Abstract
This study aimed to explore the protective mechanism of Banxia Xiexin Tang (BXXXT) on liver cell damage caused by high glucose (H-G) and to clarify its molecular regulatory pathways. First, the main components in BXXXT-containing serum were analyzed by high-performance liquid chromatography (HPLC) to provide basic data for subsequent experiments. Subsequently, the effect of BXXXT on high glucose (H-G)-induced hepatocyte activity was evaluated through screening of the optimal concentration of drug-containing serum. Experimental results showed that BXXXT significantly reduced the loss of cell activity caused by high glucose. Further research focuses on the regulatory effect of BXXXT on high glucose-induced hepatocyte apoptosis, especially its effect on the PGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α) pathway. Experimental results showed that BXXXT reduced high-glucose-induced hepatocyte apoptosis and exerted its protective effect by upregulating the activity of the PGC-1α pathway. BXXXT significantly increased the expression level of IGFBP1 (insulin-like growth factor-binding proteins) in hepatocytes under a high-glucose environment. It cleared mitochondrial ROS (reactive oxygen species) by enhancing SOD2 (superoxide dismutase) enzyme activity and maintained the survival of hepatocytes under a high-glucose environment. Finally, the regulation of PGC-1α by BXXXT is indeed involved in the regulation of IGFBP1 expression in hepatocytes and its downstream SOD2 effector signaling. Taken together, this study provides an in-depth explanation of the protective mechanism of BXXXT on hepatocytes in a high-glucose environment, focusing on regulating the expression of the PGC-1α pathway and IGFBP1, and reducing cell damage by scavenging ROS. This provides an experimental basis for further exploring the potential of BXXXT in the treatment of diabetes-related liver injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of diabetes mellitus has increased rapidly over the past few decades, making it a serious chronic metabolic disease worldwide (GBD 2021 Diabetes Collaborators 2023; Lin et al. 2020). Diabetic patients are usually accompanied by a hyperglycemic state, which has a significant adverse effect on several organs, especially the liver (Xu et al. 2023; Farokhi et al. 2012). Studies have shown that damage to hepatocytes from a high-glucose environment is one of the major causes of diabetic complications (Xu et al. 2023; Farokhi et al. 2012; Wang et al. 2020).
Diabetic patients are usually accompanied by a hyperglycemic state, which causes the liver to be exposed to a variety of unfavorable factors, including oxidative stress, apoptosis, and inflammatory responses (Mohamed et al. 2016; Frances et al. 2013). The liver is critical for maintaining the metabolic balance of the overall body, and high-glucose-induced hepatocellular injury directly affects this balance and accelerates the progression of diabetic complications (Chen et al. 2022). Studies have shown that increased apoptosis of hepatocytes in a high-glucose environment is one of the important mechanisms leading to liver injury (Santra et al. 2022; Li et al. 2019). Abnormal increase in apoptosis, a mode of programmed cell death, is closely associated with the onset and progression of a variety of diseases and especially plays a key role in the development of liver diseases (Oakley et al. 2005; Lee and Friedman 2011). Oxidative stress is a state in which the presence of excess oxidizing substances in the internal and external environment of a cell exceeds the cell’s antioxidant capacity (Kawabata 2022). In this case, the intracellular redox balance is disrupted and large amounts of reactive oxidizing substances (ROS), such as superoxide anion, hydrogen peroxide, and hydroxyl radicals, are produced (Huang and Tian 2022). Oxidative stress and excessive accumulation of ROS are closely associated with the onset and progression of a variety of diseases, including liver injury induced by hyperglycemic states (Li et al. 2015; Schwabe and Brenner 2006). Therefore, finding a method that can attenuate high glucose-induced hepatocyte apoptosis is of great clinical importance.
In traditional Chinese medicine, many herbs and prescriptions are believed to have hepatoprotective effects, some of which may have potential therapeutic effects on liver damage caused by high glucose levels (Zhao et al. 2014; Ma et al. 2021). These traditional Chinese medicines may play a role in protecting the liver by regulating redox balance, inhibiting apoptosis, reducing inflammatory responses, and other mechanisms (Li et al. 2023; Fu et al. 2021). This study is dedicated to exploring the effect of Banxia Xiexin Tang (BXXXT), a traditional Chinese medicine prescription, on high-glucose-induced liver cell injury and its potential molecular mechanisms. Previous studies have shown that traditional Chinese medicine is an important strategy for treating liver damage. Sarmentol H derived from Sedum sarmentosum Bunge directly targets FXR to mitigate cholestasis by recruiting SRC-1 (Liu et al. 2024). Schisantherin A protects hepatocyte via upregulating DDAH1 to ameliorate liver fibrosis in mice (Liang et al. 2024). As an ancient and classic Chinese medicine prescription, BXXXT has always had excellent efficacy in the treatment of heart and liver-related diseases (Xia et al. 2022). However, its role in high-glucose-induced hepatocellular injury and its specific molecular mechanisms have not been thoroughly studied. BXXXT has a complex composition, including a variety of herbal extracts. This study revealed the basic characteristics of its chemical composition for the first time by analyzing the positive and negative ion chromatograms of BXXXT samples. This provides preliminary data support for our in-depth study of the efficacy of BXXXT.
