Abstract
The association of plant beneficial Azospirillum and Bacillus spp. strains expressing different sets of PGP traits may have complementary or supplementary effects on host plants. In the present investigation, A. formosense and Bacillus spp. strains showing diverse PGP traits (IAA production, nitrogenase activity, phosphate, zinc and potassium solubilization, siderophores, antagonism against phytopathogens, osmotic stress tolerance, etc.) were assessed for compatibility by cross-streaking and co-culturing. Under co-culture (Azospirillum + Bacillus), a significant increase in the expression of PGP traits, nitrogenase activity (up to 89%), phosphate solubilization (upto 236%), siderophore production (upto 20%) was observed as compared to individual Azospirillum culture, indicating synergistic effect of co-culture. IAA production was higher in Azospirillum sp. strains as compared to Bacillus spp. strains, when cultured individually; however, when co-cultured, the IAA levels were in the mid-range indicating the contributory effects of compatible strains. The effect of individual Azospirillum and Bacillus strains and their co-inoculation was also assessed on the growth of pearl millet at early stages under moisture-deficit stress imposed using PEG6000 (0, 10, and 20%). Co-inoculation enhanced seed germination (up to 10, 3, and 6% increase under 0, 10, and 20% PEG, respectively, over individual Azospirillum treatment), root traits (increased root hair density and lateral branches), and seedling vigor indices (up to 22, 32, 43% increase in seed vigor index I and 8, 14, and 10% increase in seed vigor index II under 0, 10, 20% PEG, respectively, over individual Azospirillum treatment) under normal as well as moisture-deficit conditions suggesting the role of Bacillus spp. strains in better adaptation of the plants to stress and higher yield potential. The synergistic effect of co-cultured Azospirillum and Bacillus strains on PGP traits indicated metabolic interplay between the two strains which needs to be further understood. The positive effect of co-inoculation on plant growth under moisture-deficit stress indicated the promise of Azospirillum and Bacillus as a synergistic bioformulation for combating nutrient and drought stress in pearl millet, particularly in nutrient-poor dryland agricultural systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR) are beneficial bacteria which establish a symbiotic or non-symbiotic association with plants in the rhizosphere (Miransari 2016; Bhattacharyya and Jha 2012; Grover et al. 2021). Gram-negative Azospirillum and Gram-positive Bacillus are two such genera of rhizobacteria which express multifunctional traits such as biological nitrogen fixation, production of phytohormones like IAA, gibberellins, cytokinins, ethylene, etc., siderophores, nutrient solubilization, antagonism against phytopathogens and protection of plants against abiotic stresses such as drought, soil salinity, and toxic compounds, etc. (García et al. 2017; Akinrinlola et al. 2018; Cassan et al. 2020). The advantages of using Bacillus as inoculants are high cell viability, spore forming nature, and prolonged shelf-life of the formulation, which explains its frequent and successful commercialization (Akinrinlola et al. 2018).
The number of plant beneficial features expressed by PGP strains belonging to various genera and/or species varies. Combining two or more PGPR strains with various metabolic capacities (nutrient solubilization, nitrogen fixation, phytohormone synthesis, antimicrobials, etc.) may have additive or synergistic impacts on plant beneficial features, thereby improving the inoculum's plant beneficial potential (Santoyo et al. 2021; Bagheri et al. 2022). The strains utilized for combination inoculation, however, must be compatible with one another. Metabolites released by one organism may or may not influence the growth of other microorganisms in its vicinity. The limited reports are available on the synergistic effect of Azospirillum spp. and Bacillus spp. That demonstrated in vitro the potential of combining the two PGPR for optimizing the effects in terms of plant beneficial traits such as nitrogen fixation, IAA production, and phosphate solubilization (Drozdowicz and Santos 1987; Ribeiro et al. 2022; Bagheri et al. 2022). Furthermore, relatively few species have been reported for Azospirillum–Bacillus interactions, and investigating other less studied and native strains of Azospirillum may prove useful for developing novel bio-inoculants.
Pearl millet (Pennisetum glaucum (L.) R. Br.) is one of the oldest cultivated cereal crops and is vital for food and nutritional security for the resource poor people of the world living in adverse agro-ecological zones. It is grown in about 27 million hectares of cultivated land worldwide and is the livelihood of more than 90 million farmers (Varshney et al. 2017). In Asia, India stands first in terms of area under pearl millet cultivation (about 9 million ha) with a total production of nearly 8.3 million tons and an average productivity of 930 kg/ha during the last 3 years. Since the past few years, pearl millet demand has increased due to its nutritional characteristics and its adaptability to a wide range of climatic conditions (Tako et al. 2015). However, drought-affected regions have witnessed severely reduced pearl millet growth and the productivity (Choudhary et al. 2015). Current climate change-induced stresses are the major reasons for the massive loss of yield and quality of crops (Wang et al. 2018; Davis et al. 2019), which necessitate research in new directions to develop suitable strategies for improving growth and productivity of pearl millet. In this direction, the role of beneficial microorganisms in imparting tolerance to climate change-induced stresses in crop plants is being increasingly highlighted (Grover et al. 2011; Grover et al. 2021; Fiodor et al. 2021; Rani et al. 2022). Several PGP bacteria including Azospirillum and Bacillus spp. exert positive effect on plant growth, root development, and yield parameters of pearl millet (Tilak and Subba Rao 1987; Grover et al. 2022; Vishnu et al. 2022).
Combined use of two or more PGP bacteria may have synergistic effect in terms of plant growth and also in alleviating abiotic stress effects in host plants (Cassan et al. 2020). Limited reports on use of Azospirillum and Bacillus co-inoculations for alleviating abiotic stress effects are also available in few crops including Ocimum basilicum (basil), foxtail millet (Setaria italica (L.), wheat (Triticum aestivum), (Heidari and Golpayegani 2012; Rafi and Charyulu 2016; Akhtar et al. 2021). However, no such study has been reported in pearl millet specifically with A. formosense strains and under moisture-deficit conditions. Therefore, the present study aimed at exploring the possibilities of using combined inoculations in pearl millet crop that was planned with two objectives: 1. to study the compatibility between selected A. formosense and Bacillus spp. strains and effect of their co-culture on plant growth-promoting traits in vitro; 2. to study the co-inoculation effects of A. formosense and Bacillus spp. strains on pearl millet seedlings under moisture-deficit stress.
