Abstract
This study aimed to investigate the effects of spontaneous fermentation on physicochemical characteristics, bioactive compounds, and antioxidant activity of acerola and guava fruit industrial by-products. Viable cell counts of lactic acid bacterial (LAB) in acerola and guava by-products were ≥ 5.0 log CFU/mL from 24 h up to 120 h of fermentation. Fermented acerola and guava by-products had increased luminosity and decreased contrast. Contents of total soluble solids and pH decreased, and titrable acidity increased in acerola and guava by-products during fermentation. Ascorbic acid contents decreased in acerola by-product and increased in guava by-product during fermentation. Different phenolic compounds were found in acerola and guava by-products during fermentation. Fermented acerola and guava by-products had increased contents of total flavonoids, total phenolics, and antioxidant activity. The contents of total flavonoids and total phenolics positively correlated with antioxidant activity in fermented acerola and guava by-products. These results indicate that spontaneous fermentation could be a strategy to improve the contents of bioactive compounds and the antioxidant activity of acerola and guava by-products, adding value and functionalities to these agro-industrial residues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acerola (Malpighia glabra L.) and guava (Psidium guayaba L.) are fruit rich in phenolic compounds, vitamins, minerals, and fiber, and their consumption has been associated with several health-promoting effects (Alvarez-Suarez et al. 2018; Jaeschke et al. 2016; Paz et al. 2015). A significant part of the acerola and guava production is directed to agro-industrial processing, with the production of large amounts of by-products commonly discarded in the environment (Araújo et al. 2020; Duarte et al. 2017). Fruit processing by-products are mainly formed by peel, seeds, endocarps, and pulp remnants, becoming these materials as rich carbon sources. These by-products contain a wide variety of valued compounds in their composition, including pigments, phenolic compounds, minerals, and vitamins, with a predominance of polysaccharides, such as cellulose, hemicellulose, and pectin (Sabino et al. 2020), enabling their use as biomass in different biotechnological processes (Khubber et al. 2022; Miskinis et al. 2022).
Natural fermentation is the oldest technique to obtain food products with improved characteristics and preservation capacity (Souza et al. 2023). Natural fermentation is a simple method to improve the safety, nutritional, and sensory properties of fruits and vegetables (Kuria et al. 2021). It has also been considered a strategy to change the nutritional profile and techno-functional properties of food plant-origin products through the conversion of macronutrients and the release of antioxidant peptides and phenolic compounds (Leonard et al. 2021; Septembre-Malaterre et al. 2018; Tamang et al. 2016). Therefore, spontaneous fermentation is a bioprocess that typically adds nutritional value and functionality to fruit by-products, besides contributing to the environment and the circular economy.
This study hypothesized that spontaneous fermentation positively impacts the profile of bioactive compounds and the antioxidant activity of acerola and guava fruit processing by-products. To test this hypothesis, the effects of spontaneous fermentation on the physicochemical characteristics, contents of different bioactive compounds, and antioxidant activity of acerola and guava fruit processing by-products were evaluated.
Material and methods
Preparation of acerola and guava fruit by-product
The processing by-products of acerola (Malpighia emarginata D.C.) and guava (Pisidium guayaba L.) fruits (ACE and GUA, respectively) were obtained from three different fruit pulp processing companies (João Pessoa, PB, Brazil), where each company consisted of one repetition (n = 3) for each fruit species. Samples (approximately 500 g) from four different processing batches from each industry were pooled (a total of approximately six kg for each by-product), packaged in sterile plastic bags, and stored (– 18 ± 2 °C).
Spontaneous fermentation conditions
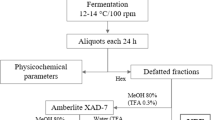
ACE and GUA were mixed with sterile water (1:5, w/v) and directly incorporated in a sterile glass flask with a lid and subjected to fermentation under aerobiosis in an orbital shaker (Tecnal, Piracicaba, SP, Brazil) under stirring (200 rpm) at 37 ± 1 ºC for 120 h. The final volume of the fermentation media was 500 mL (completed with sterilized distilled water). After zero—just after homogenization, 8, 24, 48, 72, and 120 h of spontaneous fermentation, ACE and GUA were evaluated for viable cell counts of lactic acid bacteria. Distinct physicochemical parameters, bioactive compounds, and antioxidant activity of ACE and GUA were determined before fermentation and on pre-established fermentation time intervals (zero—just after homogenization, 48, and 120 h).
Viable cell counts of lactic acid bacteria in ACE and GUA during spontaneous fermentation
The viable cell counts of lactic acid bacteria (LAB) in ACE and GUA were determined at zero (just after homogenization), 8, 24, 48, 72, and 120 h of spontaneous fermentation. A sample (1 mL) of each treatment collected in the different fermentation time intervals was diluted in sterile saline solution (NaCl 8.5 g/L), homogenized, serially diluted (1:9 v/v, 10–1–10–6) with the same diluent, and plated on de Man, Rogosa, and Sharpe (MRS) agar (HiMedia) using a micro drop inoculation technique (Herigstad et al. 2001). After an incubation period of 120 h at 37 °C under anaerobic conditions (Anaerobic System Anaerogen, Oxoid), the visible colonies were enumerated, and the results were expressed as log CFU/mL. The detection limit for viable cell counts was 2 log CFU/mL.
Determination of physicochemical characteristics of ACE and GUA before and during spontaneous fermentation
Before fermentation, ACE and GUA by-products underwent analysis to evaluate color parameters (Luminosity—L value, chroma, and hue°) (McLaren 1976), pH determination, titratable acidity (TA), and total soluble solids (TSS) (AOAC 2019). After zero—just after homogenization, 48, and 120 h of spontaneous fermentation, samples of the fermented by-products were evaluated for color parameters (Luminosity—L value, chroma, and hue°) (McLaren 1976). pH values were determined using a potentiometer equipped with a combined glass electrode (Q400AS, Diadema, São Paulo, Brazil). To determine the TA, 5 g of the substrate were transferred to a 125 mL Erlenmeyer flask using 50 mL of distilled water. Subsequently, two to four drops of 1% phenolphthalein solution were added, and the mixture was titrated with a 0.1 N NaOH solution until reaching a pH range of 8.2–8.4, which was measured using a potentiometer to ensure neutralization. TSS (expressed in °Brix, g/100 g) was measured using a digital refractometer (HI 96801, Hanna Instruments, São Paulo, Brazil) at 25 ± 1 °C.