The main objective of this study was to investigate the effects of BXXXT on high-glucose-induced hepatocyte injury and to deeply analyze its molecular mechanisms. Specifically, attention was paid to the role of BXXXT in the regulation of two key molecules, PGC-1α and IGFBP1.
Methods and materials
Reagents
The BXXXT consists of 7 medicinal herbs: Pinellia ternata (Thunb.) Ten. ex Breitenb (12 g), Scutellaria baicalensis Georgi (9 g), Coptis chinensis Franch (3 g), Panax ginseng C. A. Mey (9 g), Zingiber officinale Roscoe (9 g), Radix Glycyrrhizae Preparata (9 g), Ziziphus jujuba Mill (9 g). BXXXT was prepared by the Department of Pharmacy of Chengdu University of Traditional Chinese Medicine and the drug was prepared to be soluble in water.
Preparation of serum containing BXXXT
Specific pathogen-free-grade SD female rats were purchased from the Animal Center of DASHUO [SCXY (Chuan) 2020-034] (body weight, 200 ± 10 g, age, 5 weeks) and reared in a separate cage. The rats were given free access to water and food at 20-26 °C and 40–70% humidity, alternating between light and dark for 12/12 h. All experimental procedures were approved by the Animal Experimental Committee and the Ethics Committee of Chengdu University of TCM (No: 2021-72). BXXXT was administered at high doses for consecutive 5 days. 3 h after the last administration, rats were anesthetized with isoflurane, blood was collected from the abdominal aorta, stratified at room temperature, centrifuged (2000 r/min, 10 min), serum was extracted, inactivated in a water bath at 56℃ for 20 min, de-bacterized by filtration through a 0.22 μm microporous membrane(Nest, China), divided and stored in a −20℃ refrigerator.
Cell culture
HepG2 cells, obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were cultured at 37 °C in Dulbecco’s minimum essential medium (DMEM) (Gibco BRL, Carlsbad, CA, USA). The growth medium consisted of 10% heat-inactivated fetal bovine serum (FBS) (Gibco BRL), supplemented with penicillin (100 U/mL; Gibco BRL) and streptomycin (100 μg/mL; Gibco BRL, USA). In addition, BXXXT and negative DMEM medium with rat serum contents of 2.5%, 5%, 10%, and 20% were prepared for cell experiments. The concentration of D-glucose in the control group was 5.5 mM, and the high-glucose group (D-glucose, 33 mM) was used to experimentally establish a high-glucose-induced cell injury model.
CCK-8 assay
Cells were first seeded into 96-well plates and cultured overnight, and the medium was replaced after 12 h, with 5 repeating holes set in each group. Absorbance (A) at 450 nm at the appointed time points was recorded in accordance with the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). Finally, the survival curve was drawn according to the cell proliferation.
Transfection experiment
HepG2 cells were transfected with 50 nmol/L of si-IGFBP1, si-NC, and si-PGC-1α using Lipofectamine 2000 (Invitrogen, ThermoFisher Scientific, CA, USA) instructions until reaching 60% confluence. In a separate experiment, HepG2 cells were co-transfected with pcDNA3.1-NC/pcDNA3.1-IGFBP1, following the same Lipofectamine 2000 protocol. The complete medium was replaced once after 6 h, and cells transfected for 24–48 h were collected for in vitro experiments. SiRNA reagents were procured from Ambion (Shanghai, China), while pcDNA-IGFBP1 and pcDNA-NC were synthesized by GENERAL Biol (Anhui, China).
Real-time PCR
RNA extraction from Hepg2 was carried out using a Trizol reagent following the manufacturer’s instructions. Subsequently, equal amounts of RNA were subjected to reverse transcription into cDNA using HiScriptQ RT SuperMix for qPCR (Vazyme, Nanjing, China) as per the provided guidelines. The average cycle thresholds (Ct) were utilized for quantifying fold-change, and the relative gene expression levels were determined using the 2−△△CT method. The primer sequences for PGC-1α were as follows: Forward-cagagagtatgagaagcgagag, Reverse-agcatcacaggtataacggtag. And for GAPDH: Forward-tgacttcaacagcgacaccca, Reverse-caccctgttgctgtagccaaa.
