Naritomi et al. (1998) observed enhanced BC production due to the energy released by the oxidative reaction of lactate to pyruvate. Methionine stimulates the growth rate by reducing lag time leading to an increased cellulose production rate. El-Saied et al. (2008) reported yields of 4.47 g l−1 BC by G. xylinus (ATCC 10245) from CSL medium using glucose and 4.7 g l−1 with molasses. Further increase in the CSL concentration had no effect on BC production. Further, G. hansenii UAC09 was unable to produce a significant amount of BC from any of the inorganic nitrogen sources tested.
The addition of ethanol and acetic acid to the medium up to certain level enhanced production of BC compared to the control (4.45 g l−1). However, this yield was lower than the yield achieved with 8% CSL (Fig. 1f). A probable reason for the increase in yield is that ethanol stimulates cell growth and functions as an energy source for ATP generation. Increased levels of ATP lead to the abundant flow of G6P (a precursor of BC) into the BC biosynthetic pathway by inhibiting G6PDs. This increase in G6P increases the yield (Naritomi et al. 1998). Acetic acid is consumed as a monomeric raw material leading to a polymerization reaction and mass flow of glucose leading to the conversion of glucose to cellulose (Matsuoka et al. 1996). Repression of glucose oxidation by addition of ethanol and acetic acid as an alternate electron source has been reported to increase cellulose yield (Velasco-Bedran and Lopez-Isunza 2007). However, any further increase in the levels of ethanol and acetic acid decreased BC yield. This may be due to the increased levels of residual acetate, which inhibits cell growth, as BC production is generally associated with cell growth (Marx-Figini and Pion 1974; Naritomi et al. 1998).
These observations explain the improved BC production upon ethanol, acetic acid and CSL supplementation when used individually. BC yield was found to be maximal (7.40 g l−1) with a 4% glucose and 8% CSL combination. However, in combinations of all three (CSL, ethanol and acetic acid), the yield was reduced (5.83 g l−1) probably due to the inhibition of cell growth by the accumulated acetate. The final pH of all the optimum combinations ranged from 3.6 to 3.90 (Table 2).
FT-IR analysis of BC produced from the various carbon (Fig. 2a) and nitrogen (Fig. 2b) sources revealed typical IR spectra of the BC that were very similar to each other, indicating no change in the structure with nutritional source. Cellulose is a biopolymer comprising of β-d-glucopyranose units linked together through β-1,4-glycosidic linkages. The peak around 1640 cm−1 is due to the H–O–H bending vibration of absorbed water molecules in cellulose. The 1430 (C–H2 bending), 1162 (C–O–C stretching), 1111 (ring asymmetric stretching) and 895 cm−1 (group C1 frequency) transmittance bands can be used to study the type of crystalline cellulose because the crystalline cellulose I spectrum differs clearly in these bands from cellulose II. The 1430 cm−1 band is a characteristic of crystalline cellulose I. If a cellulose fiber has a significant amount of cellulose II, this band moves towards 1420 cm−1 and the amount of cellulose I decreases (Colom and Carrillo 2002). In the spectral region 1162 cm−1 is assigned to cellulose C–O–C bridges. In crystallized cellulose, this band is located at 1163 cm−1, while for amorphous cellulose it is located at 1156 cm−1. The band at 895 cm−1 corresponds to crystalline cellulose I. The 1335–1316 doublet is assigned to the cellulose with high crystallized cellulose I content (Colom et al. 2003). The frequency bands at 1375, 1335 (O–H plane bending), 1315 (CH2 wagging) and 1278 cm−1 (CH bending) indicate the presence of crystalline cellulose II (Carrillo et al. 2004). The characteristic predominant bands at 1428, 1163, 1111 and 897 cm−1 indicated cellulose I as the major component and 1336, 1317 and 1281 cm−1 indicates the presence of cellulose II. Hence, these results, confirms that BC is mainly composed of crystalline cellulose I with a negligible content of crystalline cellulose II.
The SEM images of the lower and upper surfaces of BC (Fig. 3) obtained from the standardized medium composition depict porous structure with extensively interwoven microfibrils. The upper surface, which is facing towards air, was found to consist of a densely packed microfibril network structure with no visible pores and open fibrils when compared to the lower surface facing towards the medium, which showed a loose network with many pores. The denser upper surface is probably due to slight drying and subsequent contraction (Bodin et al. 2007).