Determination of ascorbic acid and carotenoid contents in ACE and GUA before and during spontaneous fermentation
The ascorbic acid (AA) content (vitamin C) was quantified using a 2,6- dichlorophenol-indophenol titrimetric method (AOAC 2019). For this, 5 mL of fermented ACE and GUA filtrate were mixed with 5 mL of metaphosphoric-acetic acid solution and titrated with a 2,6-dichlorophenolindophenol dye solution (Tillmans reagent). The endpoint was reached with > 5 s persistence of light but distinct rose-pink color. The results were expressed as mg AA per 100 g of sample (mg/100 mg) (AA was used as the reference standard). The total carotenoid content was measured according to Lichtenthaler and Buschmann (2001). The absorption was measured at 470, 645, and 662 nm with a spectrophotometer (BEL Photonics, Piracicaba, SP, Brazil), and total carotenoid content was expressed as mg/100 g.
Determination of total flavonoid and total phenolic contents in ACE and GUA before and during spontaneous fermentation
For extract preparation, 2 g of in natura or fermented ACE and GUA were homogenized with 80% methanol (Sigma–Aldrich, St. Louis, MA, USA) for 10 min with a mini-Turrax apparatus (Tecnal), kept resting for 24 h, and filtered with a 125 mm-filter paper (Whatman®, GE Healthcare, Chicago, IL, USA). Total flavonoid content was measured using a previously described procedure (Guevara-Figueroa et al. 2010). A 0.5 mL aliquot of the extract was added to 2 mL of distilled water and homogenized with 150 µL of a 5% sodium nitrite. After 5 min, 150 µL of 10% aluminum chloride solution was added, and after 6 min, one mL of 1 M sodium hydroxide solution and 1.2 mL of distilled water were added to the mixture. Sample absorbance was measured at 510 nm with a spectrophotometer (BEL Photonics) against a blank without extract. The total flavonoid content was determined with a standard curve of catechin (Sigma–Aldrich) equivalent. The results were expressed as mg catechin equivalents (CE) per 100 g of sample (mg CE/100 g).
Total phenolic content was measured using the Folin-Ciocalteu method (Liu et al. 2002). A 250 µL-aliquot of the extract was homogenized with 1250 µL of 10% Folin–Ciocalteau reagent, stirred with a Vortex mixer (Quimis, Diadema, SP, Brazil), kept at room temperature (25 ± 0.5 °C) under the dark for 6 min, added with a one mL aliquot of 7.5% sodium carbonate solution, and placed in a water-bath (Raypa, Barcelona, Spain) at 50 ± 0.5 °C for 5 min. The absorbance was measured at 765 nm with a spectrophotometer (BEL Photonics). A blank was performed with the absence of extract to reset the spectrophotometer. The total phenolic content was determined with a standard curve prepared with gallic acid (Sigma–Aldrich). Results were expressed as mg equivalent of gallic acid (EGA) per 100 g of sample (mg EGA/100 g).
Determination of phenolic compounds in ACE and GUA before and during spontaneous fermentation
For extract preparation, 5 g of fermented ACE and GUA were homogenized with 5 mL of 80% methanol (Sigma–Aldrich) using a mini-Turrax apparatus (Tecnal), centrifuged (9000 × g, 15 min, 4 °C) (NT-815, Tecnal), and filtered with a 0.45 μm-filter (Millex Millipore, Barueri, SP, Brazil). The individual phenolic compounds were determined with a high-performance liquid chromatograph, with gradient adaptations and runtime to quantify different phenolic classes using an Agilent 1260 Infinity System LC liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled to a diode array detector (DAD) (G1315D). The column was a Zorbax Eclipse Plus RP-C18 (100 × 4.6 mm, 3.5 μm), and the pre-column was a Zorbax C18 (12.6 × 4.6 mm, 5 μm) (Agilent Technologies). The oven temperature was 35 °C, and the injection volume was 20 μL diluted in phase A and filtered with a 0.45 μm-filter (Millex Millipore). The solvent flow was 0.8 mL/min; the new gradient used in separation was zero to 5 min: 5% B; 5–14 min: 23% B; 14–30 min: 50% B; 30 –33 min: 80% B, where solvent A was a solution of phosphoric acid (0.1 M, pH = 2.0) and solvent B was acidified methanol with 0.5% H3PO4. The data were processed with OpenLAB CDS ChemStation Edition software (Agilent Technologies). Phenolics were detected at 220, 280, 320, 360, and 520 nm. Identification and quantification were performed by comparison with external standards (Sigma–Aldrich). The results were expressed as mg of phenolic for 100 g of sample (mg/100 g) (Dutra et al. 2018; Padilha et al. 2017).