Apoptosis determination
HepG2 cells in the logarithmic growth phase underwent trypsin digestion and were subsequently centrifuged at 1000 r/min for 5 min. The resulting supernatant was discarded, and a single wash with 1× binding buffer was performed. Following this, the cells were resuspended in 100 μL 1× binding buffer to achieve a cell density of 1 × 106 cells/mL. Subsequently, 5 μL Annexin V-FITC and 5 μL PI staining were added, and the mixture was incubated at room temperature in the absence of light for 15 min.
ELISA assay
Thehomogenate from Hepg2 cells was centrifuged to obtain the supernatant for ELISA detection. The concentrations of Caspae 3 (ZC-32471, ZCIBIO Biotechnology), as well as the concentration of Caspae 7 (ZC-32486, ZCIBIO Biotechnology) and SOD2 (S0103, Beyotime Biotechnology) in Hepg2 cells, were examined by commercial ELISA kits in keeping with the operating manual.
Western blot
Protein expression in HepG2 cells was assessed through Western blot analysis. Protein extraction was carried out using the Pierce kit from Thermo Fisher Ltd. The total protein concentration was determined using the KCTMBCA protein quantitative kit protocol. Subsequently, protein lysates were subjected to electrophoresis using SDS–PAGE and transferred onto PVDF membranes. These membranes were then blocked with 5% non-fat milk powder. For immunoblotting, primary antibodies against IGFBP1 (A11672, abclonal, 1:5000), Cytochrome C (A4912, abclonal, 1:2000), SOD2 (A1340, CST, 1:1000), PGC-1α (A11971, abclonal, 1:1000), AMPKα (A1229, abclonal, 1:1000), Srebp1 (A15586, abclonal, 1:1000), Nrf2 (A0674, abclonal, 1:500), SIRT3 (A20205, abclonal, 1:500), and β-actin (ab49900, Abcam, 1/25000) were used. Incubation with the primary antibodies took place overnight at 4℃, followed by binding to horseradish peroxidase-conjugated secondary antibodies from Santa Cruz Biotechnology, USA. Enhanced chemiluminescence (Thermo Fisher Scientific) was employed for membrane detection, and protein expression levels were analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Flow cytometry for ROS
HepG2 cells were initially distributed into 6-well plates, with 1 × 106 cells per group subsequently subjected to two washes with PBS. The cells were loaded with probes, constituting a 500 μL suspension of cells containing 5 mol/L DCFH-DA (MCE, USA). Following this, the cells underwent a 30-min reaction in the dark at 37 °C and were then washed twice with PBS. The FITC channel was selected to assess variations in reactive oxygen species (ROS) among different groups. The optical density of cells, as detected by the FITC channel, served as a measure for quantifying ROS changes.
Statistical analysis
The data were expressed as means ± standard deviation (SD). Statistical analysis was conducted using SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Group comparisons were performed through a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test and Student’s unpaired t test. A p value <0.05 was deemed statistically significant.
Results
The analysis of active ingredients of BXXXT
Figure 1 shows chromatograms illustrating the positive (Fig. 1A) and negative (Fig. 1B) ions of BXXXT. The raw LC–MS data underwent preprocessing, which involved peak extraction, noise elimination, deconvolution, and peak alignment using MS-DIAL4.60 software. The extracted peak data was cross-referenced with GNPS, Respect, and MassBank databases for analysis. Detailed information regarding the active ingredients present in the chromatograms of both positive and negative ions of BXXXT can be found in Table S1.
Effect of BXXXT on proliferation and apoptosis of hepatocellular carcinoma cells
The prepared BXXXT rat serum was used for cell proliferation and apoptosis experiments. As shown in Fig. 2A, compared with the control group, the proliferation ability of liver cancer cell Hepg2 was weakened after high glucose induction. Compared with the high glucose group (H-G), 5% and 10% BXXXT rat serum can significantly promote the proliferation of liver cancer cells (Fig. 2A). At the same time, compared with the high-glucose group, 2.5%, 5%, 10%, and 20% negative serum had no effect on the proliferation ability of Hepg2 (Fig. 2B). According to the CCK-8 cell proliferation experiment, we selected 2.5%, 5%, and 10% BXXXT serum for the apoptosis experiment. Flow cytometry apoptosis experiment results showed that Hepg2 cell apoptosis increased after high glucose induction, and 2.5%, 5%, and 10% BXXXT serum could inhibit the number of apoptotic cells in a dose-dependent manner (Fig. 2C, D). In addition, compared with the control group, the levels of Caspase 3 and Caspase 7 increased after H-G induction, while BXXXT could reduce the levels of these two apoptotic factors (Fig. 2E, F).