Materials and methods
Microbial strains and their maintenance
Three Azospirillum formosense strains isolated from pearl millet rhizosphere (Grover et al. 2022) and a set of twelve Bacillus spp. strains were procured from Division of Microbiology, IARI (Supplementary Table I). The cultures were preserved and maintained on Luria–Bertani (LB) agar slants at 4ºC. The bacterial strains were grown in LB agar or LB for conducting various experiments. The purity of the Azospirillum cultures was confirmed by observing scarlet red, dry wrinkled type colonies when streaked on Rojo Congo (Caceres 1982) agar medium and subsurface pellicle formation with change in the color of medium to blue when inoculated in semi-solid N-free malate medium (Dobereiner 1980). The phytopathogenic fungal cultures Fusarium solani, Macrophomina phaseolina, Curvularia sp., Bipolaris sp., Alternaria alternata were obtained from the Division of Microbiology and Magnaporthe grisea was obtained from Division of Plant Pathology, IARI and maintained and multiplied on potato dextrose agar medium (HiMedia).
Indole acetic acid (IAA) production
IAA production by bacterial cultures was estimated by the colorimetric assay. Exponential-phase bacterial cultures (~ 108 CFU/ml) were inoculated (1% inoculum) in LB broth containing tryptophan (50 μg/ml) and then incubated for 48 h at 28 ± 2 °C under shaking. After centrifuged at 10,000 rpm for 10 min, 1 ml of supernatant was transferred to a fresh tube followed by adding 2–3 drops of ortho-phosphoric acid and 2 ml of Salper reagent (Gordon and Paleg 1957). The reaction mixture was incubated at room temperature for 30 min and the intensity of pink color was read at 530 nm by spectrophotometer. Auxins were quantified from the calibration curve prepared using pure IAA as standard and expressed as µg/mg protein.
Nitrogenase activity by acetylene reduction assay (ARA)
The nitrogen-fixing capacity of the test organisms was evaluated under microaerophilic conditions by ARA (Bergerson 1980). Semi-solid N-free malate medium (5 ml) in 30 ml glass test tubes was sterilized and inoculated (1% inoculum) with exponential-phase bacterial cultures (~ 108 CFU/ml). For co-inoculation, each bacterial strains were inoculated at 0.5% inoculum level. The tubes were incubated under static conditions in an incubator at 37 °C for 72 h. Post-incubation, the cotton plugs in the tubes were replaced with surface sterilized Suba-seal septa and 2 ml of the air in each tube was replaced with pure acetylene followed by incubation at 37 °C for 24 h. 1 ml of gas sample was injected into the gas chromatograph fitted with flame ionization detector (FID) (Agilent 7890) to estimate the conversion of acetylene to ethylene. Nitrogenase activity was expressed in nmoles of ethylene/hr.
Siderophore production assay
Universal CAS assay of Schwyn and Neilands (1987) was used for estimating siderophore producing ability of the test bacterial strains. Qualitative estimation was done by spotting the bacterial cultures on CAS agar plates followed by incubation at 28 ± 2 °C for 24–72 h. The appearance of orange to brown hallow around the colonies indicates siderophore production. Siderophore production was quantified in the supernatant obtained from the bacterial cultures grown in LB broth medium. The bacterial cultures were centrifuged at 10,000 rpm for 10 min. 0.5 ml of supernatant was mixed with 0.5 ml of CAS reagent and a decline in absorbance at 630 nm was measured after 20 min (Murakami et al. 2021). The amount of siderophore produced in the culture was expressed in percent siderophore unit (psu) calculated using the following formula (Payne 1993):
where Ar represents the absorbance of the reference solution (CAS reagent mixed with un-inoculated medium), and As is the absorbance of the sample (CAS reagent mixed with bacterial culture supernatant).
Zn solubilization assay
Modified Bunt and Rovira (1955) medium containing 0.5 g/L of different zinc salts [ZnO, Zn3(PO4)2] was poured in petri plates and spotted with 10 µl of log-phase bacterial cultures. The plates were incubated for 7 days at 28 ± 2 °C. The presence of transparent halo around the bacterial colonies indicated the zinc solubilization.
K solubilization assay
The bacterial cultures were spotted (10 µl) on the Aleksandrov (Aleksandrov et al. 1967) agar medium containing potassium aluminium silicate as insoluble source of K. The petri plates were incubated for 7 days at 28 ± 2 °C and observed for the presence of transparent halo around the bacterial colonies which indicated the K solubilization.
Phosphate solubilization assay
The bacterial cultures were spotted (10 µl) on the Pikovskaya’s agar medium (Pikovskaya 1948) containing tri calcium phosphate as insoluble source of P. The petri plates were incubated for 7 days at 28 ± 2 °C and observed for the presence of transparent halo around the bacterial colonies which indicated the phosphate solubilization. Quantitative estimation for P solubilization was done in Pikovskaya’s broth. The test strains were inoculated and incubated at 28 ± 2 °C for 72 h. To 1 ml of culture supernatant, 10 ml of ammonium molybdate was added in a volumetric flask and the volume was made up to 45 ml. After adding 0.25 ml of chlorostannous acid, the volume in the flask was immediately made up to 50 ml. The blue color intensity of the solution was measured at 600 nm (Jackson 1973). The amount of soluble phosphate was determined from a standard curve prepared using KH2PO4.
Antagonism against fungal pathogens
The antagonistic behavior of the bacterial strains was tested by dual culture method (Kumar et al. 2002). The fungal culture (3-mm disc) was inoculated at the center of PDA plate. The bacterial cultures were streaked 3 cm away from fungal disc near the periphery of the plates. The plates were incubated for 4–5 days. Observation on inhibition of fungal growth by the bacterial cultures was recorded.
Osmotic stress tolerance
Osmotic stress test was induced by adding mannitol (0, 200, 400, 600, 800, and 1000 mM) in the LB agar medium. Mannitol-containing LB agar plates were spotted inoculated with bacterial cultures and the plates were incubated at 28 °C for 7 days after which the observation was taken for the presence/absence and extent of bacterial growth (Deeba et al. 2017; Grover et al. 2022).