Determination of antioxidant activity in ACE and GUA before and during spontaneous fermentation
The FRAP (ferric reducing ability of plasma) and ABTS (2,2-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid) methods were used to estimate the antioxidant activity of fermented ACE and GUA. For extract preparation, 2 g of fermented ACE and GUA were homogenized with 10 mL of 80% methanol (Sigma-Aldrich) for 10 min with a mini-Turrax apparatus (Tecnal), kept resting for 24 h, and filtered with a 125 mm filter (Whatman®). The ability of extracts to reduce iron was measured with FRAP method as previously described (Rockenbach et al. 2011). The FRAP reagent was prepared with three mol/L of acetate buffer (pH 3.6) + 10 mM/L of TPTZ (2,4,6-tris (2-pyridyl)-s-triazine) in a 40 mM/L HCl solution + 20 mM FeCl3. A 200 mL aliquot of the extract was added to 1800 µL of the FRAP solution, stirred with a Vortex mixer (Quimis) for 30 s, and placed in a water bath for 30 min at 37 °C. The absorbance was measured at 593 nm with a spectrophotometer (Bel Photonics). The ability of extracts to capture ABTS•+ cation was measured with ABTS method as previously described (Sariburun et al. 2010). The ABTS reagent was prepared by mixing 5 mL of 7 mM ABTS with 88 µL of 140 mM potassium persulfate (final concentration of 2.45 mM). The ABTS•+ was formed after resting the ABTS reagent for 12–16 h at room temperature (25 ± 0.5 °C) in the dark. The ABTS•+ solution was diluted with distilled water to an absorbance value of 0.800–0.900 at 734 nm. The absorbance of the reaction mixture (600 µL) with 100 µL of extract and 500 µL of ABTS•+ solution was measured at 734 nm in a spectrophotometer (Bel Photonics). A control solution with 100 µL of extracting solvent + 500 µL of ABTS radical was prepared. The negative control solution was the extracting solvent for each extract used to reset the spectrophotometer. The standard curve was created with Trolox 1 mM. The results of FRAP and ABTS methods were expressed as micromoles of Trolox equivalent antioxidant capacity (TEAC) per gram of sample (μmol TEAC/g).
Statistical analysis
The experiments were done in triplicate on three independent occasions. Results were expressed as average ± standard deviation. The Kolmogorov–Smirnov normality test was run to check the data normal distribution. Data were submitted to Student's t-test or analysis of variance (ANOVA) followed by Tukey's test considering a p-value of ≤ 0.05 for significance. Statistical analyses were performed with the computational software GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). Correlations were calculated with Pearson's correlation coefficient (r). A principal component analysis (PCA) was run to evaluate the correlation matrix among LAB viable cell counts, total carotenoids, total flavonoids, ascorbic acid, total phenolics, phenolic profile, and antioxidant activity during fermentation. XLSTAT software version 2019.2.2.59614 (Addinsoft 2019; XLSTAT statistical and data analysis solution, Boston, MA, USA) was used to run PCA and correlations analysis.
Results and discussion
The physicochemical characteristics and bioactive compounds of in natura ACE and GUA used as substrates for spontaneous fermentation were evaluated in a previous study (Oliveira et al. 2020), being reported that in natura ACE and GUA have acidic pH, low TSS content, low luminosity, more saturated red hue, high contents of ascorbic acid, carotenoids, flavonoids, and phenolics, and high antioxidant activity (Table 1). Especially the high contents of bioactive compounds and antioxidant capacity suggested the use of in natura ACE and GUA for developing new value-added products by the food industry.
LAB viable cell counts in ACE and GUA before and during spontaneous fermentation
The LAB viable cell counts in ACE and GUA were below the detection limit, i.e., < 2 log CFU/mL, before the spontaneous fermentation (time zero). There was a significant increase (p ≤ 0.05) in LAB viable cell counts in ACE and GUA at 24 h of spontaneous fermentation (ACE: 5.6 ± 0.01 log CFU/mL; GUA: 8.9 ± 0.01 log CFU/mL). The highest LAB viable cell counts (p ≤ 0.05) were found at 24 h of fermentation for GUA (8.9 log CFU/mL) and at 48 h for ACE (6.6 log CFU/mL) (Fig. 1a). These counts did not change in GUA from 48 h until 120 h of fermentation (p > 0.05), while decreased in ACE (4.9 ± 0.01 log CFU/mL) (p ≤ 0.05). Contrary to these results, a previous investigation using probiotic bacteria to ferment guava extract reported that these microorganisms reached the stationary growth phase between 12 and 16 h of fermentation (Bhat et al. 2015).
Viable cell counts (log CFU/mL) of lactic acid bacteria (LAB), and values of total soluble solids (b), titratable acidity (c), and pH (d) in acerola (ACE, red circle) and guava by-product (GUA, down black triangle) during 120 h of spontaneous fermentation. Results are expressed as average (n = 3) ± standard deviation. a, b Average ± standard deviation with different lowercase letters differs (p ≤ 0.05) among fermented fruit processing by-products, based on Student's t-test. a–c Average ± standard deviation with different uppercase letters differs (p ≤ 0.05) among fermentation times, based on Tukey's test
Fruits and vegetables have a microbial population of about 5–7 log CFU/g and LAB correspond to 2–4 log CFU/g of this population (Di Cagno et al. 2013), indicating that LAB viable cell counts detected in spontaneously fermented ACE and GUA may correspond to more LAB than to other microorganisms. Furthermore, considering that ACE and GUA are sources of simple sugars and nondigestible carbohydrates (e.g., fiber and fructooligosaccharides) (Batista et al. 2018), these by-products could function as substrates for the multiplication of autochthonous LAB. Phenolic compounds could stimulate the growth and metabolism of various LAB species (Campanella et al. 2017), which could justify the increase in LAB viable cell counts during the spontaneous fermentation of ACE and GUA (Souza et al. 2018).
Physicochemical characteristics and instrumental color of ACE and GUA during spontaneous fermentation
In general, a reduction in TSS (2.15 ± 0.07 to 0.65 ± 0.07 g/100 g) was observed in ACE and GUA, with a concomitant increase in TA (0.99 ± 0.01 to 4.40 ± 0.13 g/100 g) and a reduction in pH (4.43 ± 0.01 to 2.85 ± 0.07) (p ≤ 0.05) during 120 h of spontaneous fermentation (Fig. 1b–d). Biochemical alterations, such as increased TA and decreased pH and TSS contents (sugar), normally occur in fermented fruit by-products (Campanella et al. 2017; Oliveira et al. 2020). In this study, there was a reduction in TSS during the measured fermentation period for ACE and GUA, indicating the consumption of sugars by LAB (Kwaw et al. 2018). The fermentation of fruits and vegetables by LAB commonly decreases total sugar contents since LAB uses these compounds for cell multiplication and bioconversion into lactic acid (Verón et al. 2019).