Effect of BXXXT on high glucose-induced apoptosis and PGC-1α expression in hepatocytes. A Effect of BXXXT-containing serum on Hepg2 cell proliferation. B Effect of negative serum (NS) without BXXXT on Hepg2 cell proliferation. C Flow cytometry apoptosis statistics. D Representative pictures of flow apoptosis. E Levels of Caspase 3. F The content of Caspase 7. G Levels of PGC-1α mRNA. H Representative bands of AMPK and Serbp1. I Relative expression statistics results of AMPK and Serbp1. # Compared with control, ## p < 0.01, ### p < 0.001. * Compared with H-G group, * p < 0.05, ** p < 0.01. ns p > 0.05
Interestingly, we also detected a decrease in the mRNA content of PGC-1α in Hepg2 cells induced by high glucose. Compared with the H-G group, the mRNA content of PGC-1α increased after incubation with 5% and 10% BXXXT serum (Fig. 2G). As for Fig. 2H and I, compared with the control group, the expression of AMPK and Serbp1 was decreased. Compared with the H-G group, BXXXT could induce the expression of AMPK and Serbp1 in a dose-dependent manner. The above experimental results show that BXXXT can promote the proliferation and inhibit apoptosis of liver cancer cells, and preliminarily determined that PGC-1α may be involved in these regulations.
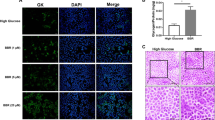
Expression changes of IGFBP1 in high glucose-induced hepatocytes
As shown in Fig. 3A and B, compared with the control group, the protein expression of IGFPB1 was significantly inhibited under high glucose induction for 24/48 h. To further evaluate the effect of IGFPB1 on Hepg2 proliferation and apoptosis, we introduced si-IGFPB1 to perform the following experiments. As shown in Fig. 3C and D, compared with the control group, the expression of IGFPB1 decreased and the expression of cytochrome increased after 24 h of high glucose induction. Compared with the high-glucose group, the expression of IGFPB1 increased and the expression of cytochrome decreased in the H-G+10% BXXXT+si-NC group. Compared with the H-G+10% BXXXT+si-NC group, transfection with si-IGFPB1 can inhibit the effect of 10% BXXXT. In addition, 10% BXXXT+si-NC can inhibit the contents of Caspase 3 and Caspase 7 in Hepg2 cells. After transfection with si-IGFPB1, the contents of Caspase 3 and Caspase 7 increased (Fig. 3F, G). Flow cytometry apoptosis results showed that compared with the high glucose group, 10% BXXXT+si-NC could inhibit the apoptosis of Hepg2 cells. After transfection with si-IGFPB1, the number of these apoptotic cells increased. These experimental results show that high glucose-induced reduction of IGFPB1 in Hepg2 cells, and IGFPB1 is involved in the regulation of BXXXT on Hepg2 cell proliferation and apoptosis.
Inhibitory effect of high glucose induction on IGFPB1 in liver cancer cells. A Representative bands of IGFPB1 protein in Hepg2 cells induced by high glucose for 24/48 h. B Statistical results of relative expression of IGFPB1 protein. C Representative protein bands of IGFPB1 and Cytochrome C. D Relative expression statistics results of IGFPB1 and Cytochrome C. E CCK-8 cell proliferation activity. F Levels of Caspase 3. G Levels of Caspase 7. H Flow cytometry apoptosis statistical results. I Representative images of apoptosis by flow cytometry. # Compared with control, # p < 0.05, ## p < 0.01, and ### p < 0.001. * Compared with H-G group, * p < 0.05, ** p < 0.01. & H-G+10% BXXXT+si-NC, & p < 0.05
IGFBP1 scavenges ROS by enhancing SOD2 enzyme activity to maintain hepatocyte survival under high glucose
The effect of IGFBP1 on SOD2 activation was further evaluated by overexpressing IGFBP1. As shown in Fig. 4A and B, compared with the control group, the expression of IGFBP1, Nrf2, SIRT3, and SOD2 proteins in the H-G+OV-NC group was reduced, while OV-IGFBP1 could upregulate the levels of IGFBP1, Nrf2, SIRT3, and SOD2. The SOD2 activity experiment showed that compared with the control group, the SOD2 activity decreased after the action of H-G+OV-NC, while OV-IGFBP1 could increase the SOD2 activity (Fig. 4C). The results of apoptosis experiments showed that compared with the control group, the number of apoptotic cells increased after the action of H-G+OV-NC, and OV-IGFBP1 could inhibit the number of apoptosis induced by high glucose (Fig. 4D, E). The results of the flow cytometry ROS experiment showed that compared with the control group, ROS increased significantly in the H-G+OV-NC group, and ROS decreased in Hepg2 cells after transfection with OV-IGFBP1 (Fig. 4F, G). These experimental results indicate that overexpression of IGFBP1 increases the activity of SOD2 to eliminate ROS and inhibit apoptosis.