Compatibility studies
For compatibility studies on solid medium, the log-phase cultures of Azospirillum and Bacillus spp. strains were streaked perpendicular to each other on the LB and Rojo Congo agar plates using an inoculation loop. The plates were incubated at 30 °C for 72 h. The plates were observed for growth inhibition zone between the Azospirillum and Bacillus cultures. For co-cultivation studies in broth, the selected bacterial strains were inoculated in LB broth at the rate of 1% inoculum (~ 106 cells ml−1) of individual and dual strains (Azospirillum and Bacillus) and incubated at 28 ± 2 °C for 72 h. The cultures were serially diluted and appropriate dilutions were spread plated on Rojo Congo medium. The plates were incubated at 28 ± 2 °C for 48—72 h and observed for Azospirillum type (small, dry, and brick red) and Bacillus type (pale, mucoid, and spreading type) colonies.
Negative staining
Bacterial culture (25 µl) was placed on one end of a clean sterilized glass slide and 25 µl of 10% nigrosin solution was added to the culture and another slide was used to spread the drop to make a thin smear. The slide was air dried for 5 min and was viewed using bright field microscope (Zeiss Scope A1) under 40 × and oil emulsion.
Co-cultivation and PGP traits
The effect of co-cultivation of compatible Azospirillum and Bacillus strains was studied on the expression of selected PGP traits in vitro. The procedures mentioned above were followed. For co-cultivations studies, the bacterial cultures of Azospirillum and Bacillus (~ 108 CFU/ml each) were inoculated in 1:1 ratio to a final inoculum rate of 1%.
Seed germination and seedling growth assay
Seeds of pearl millet (Pusa composite 443) of uniform size and color were selected and were surface sterilized with 0.1% mercuric chloride solutions for 2 min followed by immersing in 70% alcohol for 30 s. The seeds were washed thoroughly with sterile distilled water for 6–7 times and imbibed in culture broth (~ 108 CFU/ml) of Azospirillum (AIM57) and Bacillus (IMSB1, AB4, RP24, IMBJ3, IMJ4, IMJ7, IMJ12 selected based on PGP traits) strains and their combinations (Azospirillum AIM57 + each strain of Bacillus) for 60 min. For co-inoculation treatments, bacterial cultures of Azospirillum and Bacillus spp. strains were mixed in 1:1 ratio before imbibing the seeds. For control treatment, un-inoculated sterilized LB medium was used. The imbibed seeds were then air dried in laminar air flow and placed aseptically on seed germination sheets soaked in distilled water (control) or solutions containing 10%, 20% PEG6000. Each sheet was loaded with 25 seeds and 3 replicates were maintained for each treatment. The sheets were incubated at 25 ± 2 °C in a BOD (Thermotech, L7003). The seed germination was recorded after 5 days and data on root and shoot length wERE recorded. Seedling dry weight was recorded after drying to constant weight at 60 °C. Visual observations on root hair density and lateral roots were also recorded.
Seedling vigor indices
Seedling vigor indices were calculated using the formula suggested by Abdul-Baki and Anderson (1972) and expressed as a whole number.
Molecular identification based on 16SrRNA gene sequence analysis
Genomic DNA from the log-phase bacterial culture raised in LB medium was extracted by GSure Bacterial Genomic DNA isolation kit (GCC BIOTECH). PCR amplification was done using 12.5 µl of 2X PCR master mix, I µM of forward primer PA (5′-AGA GTT TGA TCC TGG CTC AG-3′), I µM of reverse primer PH (5′-AAG GAG GTG ATC CAG CCG CA-3′), 3 µl of genomic DNA in a 30 µl reaction. A reaction without DNA was included as negative control for PCR amplification. Amplification was done under standardized conditions (initial denaturation 94 °C for 3 min, 32 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 1 min, extension at 72 °C for 2 min, and final extension at 72 °C for 8 min) using a 96-well thermal cycler (MyCycler™, Bio-Rad). The PCR product was electrophoresed on 1.5% (w/v) agarose gel and viewed using gel-documentation system. The gel eluted PCR products was purified and sequenced by Barcode Biosciences, Bangalore, India. The forward and reverse sequences were aligned pairwise using AMBOSS Needle software online and contig obtained was blasted against the existing database (GenBank database) in NCBI-BLASTN to know the identity of the isolates. Test sequences and the closely matching sequences were aligned using ClustalW program and the evolutionary history of bacterial isolate was inferred using the neighbor joining method using MegaX (Kumar et al. 2018). The partial 16S rRNA sequence was submitted to NCBI, GenBank and accession number was obtained.
Statistical analysis
The data were subjected to analysis of variance, and Tukey’s HSD was calculated using Indian NARS Statistical Computing portal. Data are presented as mean ± SE (error bars).
Results
Comparative assessment of individual Azospirillum and Bacillus spp. strains for in vitro PGP traits
Variations were observed among the bacterial strains with respect to number of PGP traits positive as well as in terms of their expression level (Table 1). IAA production among the Azospirillum and Bacillus strains ranged between 6.3 and 34.94 µg/mg protein. Higher IAA production was observed in Azospirillum sp. strains as compared to Bacillus spp., with AIM57 showing highest IAA production among all the strains tested. Among the Bacillus spp. strains, IMJ12 showed highest IAA production and was at par with IMSB1. All the strains were positive for siderophore production as indicated by the yellow halo around the colonies. The strains AB1 and IMJ4 showed the maximum siderophore production. All the three Azospirillum strains were also able to produce siderophores comparable to the Bacillus strains IMSB1, AB4, RP24, and IMJ9.
When tested qualitatively on Pikovskaya’s agar medium, Bacillus spp. strains AB4, RP24, IMJ5, and IMJ7 showed P solubilization whereas rest of the strains were negative for P solubilization on Pikovskaya’s agar medium. Similarly, Bacillus spp. strains, AB4 and IMJ7 could exhibit K solubilization in vitro on Aleksandrov agar medium, whereas all other strains were found negative for K solubilization. Among the Bacillus spp. strains, except IMJ5 and IMJ10, all could solubilize ZnO with strain IMJ7 showing maximum ZnO solubilization followed by strain IMSB1 which was at par with AB3, AB4, RP24, and IMJ4. Among the Azospirillum strains, only AIM19 exhibited solubilization of ZnO. When Zn3(PO4)2 was used as Zn source, none of the Azospirillum sp. strains showed solubilization. The Bacillus sp. strain IMJ7 showed maximum solubilization of zinc phosphate followed by AB1 which was at par with AB4, IMJ9 whereas IMJ5 and IMJ10 showed no solubilization of zinc phosphate in vitro.