The L values of ACE and GUA increased (p ≤ 0.05) during spontaneous fermentation, with higher values found at 120 h. These results indicate the influence of fermentation time on this color parameter, making the fermented by-products brighter. There was a reduction in chroma value and an increase in °Hue value throughout ACE and GUA fermentation, where the °Hue values were higher (p ≤ 0.05) for GUA when compared to ACE at 120 h of fermentation (Table 2). Then, an increase in luminosity and a decrease in contrast for ACE and GUA occurred during fermentation (p ≤ 0.05), indicating a change in color from red to yellowish red. Carotenoids present in ACE could have suffered oxidation during fermentation, leading to color changes. Because of their sensitivity to acidic pH and heat, carotenoids may undergo isomerization with color loss (Amorim et al. 2022; Rodriguez-Amaya 2015), possibly linked to alterations in instrumental color parameters found for ACE and GUA.
An increase in luminosity and a decrease in contrast, with a more neutral color, was observed for ACE and GUA during fermentation (p ≤ 0.05), indicating a change in color from red to yellowish red. Isomerization and oxidation of carotenoids through hydrogen peroxide production could change the color of fruit products during fermentation (Baker and Günter 2004), possibly linked to alterations in instrumental color parameters found for ACE and GUA. Alterations in phenolic profile have also been linked to color variations in plant matrices fermented with LAB (Kwaw et al. 2018) since these matrices have various enzymes that degrade phenolic compounds. Still, the soluble phenolics present in fermented by-products would be mobilized and broken down into small molecules, reducing phenolic compound content (Ankolekar et al. 2012).
Contents of bioactive compounds in spontaneously fermented ACE and GUA
The effects of spontaneous fermentation on the contents of bioactive compounds measured in ACE and GUA were evident but not similar, mainly regarding the ascorbic acid content that decreased in ACE and increased in GUA during fermentation (p ≤ 0.05) (Table 3). Environmental factors, including oxygen concentration, temperature, light exposure, pH, water activity, and the presence of metallic ions, such as Cu2+ and Fe3+, in the medium largely influence the stability of ascorbic acid. Acidic pH values tend to inhibit the autoxidation of ascorbic acid when the redox potential fluctuates (Filannino et al. 2016; Silveira et al. 2019). GUA had a more acidic pH at 120 h of fermentation when compared to ACE, which could contribute to the increased ascorbic acid content found in GUA during fermentation, indicating the possible preservation of ascorbic acid from oxidative processes.
However, as ACE showed a reduction in ascorbic acid content even with low pH values, it is likely that factors other than acidity and more related to the matrix (e.g., sugars, mineral concentration, oxygen levels, and enzymes), are involved in the ascorbic acid depletion in fermented ACE and GUA. As verified for ACE, an early study analyzing the effect of fermentation on okra seeds for 120 h reported that as the fermentation time increased, the ascorbic acid content decreased (Adetuyi and Ibrahim 2014). The loss of ascorbic acid during fermentation may be due to the increased activity of the enzyme ascorbate oxidase since it is typically produced by fermentative microorganisms, which strongly depends on the pH of the fermentation environment. These enzymes convert ascorbic acid to dehydroascorbic acid (Adetuyi et al. 2008), which could justify the low values of vitamin C in ACE at 120 h of fermentation.
The contents of total carotenoids did not alter (p > 0.05) in ACE and GUA during the 120 h of spontaneous fermentation. Carotenoid content commonly decreases during lactic acid fermentation since volatile carotenoid cleavage derivatives can be formed, contributing to flavor and aroma development in fermented products (Mapelli-Brahm et al. 2020). Furthermore, LAB metabolism may favor the oxidation of these compounds during fermentation through the production of hydrogen peroxide (Vermeulen et al. 2007). Still, greater degradation of carotenoids may be due to their oxidation when in contact with oxygen (Antognoni et al. 2019). However, these factors did not directly impact the total carotenoid concentration throughout the ACE and GUA fermentation.
As for the content of total flavonoids, which correspond to a group of secondary metabolites of the polyphenols class, the fermentative process contributed to the increase in the flavonoid content (p ≤ 0.05) in ACE (from 35.56 ± 0.57 to 39.97 ± 0.01 mg EC/100 g) and GUA (from 22.94 ± 1.39 to 27.97 ± 0.01 mg EC/100 g). Likewise, the total phenolic contents increased in ACE (from 779.25 ± 0.01 to 1628.30 ± 133.42 mg EGA/100 g) and GUA (from 39.68 ± 0.27 to 59.68 ± 4.00 mg EGA/100 g) during the measured fermentation period. It is important to note that, at all examined fermentation periods, the total flavonoid content was higher in ACE when compared to GUA (p ≤ 0.05).
Fermentation typically releases flavonoids and phenolic compounds in plant-based foods, producing potential antioxidant activity (Oh et al. 2017; Parra-Matadamas et al. 2015). During the fermentation of plant materials with suitable microorganisms, the cell wall and/or starch are broken down by some classes of enzymes to facilitate the production of more useful compounds and to change the structure of phytochemicals present in fruits or other plant parts (Lee and Paik 2017; Michlmayr and Kneifel 2014). The LAB contribute to simple phenolic conversion and depolymerization of high molecular weight phenolic compounds during this fermentative biochemical process (Hur et al. 2014; Oh et al. 2017; Pontonio et al. 2019).
Previous studies reported increased phenolic compound content of apple, orange, and elderberry juices subjected to lactic fermentation (Multari et al. 2020; Ricci et al. 2019). The fermentation with homofermentative and heterofermentative LAB did not change the phenolic compound contents in cashew juice. However, the use of Lactiplantibacillus plantarum to ferment cashew juice increased the total phenolic compound content at 24 h, indicating that possibly the production and metabolism of phenolic compounds depends on the microorganism involved in the fermentation (Kaprasob et al. 2017).