Overexpression of IGFPB1 activates SOD2 to scavenge ROS and inhibit apoptosis of liver cancer cells. A Representative images of IGFPB1, Nrf2, SIRT3, and SOD2 proteins. B Relative expression results of IGFPB1, Nrf2, SIRT3, and SOD2 proteins. C SOD2 enzyme activity detection. D Effect of overexpression of IGFPB1 on Hepg2 cell apoptosis. E Flow cytometry apoptosis statistical results. F Effect of overexpression of IGFPB1 on ROS in Hepg2 cells. G Streaming ROS statistical results. # Compared with control, # p < 0.05, ## p < 0.01, and.### p < 0.001. * Compared with H-G+OV-NC group, * p < 0.05, ** p < 0.01, and *** p < 0.001
BXXXT regulates PGC-1α and affects IGFPB1-mediated SOD2 activity
To study the effect of PGC-1α on IGFPB1 and SOD2, we introduced si-PGC-1α to connect this relationship in series. As shown in Fig. 5A and B, compared with the high-glucose group, 10% BXXXT+si-NC can promote the expression of PGC-1α, IGFPB1, and SOD2. However, transfection with si-PGC-1α can inhibit the expression of PGC-1α, IGFPB1, and SOD2 proteins. The results of PGC-1α mRNA detection were consistent with those of protein detection, and silencing PGC-1α inhibited the effect of BXXXT (Fig. 5C). The SOD2 enzyme activity experiment showed that 10% BXXXT+si-NC can promote SOD2 enzyme activity, while the enzyme activity decreased after transfection with si-PGC-1α (Fig. 5D). Flow cytometry ROS detection showed that the increase of ROS induced by H-G could be inhibited by BXXXT while silencing PGC-1α offset the effect of BXXXT (Fig. 5E, F). These experimental results indicate that PGC-1α can regulate IGFPB1-mediated SOD2 activity.
Under a high glucose environment, the regulation of PGC-1α by BXXXT mediates the expression of IGFBP1 and the activity of downstream SOD2 in hepatocytes. A Representative bands of PGC-1α, IGFPB1, and SOD2 proteins. B Relative expression statistics of PGC-1α, IGFPB1, and SOD2 proteins. D SOD2 enzyme activity detection. E Statistical results of ROS. F Flow cytometry detection of ROS. # Compared with control, # p < 0.05, ## p < 0.01. * Compared with H-G group, * p < 0.05. & H-G+10% BXXXT+si-NC, & p < 0.05, &&& p < 0.05
Discussion
Diabetics are a high-risk group for chronic liver disease (Xu et al. 2023; Farokhi et al. 2012). Further deterioration of chronic liver damage can transform into liver fibrosis, cirrhosis, or even liver cancer, which will subsequently cause heavy social and economic burdens (GBD 2021 Diabetes Collaborators 2023). Therefore, intervention in diabetic liver damage is very important. Liver damage caused by diabetes has a higher mortality rate among diabetic complications. Its pathogenesis is complex and there is no specific drug. Since traditional Chinese medicine and its extracts have the characteristics of multiple targets and relatively small adverse reactions, a variety of traditional Chinese medicine and extracts have been used in the treatment of diabetic liver disease and have shown good efficacy (Zhao et al. 2014; Li et al. 2020). This study simulated the high-glucose-induced liver injury model by inducing hepatoma cell line Hepg2 in a high-glucose culture medium. This model well avoids the problems of individual differences in animal experiments and the long modeling cycle. In this model, traditional Chinese medicine BXXXT was used for intervention. The results showed that BXXXT effectively inhibited the apoptosis of Hepg2 cells induced by high glucose and promoted the proliferation of Hepg2 cells. The mechanism may be related to the increase in antioxidant capacity.
BXXXT not only reduces liver damage through antioxidant effects but also has a comprehensive therapeutic effect by regulating PGC-1α, IGFBP1, AMPK, Serbp1, SOD2, etc. Compared with single-target drugs, the multiple impacts of BXXXT make it more advantageous in the treatment of complex diseases (such as diabetes-related liver damage). As a traditional prescription, BXXXT is generally composed of natural products, and its side effects and toxicity are usually lower than some synthetic drugs, which makes it safer for long-term use and may have better tolerance in clinical applications.