In vitro antagonism against fungal phytopathogens
Antagonistic ability of the test strain was assessed against selected six fungal phytopathogens by dual culture method on PDA medium. All the Azospirillum strains were negative for antifungal activity. The Bacillus strains IMSB1, RP24, IMJ4 were able to inhibit all the six test fungal pathogens, followed by AB3, IMBJ3 which were able to inhibit five of the test fungal pathogens. The strains IMJ9 and AB1 were able to inhibit four and three of the test fungal pathogens, respectively. The strains IMJ12, IMJ7, AB4 showed lesser inhibition potential than other strains and the strains IMJ10 and IMJ5 showed no inhibition of any of the six test fungal pathogens (Table 2, Supplementary Fig. 1).
Tolerance of bacterial strains to osmotic stress
All the three A. formosense strains exhibited luxurious growth on the medium amended with concentrations up to 600 mM mannitol. The strains were able to grow at 800 and 1000 mM concentrations also; however, reduction in growth was observed as compared to that at lower concentrations. The Bacillus spp. strains AB1, IMSB1, IMBJ3, and IMJ4 showed luxuriant growth up to 1000 mM mannitol whereas the strains AB3, AB4, RP24, IMJ9 which showed luxuriant growth up to 800 mM mannitol showed slight reduction in colony size at 1000 mM mannitol and the strain IMJ7 that could grow efficiently up to 800 mM mannitol showed significantly reduced growth at 1000 mM mannitol. The strains IMJ5, IMJ10, IMJ12 were able to grow only up to 400 mM mannitol concentrations beyond which the bacterial colony growth was reduced drastically (Table 3).
Compatibility studies
The compatibility as tested by streaking method indicated that all the tested Bacillus spp. strains (except IMJ9) were compatible with all the three A. formosense strains (Fig. 1). The Bacillus spp. strain IMJ9 showed inhibition of the growth of AIM38 on both LB agar and Rojo Congo agar media indicating the incompatibility between the two strains.
The selected Azospirillum and Bacillus strains when co-cultivated in LB broth for 48 h and plated on Rojo Congo plates showed the presence of both Azospirillum (small red colonies) type and Bacillus type (pale mucoid, spreading type) colonies. However, Azospirillum type population showed reduction under co-cultivation conditions as compared to individual Azospirillum culture. Similarly, Bacillus type population also showed decrease under co-cultivation conditions as compared to respective individual strain culture, except for IMJ4 which showed higher population under co-cultivation (Table 4). Another interesting observation was the presence of unique colony types with red center (Azospirillum type) and pale periphery (Bacillus type) indicating a mixed colony of two bacterial strains (Supplementary Fig. 2a). The mixed type colonies when streaked on Rojo agar plates showed both Azospirillum type and Bacillus type colonies (Supplementary Fig. 2b) indicating the compatibility between the strains of two different genera. The co-existence of the co-cultured strains was also confirmed microscopically by negative staining which showed the presence of both Azospirillum type and Bacillus type cells (Fig. 2).
Effect of co-cultivation of Azospirillum and Bacillus spp. strains on PGP traits in vitro
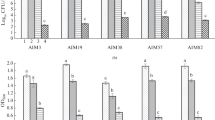
Bacillus spp. strains showed IAA production in the range of 7.50 to 16.28 µg/mg protein which was significantly lower as compared to that observed for A. formosense strains AIM57 (36.55 µg/mg protein). IAA production under co-culture conditions ranged between 15.07 and 23.84 µg/mg protein for different combinations indicating that IAA production was in the middle range (lower than individual AIM57 culture, but higher than the individual cultures of respective Bacillus strains). The combination AIM57 + IMJ12 showed the highest IAA production followed by AIM57 + IMJ4 and AIM57 + IMJ7 (Fig. 3a). The results indicated the contribution of both the strains in the production of IAA. Nitrogenase activity among the individual strains ranged between 0.25 and 65.30 nmoles of ethylene/hr with AIM57 showing the highest activity and the individual Bacillus spp. strains showing negligible ARA. The nitrogenase activity under co-cultured conditions ranged between 79.63 and 123.65 nmoles of ethylene/hr with the combination AIM57 + IMJ4 showing the highest nitrogenase activity followed by AIM57 + IMBJ3 and AIM57 + IMJ7, which was at par with AIM57 + IMJ12 (Fig. 3b). Phosphate solubilization among the individual strains ranged between 2.60 and 4.79 µg/mg protein with IMJ12 showing the highest activity. The phosphate solubilization under co-cultured conditions ranged between 3.11 and 8.74 µg/mg protein with the combination AIM57 + IMJ12 showing the highest solubilized phosphate followed by AIM57 + IMJ4. The results indicated variation in the response of co-cultured combination; however, in majority of the co-inoculated combination, a positive effect in phosphate solubilization was observed (Fig. 3c). Siderophore activity among the individual strains ranged between 8.08 and 40.92 psu with AIM57 showing the highest activity and IMJ7 showing the least activity. The siderophore production under co-cultured conditions ranged between 40.62 and 48.95 psu showing significant increase over respective individual strains. The combination AIM57 + IMBJ3 showed the highest siderophore production followed by AIM57 + IMSB1 which was at par with AIM57 + AB4. Under co-cultured conditions, the combination AIM57 + RP24 showed the least siderophore activity; however, it was significantly high than that of individual RP24 and was at par with that expressed by individual AIM57 strain (Fig. 3d).
Seed bioassay
Effect of individual and co-inoculated bacterial treatments on seed germination and root traits
Effect of inoculation was observed on seed germination and related parameters under 0%, 10%, and 20% PEG conditions (Fig. 4). Under 0% PEG, germination percent ranged between 82.67 and 98.67% with all the inoculated treatment showing positive effect on percent seed germination. However, variation was found among the treatments. Individual treatments with strains IMJ3, AIM57, RP24, and IMJ4 showed significantly higher percent seed germination as compared to control. Co-inoculation improved germination over control as well as respective individual treatments (with exception of IMJ4 and IMJ7). Treatment AIM57 + AB4 followed by AIM57 + RP24 showed highest % seed germination among all the treatments.