Concomitantly with the increase in total flavonoids and total phenolic contents in ACE and GUA through the spontaneous fermentation, there was an increase in the antioxidant activity (p ≤ 0.05) in ACE and GUA at 120 h of fermentation when measured with either FRAP or ABTS method. During fermentation, different phenolic compounds were found in ACE and/or GUA (Table 4). Hesperidin, kaempferol, myricetin, quercitin, caffeic acid, caftaric acid, chlorogenic acid, procyanidin B1, procyanidin A2, trans-resveratrol, and epicatechins were found in ACE and/or GUA in most of the measured fermentation periods, which could have contributed to the increased antioxidant activity in fermented by-products.
Kaempferol and epicatechins were the phenolic compounds found in both ACE and GUA, regardless of the fermentation time. These results agree with a previous study where kaempferol and epicatechins were found in acerola and guava by-products fermented by inoculation with probiotic LAB for 120 h (Oliveira et al. 2020). Hesperidin and other phenolic compounds, such as catechin, naringenin, caffeic acid, caftaric acid, and chlorogenic acid, were previously found in in natura ACE and GUA by-products (Araújo et al. 2020).
The bioactive compounds of tropical fruits have shown antioxidant and free-radical-sequestering properties, which can slow or inhibit the oxidative damage of proteins and lipids in cells (Ayala-Zavala et al. 2011; González-Aguilar et al. 2008; Routray and Orsat 2019) and thereby offer several beneficial health effects (Can-Cauich et al. 2017). The phenolic profile found in ACE and GUA may explain the ability of these by-products to have a high radical scavenging activity in antioxidant assays. The microorganisms naturally present in the substrate develop their fermentative activity, causing a transformation in the initial material and modifying the biochemical composition. These changes affect the sensory properties, nutritional value, and microbial safety of fermented materials (Azam et al. 2017; Di Cagno et al. 2015). In addition, other functional properties resulting from fermentation deserve attention, such as the synthesis of bioactive molecules, including antioxidants, and the increased bioavailability of nutritive compounds (Gibson et al. 2017; Hussain et al. 2016; Souza et al. 2018). Changes in antioxidant activity can occur during fruit fermentation linked to the release of bioactive compounds from conjugated phytochemicals (Rodríguez et al. 2009; Szutowska 2020). Microorganisms naturally present in these matrices, including LAB, can deconjugate these phytochemicals and positively impact the fermented materials from a health promotion perspective (Kwaw et al. 2018; Rodríguez et al. 2021; Septembre-Malaterre et al. 2018).
The PCA demonstrated the similarities between ACE and GUA concerning LAB viable cell counts, phenolics, flavonoids, carotenoids, ascorbic acid, and antioxidant activity (Fig. 2). The data variance was explained by 59.58% for PC1 and 23.97% for PC2. The strongest negative correlation with PC2 was found for LAB viable cell counts, while the weakest positive correlation with PC2 was found for total carotenoids. An average positive correlation for ascorbic acid, a strong positive correlation for total flavonoids and antioxidant activity measured with ABTS method, and a very strong positive correlation for total phenolics and antioxidant activity measured with FRAP with PC1 was found. As can be seen in the PCA, the ABTS radical scavenging activity was strongly correlated with total phenolics (r = 0.84), explaining the higher antioxidant activity in ACE since it showed higher content of total phenolics and flavonoids at 120 h of fermentation. Similar results were previously reported for acerola and guava by-products submitted for a controlled fermentation with probiotic LAB for 120 h, where fermented acerola by-products had higher contents of total flavonoids and total phenolics than guava by-products (Oliveira et al. 2020). Spontaneous fermentation, a relatively simple biotechnological process, could enhance the potential bioactivities of ACE and GUA, especially regarding antioxidant properties. Furthermore, the results indicate new opportunities to produce functionalized foods and/or dietary supplements using spontaneously fermented ACE and GUA as valued added ingredients.
Principal component analysis (PCA) plot ordainment graph of lactic acid bacteria (LAB) viable cell counts (log CFU/mL), contents of total phenolics, total flavonoids, total carotenoids, ascorbic acid, and antioxidant activity (FRAP and ABTS) of fermented acerola (n = 3) and guava by-product (n = 3) at zero, 48, and 120 h of spontaneous fermentation. A: acerola by-product spontaneously fermented at zero, 48, and 120 h; G: guava by-product spontaneously fermented at zero, 48, and 120 h
Conclusions
The results showed that ACE and GUA subjected to spontaneous fermentation have increased contents of various bioactive compounds and enhanced antioxidant activity, with viable cell counts of LAB ≥ 5 log CFU/mL from 24 h until 120 h of fermentation. Fermented ACE had higher contents of total flavonoids and phenolics than GUA, possibly contributing to the higher antioxidant activity found in ACE through the examined fermentation period. Finally, these results indicate that spontaneous fermentation of ACE and GUA is a good alternative for the functionalization of these by-products as sources of phenolic compounds and total flavonoids with high antioxidant activity, enhancing their potential health-promoting properties and converting these agro-industrial residues into sustainable, circular, and value-added ingredients to the food industry sector. Furthermore, additional investigations are warranted to optimize the fermentation conditions and measure their effects on physicochemical characteristics, the profile of bioactive compounds, and the bioactivities of ACE and GUA.
Data availability
All data have were included in the manuscript.