The mechanism of liver damage caused by diabetes is complex. Among them, the excessive oxidative stress response of the diabetic body is considered to be involved in the occurrence of liver damage. Antioxidative stress response is considered to be a beneficial strategy for the treatment of diabetic liver damage (Mohamed et al. 2016; Li et al. 2015). This study conducted a detailed analysis of the active ingredients of BXXXT through LC–MS, and found many natural products, such as flavonoids (Yufang et al. 2023) and sesquiterpenes (Ren et al. 2021), with antioxidant capabilities. Oxidative stress refers to the excessive production of highly reactive molecules such as reactive oxygen species when the body is exposed to various harmful stimuli (Cichoż-Lach and Michalak 2014). The degree of oxidation exceeds the body’s ability to remove oxides, causing an imbalance between the oxidation system and the antioxidant system, leading to tissue damage (Cichoż-Lach and Michalak 2014). ROS acts on unsaturated fatty acids on the biological membrane of body cells, and its product is mainly MDA, which will aggravate the degree of damage to body cells (Barrera 2012). The MDA content can indirectly reflect the amount of oxygen free radicals generated and the degree of tissue oxidative damage to a certain extent (Barrera 2012; Katerji et al. 2019). SOD2 is an important antioxidant enzyme in mitochondria. It can scavenge reactive oxygen species in mitochondria and protect mitochondria from oxidative stress damage (Katwal et al. 2018; Zou et al. 2022). Recent studies have shown that SOD2 can reduce ROS and reduce liver ischemia–reperfusion injury (Katwal et al. 2018; Li et al. 2021). This experiment detected SOD2 activity and ROS levels and found that in a high-glucose environment, BXXXT effectively cleared ROS and reduced oxidative stress damage to liver cells by regulating SOD2 activity.
The liver is the primary organ responsible for glucose homeostasis, oxidative processes, and detoxification sites, and major metabolites are affected by ROS (Kapoor and Kakkar 2014). Hepatocyte apoptosis is a common phenomenon during the progression of diabetes. Excessive free radical production due to hyperglycemia and the occurrence of apoptosis has been reported in the scientific literature (Sun et al. 2012; Xu et al. 2012). Apoptosis is a tightly controlled mode of cell suicide characterized by nuclear pyknosis, cell shrinkage, cell membrane blebbing, and DNA fragmentation (Elmore 2007). Promoter caspases (including caspase-2, -8, -9, -10, -11, and -12) are tightly bound to pro-apoptotic signals, and these caspases cleave and activate downstream effector caspases (including caspase-3, and -7), thereby executing apoptosis (Kesavardhana et al. 2020). In addition, mitochondria simultaneously release a variety of pro-apoptotic molecules, such as cytochrome C (Gupta et al. 2009). In cell experiments, rat serum prepared by BXXXT was used to conduct proliferation and apoptosis experiments. The results showed that BXXXT can significantly promote the proliferation of liver cancer cells Hepg2 while inhibiting high glucose-induced apoptosis. The experiment also revealed the regulatory effect of BXXXT on the apoptosis-related factors Caspase 3 and Caspase 7, further confirming its anti-apoptotic effect.
PGC-1α plays a key role in energy metabolism and mitochondrial biogenesis (Rohas et al. 2007; Yousefi et al. 2020), while IGFBP1 is involved in apoptosis and antioxidant defense (Cai et al. 2023). PGC-1α is closely related to glucose and lipid metabolism, participates in the synthesis, metabolism, and output of fatty acids and glucose in the body, and plays an important role in the pathogenesis of diabetic liver damage (Yousefi et al. 2020). In addition, PGC-1α activity is regulated by SIRT1, and both are key to maintaining cellular energy homeostasis (Rohas et al. 2007). IGFBP1 is lowly expressed in the serum of T2DM patients (Hasan et al. 2023), and transcriptional activation of the IGFBP1 gene inhibits the development of type 2 diabetes (Cheng et al. 2022). A prospective study showed that increased IGFBP1 levels can reduce the risk of type 2 diabetes by more than 85% (Lu et al. 2016). The results of previous studies combined with the results of this study suggest that increasing IGFBP1 levels may be a new treatment strategy for diabetic liver injury. In addition, Cai (Cai et al. 2023) reported that IGFBP1 can resist spatial restriction-induced apoptosis, and the involved mechanism is to enhance SOD2 enzyme activity to clear ROS accumulated in mitochondria. Through experimental observation, this study found that BXXXT significantly reduced the apoptosis rate of hepatocytes in a high-glucose environment and increased the expression level of PGC-1α. This inspired us to further study the specific role of PGC-1α in the protective effect mediated by BXXXT. At the same time, we noticed that a high-glucose environment inhibited the expression of IGFBP1 in liver cancer cells, and BXXXT significantly reversed this trend. Further experiments confirmed that overexpression of IGFBP1 can activate SOD2 and scavenge ROS, thereby inhibiting the apoptosis of liver cancer cells.