Similarly, positive effect of inoculation (individual and combined treatments) was observed on percent seed germination under 10% PEG conditions. All the inoculated treatments except AIM57 + IMJ12 showed significantly higher seed germinations as compared to un-inoculated control. Highest percent seed germination was observed in individual bacterial treatments IMJ4 and combined treatments AIM57 + IMBJ3 and AIM57 + IMJ4. However, all the inoculated treatments were statistically at par. Significant reduction in percent seed germination was observed with increasing stress level to 20% PEG. However, effect of inoculation was observed on percent seed germination. Among the individual treatments, IMJ4 showed highest percent seed germination and treatment with AB4 and IMJ4 showed significant reduction in percent seed germination under 20% PEG conditions. All other individual treatments were at par and non-significantly higher than the control treatment. Among the co-inoculated treatments, all except AIM57 + IMBJ3 showed higher percent germination over control treatment. Treatments with AIM57 + IMSB1, AIM57 + RP24, and AIM57 + IMJ4 were at par with each other and significantly higher than the control treatment.
Effect of inoculation was also visible on root hair density and number of lateral roots. Individual inoculation of Azospirillum strain AIM57 increased root hair density and number of lateral roots as compared to un-inoculated control. Inoculation with Bacillus spp. strains in general showed increased root length and increased number of lateral roots and branching. Co-inoculated treatments (AIM57 + IMSB1, AIM57 + RP24, and AIM57 + IMJ4) showed enhanced root hair density, increased number of lateral roots and root length as shown in (Supplementary Fig. 3). Higher stress level (20% PEG) showed visible negative effect on root traits in terms of reduced length and number of lateral roots that was observed. Inoculated treatments showed positive effect on root growth as compared to control.
Effect of individual and co-inoculated bacterial treatments on seedling vigor indices
The SVI-1 calculated on the basis of percent seed germination and seedling length showed significant variation among the treatments (Table 5). Under 0% PEG conditions, all the treatments except AIM57 + IMJ7 showed positive effect on SVI-1 as compared to control treatment. Among the individual treatments, IMJ4 followed by IMJ7 and RP24 showed highest SVI-1. Co-inoculation (with few exceptions) further improved SVI-1 over individual treatments with AIM57 + IMSB1 showing highest SVI-1 followed by AIM57 + IMJ4 and AIM57 + RP24.
Under 10% PEG conditions, un-inoculated control showed lowest SVI-1 among all the treatments. Among the individual treatments, RP24 showed highest SVI-1 followed by IMBJ3. Co-inoculation of AIM57 with Bacillus spp. strains further improved SVI-1. Treatment AIM57 + IMBJ3 showed significantly highest SVI-1 among all the treatments and was followed by AIM57 + RP24 and AIM57 + IMSB1. Significant reduction in SVI-1 was observed under 20% PEG conditions as compared to 0 and 10% PEG conditions. However, inoculation with individual as well as combinations improved SVI-1. Among the individual treatments except AB4, IMJ12, all the others showed positive effect with statistically significant effect. IMJ4 followed by RP24 showed highest SVI-1. Among the co-inoculation treatments, AIM57 + IMJ7 followed by AIM57 + RP24 and AIM57 + IMJ4 showed highest SVI-1.
The positive effect of inoculation was also observed on SVI-2 under 0, 10, and 20% PEG conditions with few exceptions (Table 6). Under 0% PEG conditions, IMSB1 and IMBJ3 and RP24 were at par and significantly higher than control. Among the co-inoculated treatments, AIM57 + RP24, followed by AIM57 + IMJ4 and AIM57 + IMSB1 were found best in terms of SVI-2. Similar trends were observed under 10% PEG with IMJ4, IMBJ3, IMSB1 showing highest values among the individual treatments and treatments AIM57 + AB4, AIM57 + IMJ4, and AIM57 + IMJ12 showing highest SVI-2 among the co-inoculated treatments.
Significant reduction in SVI-2 was observed under 20% PEG conditions; however, effect of inoculation was observed for both individual as well as combined inoculation treatments with few exceptions. Among the individual treatments, IMJ4 followed by IMJ7 and RP24 showed highest SVI-2, whereas co-inoculation treatments AIM57 + RP24, AIM57 + IMSB1, and AIM57 + IMJ4 showed highest SVI-2.
Molecular identification of the strain IMSB1
Among the strains showing promising results in seed bioassay, the strain IMSB1 was not identified previously; hence, molecular characterization of this strain was done. The PCR amplification of 16SrRNA gene sequence resulted in 1500 bp amplified product. The product was sequenced and the contig prepared (1484 bp), when blasted in the NCBI Nucleotide BLAST database of 16S rRNA sequences of Typed strains, showed that strain IMSB1 has maximum identity (99.66%) with Bacillus subtilis strain SBMP4 (NR 118,383.1). Phylogenetic tree constructed with closest match sequences showed that IMSB1 and SBMP4 were grouped together in a separate clade from other reference sequences indicating their close evolutionary relationship (Supplementary Fig. 4). Partial 16SrRNA gene sequence of Bacillus subtilis strain IMSB1 has been submitted to NCBI under the Accession number OP012865.
Discussion
The present study explored the possibilities of using Azospirillum–Bacillus associations for synergistic effects on plant beneficial traits. Azospirillum spp. are Gram negative, non-fermentative, microaerophilic, associative symbiotic nitrogen-fixing bacteria. The most important PGP traits identified in Azospirillum spp. include biological nitrogen fixation and phytohormones production; additionally, phosphate solubilization, siderophore production, and antagonism has also been observed in some strains (Cassan et al. 2020). In the present study, biological nitrogen fixation, IAA production, and siderophore production were recorded as prominent traits of A. formosense strains. Bacillus spp. strains showed IAA production, siderophore production, nutrient (P, Zn, K) solubilization as prominent traits, however, with variation among the tested strains with 4, 10, and 2 strains exhibiting P, Zn, and K solubilization, respectively, and majority (ten) of strains exhibiting antagonism. Interestingly, Bacillus spp. strains IMSB1, RP24, IMJ4 could inhibit all the six tested fungal pathogens. The biocontrol potential of Bacillus spp. strains against plant pathogens including bacteria, fungi, nematodes, viruses, and pests in plants and agricultural lands, by producing cell-wall-degrading substances, like chitosanases, glucanases, cellulases, proteases, lipopeptides, and hydrogen cyanide, etc., is well documented (Radhakrishnan et al. 2017). Variation in the PGP traits expressed by strains belonging to different genera and/or species has been observed commonly and these variations in PGP traits indicate the prospects of using combinations of PGP strains for additive/synergistic effects on plants (Kuan et al. 2016; Ribeiro et al. 2018; Santoyo et al. 2021; Bagheri et al. 2022).