References
Adetuyi FO, Ibrahim TA (2014) Effect of fermentation time on the phenolic: flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger Food J 32:128–137. https://doi.org/10.1016/S0189-7241(15)30128-4
Adetuyi FO, Osagie AU, Adekunle AT (2008) Antioxidant degradation in six indigenous okra (Abelmoschus esculentus (L) Moench) varieties during storage in Nigeria. J Food Technol 6:227–230
Alvarez-Suarez JM, Giampieri F, Gasparrini M, Mazzoni L, Forbes-Hernández TY, Afrin S, Battino M (2018) Guava (Psidium guajava L. cv. Red Suprema) crude extract protect human dermal fibroblasts against cytotoxic damage mediated by oxidative stress. Plant Foods Hum Nutr 73(1):18–24. https://doi.org/10.1007/s11130-018-0657-2
Amorim IS, Almeida MCS, Chaves RPF, Chisté RC (2022) Technological applications and color stability of carotenoids extracted from selected Amazonian fruits. Food Sci Technol 42:e01922. https://doi.org/10.1590/fst.01922
Ankolekar C, Johnson K, Pinto M, Johnson D, Labbe RG, Greene D, Shetty K (2012) Fermentation of whole apple juice using Lactobacillus acidophilus for potential dietary management of hyperglycemia hypertension, and modulation of beneficial bacterial responses. J Food Biochem 36(6):718–738. https://doi.org/10.1111/j.1745-4514.2011.00596.x
Antognoni F, Mandrioli R, Potente G, Saa DLT, Gianotti A (2019) Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem 292:211–216. https://doi.org/10.1016/j.foodchem.2019.04.061
AOAC (2019) Official methods of analysis, 21st edn. AOAC International, Arlington (ISBN: 978-0935584899)
Araújo CM, Sampaio KB, Menezes FNDD, Almeida ETC, Lima MS, Viera VB, Garcia EF, Gómez-Zavaglia A, Souza EL, Oliveira MEG (2020) Protective effects of tropical fruit processing by-products on probiotic Lactobacillus strains during freeze-drying and storage. Microorganisms 8(1):96. https://doi.org/10.3390/microorganisms8010096
Ayala-Zavala JF, Vega-Vega V, Rosas-Domínguez C, Palafox-Carlos H, Villa-Rodriguez JA, Siddiqui MW, Dávila-Aviña JE, González-Aguilar GA (2011) Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res Int 44(7):1866–1874. https://doi.org/10.1016/j.foodres.2011.02.021
Azam M, Mohsin M, Ijaz H, Tulain R, Ashraf MA, Fayyaz A, Abadeen ZU, Kamran Q (2017) Lactic acid bacteria in traditional fermented Asian foods. Pak J Pharm Sci 30(5):1803–1814
Baker R, Günter C (2004) The role of carotenoids in consumer choice and likely benefits from their inclusion into products for human consumption. Trends Food Sci Technol 15(10):484–488. https://doi.org/10.1016/j.tifs.2004.04.0094
Batista KS, Alves AF, Lima MS, Silva LA, Lins PP, Gomes JAS, Silva AS, Toscano LT, Meireles BRLA, Cordeiro AMTM, Conceição ML, Souza EL, Aquino JS (2018) Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br J Nutr 119(1):30–41. https://doi.org/10.1017/S0007114517003282
Bhat R, Suryanarayana LC, Chandrashekara KA, Krishnan P, Kush A, Ravikumar P (2015) Lactobacillus plantarum mediated fermentation of Psidium guajava L. fruit extract. J Biosci Bioeng 119(4):430–432. https://doi.org/10.1016/j.jbiosc.2014.09.007
Campanella D, Rizzello CG, Fasciano C, Gambacorta G, Pinto D, Marzani B, Scarano N, Angelis M, Gobbetti M (2017) Exploitation of grape marc as functional substrate for lactic acid bacteria and bifidobacteria growth and enhanced antioxidant activity. Food Microbiol 65:25–35. https://doi.org/10.1016/j.fm.2017.01.019
Can-Cauich CA, Sauri-Duch E, Betancur-Ancona D, Chel-Guerrero L, González-Aguilar GA, Cuevas-Glory LF, Pérez-Pacheco E, Moo-Huchin VM (2017) Tropical fruit peel powders as functional ingredients: evaluation of their bioactive compounds and antioxidant activity. J Funct Foods 37:501–506. https://doi.org/10.1016/j.jff.2017.08.028
Di Cagno R, Coda R, Angelis M, Gobbetti M (2013) Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol 33(1):1–10. https://doi.org/10.1016/j.fm.2012.09.003
Di Cagno R, Filannino P, Gobbetti M (2015) Vegetable and fruit fermentation by lactic acid bacteria. In: Mozzi F, Raya RR, Vignolo GM (eds) Biotechnology of lactic acid bacteria: novel applications. Wiley, Chichester, pp 216–230. https://doi.org/10.1002/9781118868386.ch14
Duarte FND, Rodrigues JB, Lima MC, Lima MS, Pacheco MTB, Pintado MME, Aquino JS, Souza EL (2017) Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus species. J Sci Food Agr 97(11):3712–3719. https://doi.org/10.1002/jsfa.8232
Dutra MCP, Rodrigues LL, Oliveira D, Pereira GE, Lima MS (2018) Integrated analyses of phenolic compounds and minerals of Brazilian organic and conventional grape juices and wines: Validation of a method for determination of Cu, Fe and Mn. Food Chem 269:157–165. https://doi.org/10.1016/j.foodchem.2018.07.014
Filannino P, Cavoski I, Thlien N, Vincentini O, De Angelis M, Silano M, Gobbetti M, Di Cagno R (2016) Lactic acid fermentation of cactus cladodes (Opuntia ficus-indica L.) generates flavonoid derivatives with antioxidant and anti-inflammatory properties. PLoS One 11(3):e0152575. https://doi.org/10.1371/journal.pone.0152575
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G (2017) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75
González-Aguilar G, Robles-Sánchez RM, Martínez-Téllez MA, Olivas GI, Álvarez-Parrilla E, De La Rosa LA (2008) Bioactive compounds in fruits: health benefits and effect of storage conditions. Stewart Postharvest Rev 4(3):1–10. https://doi.org/10.2212/spr.2008.3.8