Conclusions
In summary, the present study revealed the molecular mechanism of BXXXT in protecting hepatocytes from oxidative stress injury under a high glucose environment. It increases the expression of IGFBP1 by activating PGC-1α, which activates SOD2 to scavenge ROS and ultimately inhibits hepatocyte apoptosis. This provides an experimental basis for the use of BXXXT as a potential drug for the treatment of diabetic complications and offers new ideas for a deeper understanding of the mechanism of action of traditional Chinese medicine in the treatment of diabetes mellitus. However, we also realize that there are some limitations in the current study, such as the singularity of the experimental design and the limited number of samples, and further studies are needed to validate our findings. It is hoped that this study will provide a useful reference for future research on TCM and diabetes treatment.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barrera G (2012) Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012:137289
Cai G, Qi Y, Wei P et al (2023) IGFBP1 Sustains cell survival during spatially-confined migration and promotes tumor metastasis. Adv Sci (Weinh) 10(21):e2206540
Chen L, Lv X, Kan M et al (2022) Critical overview of hepatic factors that link non-alcoholic fatty liver disease and acute kidney injury: physiology and therapeutic implications. Int J Mol Sci 23(20):12464
Cheng Y, Yang R, Zhou Y et al (2022) HBP1 inhibits the development of type 2 diabetes mellitus through transcriptional activation of the IGFBP1 gene. Aging (Albany NY) 14(21):8763–8782
Cichoż-Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 20(25):8082–8091
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Farokhi F, Kaffash Farkhad N, Togmechi A et al (2012) Preventive effects of Prangos ferulacea (L.) Lindle on liver damage of diabetic rats induced by alloxan. Avicenna J Phytomed 2(2):63–71
Frances DEA, Ingaramo PI, Ronco MT et al (2013) Diabetes, an inflammatory process: oxidative stress and TNF-alpha involved in hepatic complication. J Biomed Sci Eng 6:645–653
Fu K, Wang C, Ma C et al (2021) The potential application of Chinese medicine in liver diseases: a new opportunity. Front Pharmacol 12:771459
GBD 2021 Diabetes Collaborators (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402(10397):203–234
Gupta S, Kass GE, Szegezdi E et al (2009) The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med 13(6):1004–1033
Hasan NS, El Dine HG, Kamel SA et al (2023) Association of genetic and epigenetic changes of insulin like growth factor binding protein-1 in Egyptian patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 200:110677
Huang Q, Tian C (2022) Visualizing time-varying effect in survival analysis: 5 complementary plots to Kaplan-Meier curve. Oxid Med Cell Longev 2022:3934901
Kapoor R, Kakkar P (2014) Naringenin accords hepatoprotection from streptozotocin induced diabetes in vivo by modulating mitochondrial dysfunction and apoptotic signaling cascade. Toxicol Rep 1:569–581
Katerji M, Filippova M, Duerksen-Hughes P (2019) Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid Med Cell Longev 2019:1279250
Katwal G, Baral D, Fan X et al (2018) SIRT3 a major player in attenuation of hepatic ischemia-reperfusion injury by reducing ROS via its downstream mediators: SOD2, CYP-D, and HIF-1α. Oxid Med Cell Longev 2018:2976957
Kawabata T (2022) Iron-induced oxidative stress in human diseases. Cells 11(14):2152
Kesavardhana S, Malireddi RKS, Kanneganti TD (2020) Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol 38:567–595
Lee UE, Friedman SL (2011) Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 25(2):195–206
Li S, Tan H-Y, Wang N et al (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16(11):26087–26124
Li X, Cheng T, He Y et al (2019) High glucose regulates ERp29 in hepatocellular carcinoma by LncRNA MEG3-miRNA 483–3p pathway. Life Sci 232:116602
Li Y-T, Cui H-T, Yang L et al (2020) Hua-Zhuo-Kai-Yu decoction inhibits apoptosis in nonalcoholic fatty liver disease. Tradit Med Res 6:5
Li Q, Zhang W, Xiao E (2021) SOD2 overexpression in bone marrow-derived mesenchymal stem cells ameliorates hepatic ischemia/reperfusion injury. Mol Med Rep 24(3):671
Li Z, Zhu JF, Ouyang H (2023) Progress on traditional Chinese medicine in improving hepatic fibrosis through inhibiting oxidative stress. World J Hepatol 15(10):1091–1108