The use of PGP strains with ability to tolerate abiotic stress is advantageous under stressful conditions (Grover et al. 2021, 2022). Therefore, the bacterial strains expressing PGP traits were also evaluated for in vitro osmotic stress tolerance. All the three Azospirillum strains were able to grow efficiently on the media with up to 600 mM mannitol above which the growth started to reduce although growth was observed even at 800 and 1000 mM mannitol. The Bacillus strains AB1, IMSB1, IMBJ3, IMJ4, RP24, AB3, and AB4 showed luxuriant growth up to 1000 mM mannitol. The bacteria possessed various traits like exopolysaccharide production, osmolyte accumulation, etc., that help in their survival under unfavorable conditions (Sandhya et al. 2009; Ilyas et al. 2020; Jayasurya and Grover 2022). Moreover, members of genus Bacillus also have spore forming ability whereas azospirilla exhibit cyst formation under unfavourable conditions. Both the traits can help the microbes in escaping adverse conditions, thus their application is advantageous for stressed ecosystems.
However, the strains used for combined inoculation must be compatible with each other. Some metabolites secreted by an organism (energy source, growth factors, etc.) may have positive effect of the growth of neighboring populations whereas others (for example, antimicrobials) may inhibit the growth. The negative effect of co-inoculation has been reported in few studies. For example, co-inoculation of Rhizobium sp. strain with Azospirillum sp. strain in clover prevented the nodule formation. It was observed that Azospirillum by colonizing the root hair blocked the sites for the Rhizobium to cause infection and develop nodules (Plazinski and Rolfe 1985). However, in the present studies, all (except IMJ9) Bacillus spp. strains were compatible with the A. formosense strains indicating their possible use together.
The co-culturing effect of selected Azospirillum (AIM57) and Bacillus spp. strains (IMSB1, AB4, RP24, IMBJ3, IMJ4, IMJ7, and IMJ12) was studied on selected PGP traits in vitro. It was observed that the IAA production in co-culture was higher than the individual Bacillus strain but lower than that expressed by AIM57, indicating contribution from both the partners. Bagheri et al. (2022) investigated the in vitro synergistic interactions between Azospirillum and Bacillus strains and found that Azospirillum produced the highest IAA in a monoculture as compared to Bacillus monoculture or Azospirillum + Bacillus co-culture. However, IAA production in co-culture system was higher than the Bacillus monoculture. Similarly, an increase in phosphate solubilization was observed under co-culture indicating synergistic interaction between the two strains. Similar results have been observed in previous studies. El-Komy (2005) co-mobilized two P-solubilizing bacteria, Bacillus megaterium (higher solubilization efficiency) and Azospirillum lipoferum strain 137 (lower solubilization efficiency) in alginate and agar and found better P solubilization than the separate strains, indicating synergistic interactions between the strains. Similarly, Ribeiro et al., (2022) observed the synergistic effect of Bacillus and Azospirillum strains on phosphate solubilization efficiency and suggested the production of organic acids by both the co-inoculated strains for better P solubilization. Similar effects were observed on siderophore production with majority of co-culture combinations showing higher siderophore production as compared to respective mono-cultures. Best co-culture treatment was AIM57 + IMBJ3 which was at par with AIM57 + IMBS1 and AIM57 + AB4. Enhanced siderophore production under co-culture may indicate competition among the strains; however, as bio-inoculants, it can be advantageous for the host plant for better iron nutrition and also in competition with other rhizosphere microorganisms (Crowley 2006). A significant increase in nitrogenase activity observed under co-culture conditions indicated synergistic effect of non-nitrogen fixing Bacillus spp. strains on nitrogen-fixing A. formosense strain AIM57. Drozdowicz and Santos (1987) were among initial workers to report interaction in vitro between Azospirillum and Bacillus. They reported that co-culturing Bacillus with Azospirillum had stimulatory effect on the nitrogenase activity of Azospirillum in the mixed culture. Holguin and Bashan (1996) investigated the interaction of a non-nitrogen-fixing strain of Staphylococcus sp. obtained from mangrove rhizosphere with a nitrogen-fixing A. brasilense sp. strain. It was observed that when cultivated in co-culture, A. brasilense was capable of fixing more nitrogen than when grown individually. The aspartic acid generated by Staphylococcus sp. in the medium was found to be associated with increased nitrogen-fixing efficiency of A. brasilense, however only in a concentration-dependent way. The observations in present study also indicate the role of Bacillus strains in providing some favourable conditions like providing some suitable metabolites or creating microaerophilic conditions for nitrogen fixation by Azospirillum strains, which is a matter of further investigation. The studies on synergistic effect of Azospirillum spp. and Bacillus spp. are limited, but clearly indicate the potential of combined use of the two PGPR for maximizing the effects in terms of plant beneficial traits.
The use of synergistic microorganisms can be a suitable and sustainable strategy for improving the productivity of crops like pearl millet grown in stressed ecosystems. Therefore, the selected combinations were evaluated for the inoculation effect on seed germination and vigor indices. Polyethylene glycol with a high molecular weight has been utilized to induce moisture deprivation stress in vitro (Kaufmann and Eckard 1978). In the present study, the seedling germination assay revealed that bacterial inoculation had a positive effect on seed germination, seedling length, and dry biomass. The seed germination percent and seed vigor indices were not much affected at 10% PEG6000; however, significant reduction was observed under 20% PEG6000 when compared with 0% PEG6000. Under 20% PEG conditions, AIM57 + IMSB1, AIM57 + RP24, and AIM57 + IMJ4 showed highest percent germination among the co-inoculation treatments. Seed vigor indices calculated based on seedling length, dry weight, and percent seed germination showed variation among the treatments and positive effect of inoculation was observed in both single and co-inoculation treatments. The co-inoculation treatments AIM57 + IMSB1, AIM57 + IMJ4, and AIM57 + RP24 performed well and showed higher seed vigor indices under all the PEG conditions and also showed higher root hair density and lateral roots as observed visually and under the microscope. Negative effect of moisture-deficit stress on seed germination and seedling growth is well reported (Jamil et al. 2006). Inoculation with Azospirillum has been shown to promote the number and length of root hair and lateral branches; however, it is has inhibitory effect on elongation of main primary root (Cassan et al. 2020). We also observed in our study that inoculation with Azospirillum boosted root hair density and lateral roots, whereas inoculation with Bacillus improved the root length and branching. Co-inoculation of Azospirillum and Bacillus showed improvement in both root hair density and root length. The improvement in root traits which can be related with the bacterial IAA production increases the plant's access to water and nutrients stored in deeper layers of soil, and the accumulation of solutes including sugars and free amino acids, which help in maintaining the cellular structure (Saharan and Nehra 2011; Hungria et al. 2015). Ilyas et al (2020) reported that co-inoculating wheat seeds with B. subtilis (MT742976) and A. brasilense (MT742977) improved seed germination, seedling vigor index, and promptness index by 18.2%, 23.7%, and 61.5%, respectively, under 20% PEG6000. They also reported the synergistic effect of bacterial strains co-inoculation on plant growth and physiological attributes indicating the potential of synergistic interactions in alleviating abiotic stress in crop plants. Although pearl millet is drought tolerant and is generally grown in low rainfall areas where other cereals like maize and sorghum cannot grow, its yield suffers greatly when water is insufficient. Drought stress is a major constraint on crop output since it affects nearly all plant activities (Shao et al. 2007). As a result, microbial interventions for combating water deficit circumstances in pearl millet can prove to be a boon for sustainable production.