Guevara-Figueroa T, Jiménez-Islas H, Reyes-Escogido ML, Mortensen AG, Laursen BB, Lin LW, León-Rodríguez A, Fomsgaard IS, De la Rosa APB (2010) Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J Food Compost Anal 23(6):525–532. https://doi.org/10.1016/j.jfca.2009.12.003
Herigstad B, Hamilton M, Heersink J (2001) How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods 44(2):121–129. https://doi.org/10.1016/S0167-7012(00)00241-4
Hur SJ, Lee SY, Kim YC, Choi I, Kim GB (2014) Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem 160:346–356. https://doi.org/10.1016/j.foodchem.2014.03.112
Hussain A, Bose S, Wang JH, Yadav MK, Mahajan GB, Kim H (2016) Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res Int 81:1–16. https://doi.org/10.1016/j.foodres.2015.12.026
Jaeschke DP, Marczak LDF, Mercali GD (2016) Evaluation of non-thermal effects of electricity on ascorbic acid and carotenoid degradation in acerola pulp during ohmic heating. Food Chem 199:128–134. https://doi.org/10.1016/j.foodchem.2015.11.117
Kaprasob R, Kerdchoechuen O, Laohakunjit N, Sarkar D, Shetty K (2017) Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem 59(Part B):141–149. https://doi.org/10.1016/j.procbio.2017.05.019
Khubber S, Marti-Quijal FJ, Tomasevic I, Remize F, Barba FJ (2022) Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr Opin Food Sci 43:189–198. https://doi.org/10.1016/j.cofs.2021.11.013
Kuria MW, Matofari JW, Nduko JM (2021) Physicochemical, antioxidant, and sensory properties of functional mango (Mangifera indica L.) leather fermented by lactic acid bacteria. J Agric Food Res 6:100206. https://doi.org/10.1016/j.jafr.2021.100206
Kwaw E, Ma Y, Tchabo W, Apaliya MT, Wu M, Sackey AS, Xiao L, Tahir HE (2018) Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem 250:148–154. https://doi.org/10.1016/j.foodchem.2018.01.009
Lee NK, Paik HD (2017) Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. J Microbiol Biotechnol 27(5):869–877. https://doi.org/10.4014/jmb.1612.12005
Leonard W, Zhang P, Ying D, Adhikari B, Fang Z (2021) Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol Adv 49:107763. https://doi.org/10.1016/j.biotechadv.2021.107763
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:F4–F3. https://doi.org/10.1002/0471142913.faf0403s01
Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH (2002) Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem 50(10):2926–2930. https://doi.org/10.1021/JF0111209
Mapelli-Brahm P, Barba FJ, Remize F, Garcia C, Fessard A, Khaneghah AM, Sant’Ana AS, Lorenzo JM, Montesano D, Meléndez-Martínez AJ (2020) The impact of fermentation processes on the production, retention and bioavailability of carotenoids: an overview. Trends Food Sci Technol 99:389–401. https://doi.org/10.1016/j.tifs.2020.03.013
McLaren K (1976) The development of CIE (L, a, b) uniform color space and color difference formula. J Soc Dye Colour 92(9):338–341. https://doi.org/10.1111/j.1478-4408.1976.tb03301.x
Michlmayr H, Kneifel W (2014) β-Glucosidase activities of lactic acid bacteria: mechanisms, impact on fermented food and human health. FEMS Microbiol Lett 352(1):1–10. https://doi.org/10.1111/1574-6968.12348
Miskinis RAS, Nascimento LÁ, Colussi R (2022) Bioactive compounds from acerola pomace: a review. Food Chem 404(Part A):134613. https://doi.org/10.1016/j.foodchem.2022.134613
Multari S, Carafa I, Barp L, Caruso M, Licciardello C, Larcher R, Tuohy K, Martens S (2020) Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel.’ LWT Food Sci Technol 125:109205. https://doi.org/10.1016/j.lwt.2020.109205
Oh BT, Jeong SY, Velmurugan P, Park JH, Jeong DY (2017) Probioticmediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J Biosci Bioeng 124(5):542–550. https://doi.org/10.1016/j.jbiosc.2017.05.011
Oliveira SD, Araújo CM, Borges GDSC, Lima MS, Viera VB, Garcia EF, Souza EL, Oliveira MEG (2020) Improvement in physicochemical characteristics, bioactive compounds and antioxidant activity of acerola (Malpighia emarginata DC) and guava (Psidium guajava L.) fruit by-products fermented with potentially probiotic lactobacilli. LWT Food Sci Technol 134:110200. https://doi.org/10.1016/j.lwt.2020.110200
Padilha CVS, Miskinis GA, Souza MEAO, Pereira GE, Oliveira D, Bordignon-Luiz MT, Lima MS (2017) Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem 228:106–115. https://doi.org/10.1016/j.foodchem.2017.01.137
Parra-Matadamas A, Mayorga-Reyes L, Pérez-Chabela M (2015) In vitro fermentation of agro-industrial by-products: grapefruit albedo and peel, forage palm peel and pineapple peel by lactic acid bacteria. Int Food Res J 22(2):859–865
Paz M, Gúllon P, Barroso MF, Carvalho AP, Domingues VF, Gomes AM, Becker H, Longhinotti E, Delerue-Matos C (2015) Brazilian fruit pulps as functional foods and additives: evaluation of bioactive compounds. Food Chem 172:462–468. https://doi.org/10.1016/j.foodchem.2014.09.102
Pontonio E, Montemurro M, Pinto D, Marzani B, Trani A, Ferrara G, Mazzeo A, Gobbetti M, Rizzello CG (2019) Lactic acid fermentation of pomegranate juice as a tool to improve antioxidant activity. Front Microbiol 10:1550. https://doi.org/10.3389/fmicb.2019.01550
Ricci A, Cirlini M, Calani L, Bernini V, Neviani E, Del Rio D, Galaverna G, Lazzi C (2019) In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem 276:692–699. https://doi.org/10.1016/j.foodchem.2018.10.046