Liang Y, Fang J, Zhou X et al (2024) Schisantherin A protects hepatocyte via upregulating DDAH1 to ameliorate liver fibrosis in mice. Phytomedicine 124:155330
Lin X, Xu Y, Pan X et al (2020) Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 10(1):14790
Liu Z, Chen L, Chen M et al (2024) Sarmentol H derived from Sedum sarmentosum Bunge directly targets FXR to mitigate cholestasis by recruiting SRC-1. Phytomedicine 130:155759
Lu J, Liu KC, Schulz N et al (2016) IGFBP1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. EMBO J 35(18):2026–2044
Ma W, Tang S, Xie D et al (2021) The protective effect of traditional Chinese medicine on liver ischemia-reperfusion injury. Evid Based Complement Alternat Med 2021:5564401
Mohamed J, Nazratun Nafizah AH, Zariyantey AH et al (2016) Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J 16(2):e132–e141
Oakley F, Meso M, Iredale JP et al (2005) Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 128(1):108–120
Ren Y, Jiang W, Luo C et al (2021) Atractylenolide III ameliorates TNBS-induced intestinal inflammation in mice by reducing oxidative stress and regulating intestinal flora. Chem Biodivers 18(8):e2001001
Rohas LM, St-Pierre J, Uldry M et al (2007) A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc Natl Acad Sci USA 104(19):7933–7938
Santra A, Bishnu D, Santra S et al (2022) Arsenic-Induced injury of mouse hepatocytes through lysosome and mitochondria: an in vitro study. Int J Hepatol 2022:1546297
Schwabe RF, Brenner DA (2006) Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290(4):583–589
Sun LQ, Zhao J, Zhang TT et al (2012) Protective effects of Salvianolic acid B on Schwann cells apoptosis induced by high glucose. Neurochem Res 37(5):996–1010
Wang Y, Fan X, Fan B et al (2020) Scutellarin Reduce the homocysteine level and alleviate liver injury in type 2 diabetes model. Front Pharmacol 11:538407
Xia Q-S, Gao Y, Wen-Bin W et al (2022) Ban-Xia-Xie-Xin-Tang ameliorates hepatic steatosis by regulating Cidea and Cidec expression in HFD-fed mice. Phytomedicine 105:154351
Xu Y-Y, Bao Y-Y, Zhou S-H et al (2012) Effect on the Expression of MMP-2, MT-MMP in laryngeal carcinoma hep-2 cell line by antisense glucose transporter-1. Arch Med Res 43(5):395–401
Xu K, Lu G, Feng Q et al (2023) Hepatoprotective effect of protocatechuic acid against type 2 diabetes-induced liver injury. Pharm Biol 61(1):737–745
Yousefi Z, Nourbakhsh M, Abdolvahabi Z et al (2020) microRNA-141 is associated with hepatic steatosis by downregulating the sirtuin1/AMP-activated protein kinase pathway in hepatocytes. J Cell Physiol 235(2):880–890
Yufang W, Mingfang L, Nan H et al (2023) Quercetin-targeted AKT1 regulates the Raf/MEK/ERK signaling pathway to protect against doxorubicin-induced nephropathy in mice. Tissue Cell 85:102229
Zhao C-Q, Zhou Y, Ping J et al (2014) Traditional Chinese medicine for treatment of liver diseases: progress, challenges and opportunities. Journal of Integrative Medicine 12(5):401–408
Zou Y, Grigorian A, Kennedy KG et al (2022) Differential association of antioxidative defense genes with white matter integrity in youth bipolar disorder. Transl Psychiatry 12(1):504
Funding
This study was supported by 1. MPRC2023024, Chengdu University of Traditional Chinese Medicine Xinglin Scholars Nursery Talent Special Fund; 2. 2022YFS0389, a key R&D project of the Sichuan Provincial Department of Science and Technology; 3.81774279, National Natural Science Foundation of China general project; 4.82274486, National Natural Science Foundation of China general project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
All authors agree to the publication of this article.
Conflict of interest
All authors have no potential conflicts of interest.
Research involving human participants and/or animals
All experimental procedures were approved by the Animal Experimental Committee and the Ethics Committee of Chengdu University of TCM (No: 2021-72).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Yue, R., Zhao, L. et al. Banxia Xiexin Tang attenuates high glucose-induced hepatocyte injury by activating SOD2 to scavenge ROS via PGC-1α/IGFBP1. 3 Biotech 14, 216 (2024). https://doi.org/10.1007/s13205-024-04060-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-04060-0