Conclusion
This study indicates the feasibility of various carbon and nitrogen sources for the production of BC by G. hansenii UAC09. Optimized conditions of 4% glucose with 8% CSL at pH 5.5 and 27°C produced 7.40 g l−1 BC compared to 1.5 g l−1 in the control. Irrespective of carbon and nitrogen source, the structure of BC produced was similar, indicating the intrinsic ability of the organism to produce BC. The ability of G. hansenii UAC09 to metabolize a wide range of nutritional sources will help design a better strategy for the production of BC.
References
Bodin A, Backdahl H, Fink H, Gustafsson L, Risberg B, Gatenholm P (2007) Influence of cultivation conditions on mechanical and morphological properties of bacterial cellulose tubes. Biotechnol Bioeng 97:425–434
Carrillo F, Colom X, Sunol JJ, Saurina J (2004) Structural FTIR analysis and thermal characterization of lyocell and viscose-type fibres. Eur Polym J 40:2229–2234
Chawla PR, Bajaj IB, Survase AS, Singhal RS (2009) Microbial cellulose: fermentative production and application. Food Technol Biotechnol 47:107–124
Colom X, Carrillo F (2002) Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur Polym J 38:2225–2230
Colom X, Carrillo F, Nogues F, Garriga P (2003) Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym Degrad Stab 80:543–549
Czaja W, Krystynowicz A, Bielecki S, Brown MR (2006) Microbial cellulose-the natural power to heal wounds. Biomaterials 27:145–151
El-Saied H, El-Diwany AI, Basta AH, Atwa NA, El-Ghwas DE (2008) Production and characterization of economical bacterial cellulose. Bioresources 3:1196–1217
George J, Ramana KV, Sabapathy SN, Jagannath JH, Bawa AS (2005) Characterization of chemically treated bacterial (Acetobacter xylinum) biopolymer: some thermo-mechanical properties. Int J Biol Macromol 37:189–194
Jonas R, Farah LF (1998) Production and application of microbial cellulose. Polym Degrad Stab 59:101–106
Marx-Figini M, Pion BG (1974) Kinetics investigation on biosynthesis of cellulose by Acetobacter xylinum. Biochim Biophys Acta 338:382–386
Masaoka S, Ohe T, Sakota N (1993) Production of cellulose from glucose by Acetobacter xylinum. J Ferment Bioeng 75:18–22
Matsuoka M, Tsuchida T, Matsushita K, Adachi O, Yoshinaga F (1996) A synthetic medium for bacterial cellulose production by Acetobacter xylinum subsp. sucrofermentans. Biosci Biotechnol Biochem 60:575–579
Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus stain ATCC 53524. J Appl Microbiol 107:576–583
Naritomi T, Kouda T, Yano H, Yoshinaga F (1998) Effect of ethanol on bacterial cellulose production from fructose in continuous culture. J Ferment Bioeng 85:598–603
Ochaikul D, Chotirittikrai K, Chantra J, Wutigornsombatkul S (2006) Studies on fermentation of Monascus purpureus TISTR 3090 with bacterial cellulose from Acetobacter xylinum TISTR 967. KMITL Sci Technol J 6:13–17
Ramana KV, Tomar A, Singh L (2000) Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World J Microbiol Biotechnol 16:245–248
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schramm M, Hestrin S (1954) Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol 11:123–129
Sievers M, Swings J (1984) Bergey’s manual of systemic bacteriology. Williams and Wilkins, Baltimore
Son HJ, Heo MS, Kim YG, Lee SJ (2001) Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp.A9 in shaking cultures. Biotechnol Appl Biochem 33:1–5
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M, Gateholm P (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26:419–431
Velasco-Bedran H, Lopez-Isunza F (2007) The unified metabolism of Gluconacetobacter entanii in continuous and batch processes. Process Biochem 42:1180–1190
Yoshino T, Asakura T, Toda K (1996) Cellulose production by Acetobacter pasteurianus on silicone membrane. J Ferment Bioeng 81:32–36
Acknowledgments
The first author wishes to acknowledge CSIR, New Delhi for awarding a Senior Research Fellowship. The authors are thankful to the Director, CFTRI, Mysore, and to the Head of the Food Microbiology Department, for providing facilities and for their support and help to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 154 kb)
Rights and permissions
About this article
Cite this article
Rani, M.U., Appaiah, A. Optimization of culture conditions for bacterial cellulose production from Gluconacetobacter hansenii UAC09. Ann Microbiol 61, 781–787 (2011). https://doi.org/10.1007/s13213-011-0196-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0196-7