Rockenbach II, Rodrigues E, Gonzaga LV, Caliari V, Genovese MI, Gonçalves AESS, Fett R (2011) Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem 127(1):174-179. https://doi.org/10.1016/j.foodchem.2010.12.137
Rodríguez H, Curiel JA, Landete JM, Rivas B, Felipe FL, Gómez-Cordovés C, Mancheño JM, Muñoz R (2009) Food phenolics and lactic acid bactéria. Int J Food Microbiol 132(2–3):79–90. https://doi.org/10.1016/j.ijfoodmicro.2009.03.025
Rodríguez LGR, Gasga VMZ, Pescuma M, Van Nieuwenhove C, Mozzi F, Burgos JAS (2021) Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res Int 140:109854. https://doi.org/10.1016/j.foodres.2020.109854
Rodriguez-Amaya DB (2015) Food carotenoids: chemistry, biology and technology. Wiley, Chichester (978-1-118-73330-1)
Routray W, Orsat V (2019) Agricultural and food industry by-products: source of bioactive components for functional beverages. In: Grumezescu AM, Holban AM (eds) Nutrients in beverages. Academic Press, Cambridge, pp 543–589. https://doi.org/10.1016/B978-0-12-816842-4.00015-0
Sabino LBS, Gonzaga MLC, Oliveira LS, Duarte ASG, Silva LMA, Brito ES, Figueiredo RW, Silva LMR, Sousa PHM (2020) Polysaccharides from acerola, cashew apple, pineapple, mango and passion fruit co-products: structure, cytotoxicity and gastroprotective effects. Bioact Carbohydr Diet Fibre 24:100228. https://doi.org/10.1016/j.bcdf.2020.100228
Sariburun E, Sahin S, Demir C, Turkben C, Uylaşer V (2010) Phenolic content and antioxidant activity of raspberry cultivars. J Food Sci 75(4):328–335. https://doi.org/10.1111/j.1750-3841.2010.01571.x
Septembre-Malaterre A, Remize F, Poucheret P (2018) Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res Int 104:86–99. https://doi.org/10.1016/j.foodres.2017.09.031
Silveira MR, Coutinho NM, Esmerino EA, Moraes J, Fernandes LM, Pimentel TC, Freitas MQ, Silva MC, Raices RSL, Ranadheera CS, Borges FO, Neto RPC, Tavares MIB, Fernandes FAN, Fonteles TV, Nazzaro F, Rodrigues S, Cruz AG (2019) Guava-flavored whey beverage processed by cold plasma technology: bioactive compounds, fatty acid profile and volatile compounds. Food Chem 279:120–127. https://doi.org/10.1016/j.foodchem.2018.11.128
Souza EL, Albuquerque TMR, Santos AS, Massa NML, Brito Alves JL (2018) Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—a review. Crit Rev Food Sci Nutr 59(10):1645–1659. https://doi.org/10.1080/10408398.2018.1425285
Souza EL, Oliveira KAR, Oliveira MEG (2023) Influence of lactic acid bacteria metabolites on physical and chemical food properties. Curr Opin Food Sci 49:100981. https://doi.org/10.1016/j.cofs.2022.100981
Szutowska J (2020) Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: a systematic literature review. Eur Food Res Technol 246(3):357–372. https://doi.org/10.1007/s00217-019-03425-7
Tamang JP, Watanabe K, Holzapfel WH (2016) Review: diversity of microorganisms in global fermented foods and beverages. Front Microbiol 7:377. https://doi.org/10.3389/fmicb.2016.00377
Vermeulen N, Czerny M, Gänzle MG, Schieberle P, Vogel RF (2007) Reduction of (E)-2-nonenal and (E, E)-2, 4-decadienal during sourdough fermentation. J Cereal Sci 45(1):78–87. https://doi.org/10.1016/j.jcs.2006.07.002
Verón HE, Cano PG, Fabersani E, Sanz Y, Isla MI, Espinar MTF, Ponce JVG, Torres S (2019) Cactus pear (Opuntia ficus-indica) juice fermented with autochthonous Lactobacillus plantarum S-811. Food Funct 10(2):1085–1097. https://doi.org/10.1039/c8fo01591k
Acknowledgements
The authors thank the CAPES—Coordination for the Improvement of Higher Education Personnel (Brazil) for partially funding this research (Grant number: 001).
Author information
Authors and Affiliations
Contributions
SDO: Conceptualization, Methodology, Formal Analysis, Writing—Original Draft Preparation and Writing—Review and Editing. ELS: Methodology, Formal Analysis, Writing—Review and Editing, Supervision, and Resources. CMA: Methodology and Formal Analysis. ACSM: Methodology and Formal Analysis. GSCB: Methodology and Formal Analysis. MSL: Methodology and Formal Analysis. VBV: Methodology and Formal Analysis. EFG: Methodology and Formal Analysis. MLC: Methodology and Formal Analysis. ALS: Writing—Review and Editing. MEGO: Conceptualization, Data Curation, Resources, Writing—Original Draft Preparation, Writing—Review and Editing, Supervision and Project administration. All authors critically revised the document and agreed on its content.
Corresponding author
Ethics declarations
Conflict of interest
Partial financial support was received from CAPES—Coordination for the Improvement of Higher Education Personnel (Brazil), grant number: 001.
Research involving human participants and/or animals
Human participants or animals were not involved in this research.
Informed consent
All authors are informed about the paper and have consented to publish the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, S.D., de Souza, E.L., Araújo, C.M. et al. Spontaneous fermentation improves the physicochemical characteristics, bioactive compounds, and antioxidant activity of acerola (Malpighia emarginata D.C.) and guava (Psidium guajava L.) fruit processing by-products. 3 Biotech 13, 315 (2023). https://doi.org/10.1007/s13205-023-03738-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03738-1