To conclude, the study revealed in vitro compatibility between Azospirillum and Bacillus spp. strains and illustrated their synergistic effects on PGP traits. The synergistic effect of co-cultured Azospirillum and Bacillus strains on PGP traits indicate metabolic interplay between the two strains which needs to be understood. The co-inoculation of Azospirillum + Bacillus spp. strains illustrated the stimulatory effect in terms of seed germination and vigor index under moisture-deficit stress, thus has potential for developing a bacterial consortium for cereals and millets cultivated under drought-affected agro-ecosystem with reduced inputs of chemical nitrogen.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
References
Abdul-Baki AA, Anderson JD (1972) Physiological and biochemical deterioration of seeds. In: Koziowski TT (ed) Seed Biology, vol II. Academic Press, New York, pp 283–315
Akhtar N, Ilyas N, Mashwani ZR, Hayat R, Yasmin H, Noureldeen A, Ahmad P (2021) Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol Biochem 166:160–176. https://doi.org/10.1016/j.plaphy.2021.05.039
Akinrinlola RJ, Yuen GY, Drijber RA, Adesemoye AO (2018) Evaluation of Bacillus strains for plant growth promotion and predictability of efficacy by in vitro physiological traits. Int J Microbiol. https://doi.org/10.1155/2018/5686874
Aleksandrov VG, Blagodyr RN, Ilev IP (1967) Phosphorus acid isolation from apatite produced by silicate bacteria. Mikrobiol Zh 29:111–114
Bagheri N, Ahmadzadeh M, Mariotte P et al (2022) Behavior and interactions of the plant growth-promoting bacteria Azospirillum oryzae NBT506 and Bacillus velezensis UTB96 in a co-culture system. World J Microbiol Biotechnol 38:101. https://doi.org/10.1007/s11274-022-03283-8
Bergerson FJ (1980) Methods for evaluating biological nitrogen fixation. Wiley, New York
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350. https://doi.org/10.1007/s11274-011-0979-9
Bunt JS, Rovira AD (1955) Microbiological studies of some sub antarctic soils. J Soil Sci 6:119–128. https://doi.org/10.1111/j.1365-2389.1955.tb00836.x
Caceres ER (1982) Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44:990–991. https://doi.org/10.1128/aem.44.4.990-991.1982
Cassan F, Coniglio A, Lopez G et al (2020) Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol Fertil Soil 56:461–479. https://doi.org/10.1007/s00374-020-01463-y
Choudhary M, Jayanand PJC (2015) Transcriptional profiling in pearl millet (Pennisetum glaucum L.R. Br.) for identification of differentially expressed drought responsive genes. Physiol Mol Biol Plants 21:187–196. https://doi.org/10.1007/s12298-015-0287-1
Crowley DE (2006) Microbial siderophores in the plant rhizosphere. In: Barton LL, Abadia J (eds) Iron nutrition in plants and rhizospheric microorganisms, 1st edn. Springer, Dordrecht, pp 169–189
Davis KF, Chhatre A, Rao ND, Singh D, Defries R (2019) Sensitivity of grain yields to historical climate variability in India. Environ Res Lett. https://doi.org/10.1088/1748-9326/ab22db
Deeba F, Sultana T, Javaid B, Mahmood T, Naqvi SMS (2017) Molecular characterization of a MYB protein from Oryza sativa for its role in abiotic stress tolerance. Braz Arch Biol Technol 60:1–12. https://doi.org/10.1590/1678-4324-2017160352
Dobereiner J (1980) Forage grasses and grain crops. In: Bergersen FJ (ed) Methods of evaluating biological nitrogen-fixation, 1st edn. John Wiley and Sons, New York, pp 541–555
Drozdowicz A, Ferreira SGM (1987) Nitrogenase activity in mixed cultures of Azospirillum with other bacteria. Zentralbl Mikrobiol 142:487–493. https://doi.org/10.1016/S0232-4393(87)80057-4
El-Komy HMA (2005) Coimmobilization of A. lipoferum and B. megaterium for plant nutrition. Food Technol Biotechnol 43:19–27
Fiodor A, Singh S, Pranaw K (2021) The contrivance of plant growth promoting microbes to mitigate climate change impact in agriculture. Microorganisms 9:1841. https://doi.org/10.3390/microorganisms9091841
García JE, Maroniche G, Creus C, Suárez-Rodríguez R, Ramirez-Trujillo JA, Groppa MD (2017) In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol Res 202:21–29. https://doi.org/10.1016/j.micres.2017.04.007
Gordon SA, Paleg LG (1957) Observations on the quantitative determination of indoleacetic acid. Physiol Plant 10:39–47. https://doi.org/10.1111/j.1399-3054.1957.tb07608.x
Grover M, Ali Sk Z, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agricultural crops to abiotic stresses. World J Microbiol Biotechnol 27:1231–1240. https://doi.org/10.1007/s11274-010-0572-7
Grover M, Bodhankar S, Sharma A, Sharma P, Singh J, Nain L (2021) PGPR mediated alterations in root traits: way toward sustainable crop production. Front Sustain Food Syst 4:618230. https://doi.org/10.3389/fsufs.2020.618230
Grover M, Bana RS, Bodhankar S (2022) Prevalance of multifunctional Azospirillum formosense strains in the rhizosphere of pearl millet across diverse edaphoclimatic regions of India. Vegetos. https://doi.org/10.1007/s42535-022-00537-6
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci 11:57–61. https://doi.org/10.1016/j.jssas.2011.09.001
Holguin G, Bashan Y (1996) Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.). Soil Biol Biochem 28:1651–1660. https://doi.org/10.1016/S0038-0717(96)00251-9
Hungria M, Nogueira MA, Araujo RS (2015) Soybean seed coinoculation with Bradyrhizobium spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. Am J Plant Sci 6:811–817. https://doi.org/10.4236/ajps.2015.66087
Ilyas N, Mumtaz K, Akhtar N, Yasmin H, Sayyed RZ, Khan W, Enshasy HAE, Dailin DJ, Elsayed EA, Ali Z (2020) Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 12:8876. https://doi.org/10.3390/su12218876
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd, New Delhi
Jamil M, Deog Bae L, Kwang Yong J, Ashraf M, Sheong Chun L, Eui Shik R (2006) Effect of salt (NaCl) stress on germination and early seedling growth of four vegetable species. J Central Eur Agric 7:273–282
Jayasurya A, Grover M (2022) Studies on endurance of Azospirillum formosense strains to osmotic stress, underlying mechanisms and modulation of early stage pearl millet growth. Appl Biochem Microbiol. (Accepted)
Kaufmann MR, Eckard AN (1978) Evaluation of water stress control with polyethylene glycols by analysis of guttation. Plant Physio 47:453–456. https://doi.org/10.1104/pp.47.4.453
Kuan KB, Othman R, Abdul Rahim K, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS One 11:1–19. https://doi.org/10.1371/journal.pone.0152478
Kumar NR, Arasu V, Gunasekaran P (2002) Genotyping of antifungal compounds producing plant promoting rhizobacteria, Pseudomonas fluorescens. Curr Sci 82:1463–1466
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Miransari M (2016) Environmental stresses in soybean production. Academic Press, USA
Murakami C, Tanaka AR, Sato Y, Kimura Y, Morimoto K (2021) Easy detection of siderophore production in diluted growth media using an improved CAS reagent. J Microbiol Methods 189:106310. https://doi.org/10.1016/j.mimet.2021.106310
Payne SM (1993) Iron acquisition in microbial pathogenesis. Trends Microbiol 1:66–69. https://doi.org/10.1016/0966-842x(93)90036-q
Pikovskaya RI (1948) Mobilization of phosphorus and soil in connection with the vital activity of some microbial species. Mikrobiologia 17:362–370
Plazinski J, Rolfe BG (1985) Analysis of the pectolytic activity of Rhizobium and Azospirillum strains isolated from Trifolium repens. J Plant Physiol 120:181–187. https://doi.org/10.1016/S0176-1617(85)80021-3
Radhakrishnan R, Hashem A, Abd Allah EF (2017) Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:667. https://doi.org/10.3389/fphys.2017.00667
Rafi MM, Charyulu PBBN (2016) Synergistic effect of Azospirillum and PSB inoculation on growth and yield of foxtail millet. Internat J Plant Ani Environ Sci 6:139–147
Rani A, Rana A, Dhaka RK, Singh AP, Chahar M, Singh S, Nain L, Singh KP, Minz D (2022) Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: Current understanding and future scope. Biotechnol Adv 63:108078. https://doi.org/10.1016/j.biotechadv.2022.108078
Ribeiro VP, Marriel IE, Sousa SM, Lana UGP, Mattos BB, Oliveira CA, Gomes EA (2018) Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz J Microbiol 49:40–46. https://doi.org/10.1016/j.bjm.2018.06.005
Ribeiro VP, Gomes EA, de Sousa SM, de Paula Lana UG, Coelho AM, Marriel IE, de Oliveira-Paiva CA (2022) Co-inoculation with tropical strains of Azospirillum and Bacillus is more efficient than single inoculation for improving plant growth and nutrient uptake in maize. Arch Microbiol 204:143. https://doi.org/10.1007/s00203-022-02759-3
Saharan B, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Sandhya V, Ali SKZ, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought tress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soil 46:17–26. https://doi.org/10.1007/s00374-009-0401-z
Santoyo G, Guzmán-Guzmán P, Parra-Cota FI, Santos-Villalobos S, Orozco-Mosqueda MC, Glick BR (2021) Plant growth stimulation by microbial consortia. Agronomy 11:219. https://doi.org/10.3390/agronomy11020219
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophore. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Shao HB, Chu LY, Wu G, Zhang JH, Lu ZH, Hu YC (2007) Changes of some anti-oxidative physiological indices under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at tillering stage. Colloids Surf B Biointerfaces 54:143–149. https://doi.org/10.1016/j.colsurfb.2006.09.004
Tako E, Reed SM, Budiman J, Hart JJ, Glahn RP (2015) Higher iron pearl millet (Pennisetum glaucum L.) provides more absorbable iron that is limited by increased polyphenolic content. Nutr J 14:11. https://doi.org/10.1186/1475-2891-14-11
Tilak KVBR, Subba Rao NS (1987) Association of Azospirillum brasilense with pearl millet (Pennisetum americanum (L.) Leeke). Biol Fert Soil 4:97–102. https://doi.org/10.1007/BF00280358
Varshney R, Shi C, Thudi M et al (2017) Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat Biotechnol 35:969–976. https://doi.org/10.1038/nbt.3943
Vishnu P, Yasasvi B, Tarate SB (2022) Influence of biofertilizers on millet production. Pharma Innov J 11:950–953
Wang Q, Liu J, Li H, Yang S, Körmöczi P, Kereszt A, Zhu H (2018) Nodule-specific cysteine-rich peptides negatively regulate nitrogen-fixing symbiosis in a strain-specific manner in Medicago truncatula. Mol Plant Microbe Interact 31:240–248. https://doi.org/10.1094/mpmi-08-17-0207-r
Acknowledgements
The authors would like to acknowledge Director and Dean (PG, School), ICAR-Indian Agricultural Research Institute, New Delhi and Head, Division of Microbiology, ICAR-Indian Agricultural Research Institute for providing necessary facilities.
Author information
Authors and Affiliations
Contributions
SY has performed the experiments, analysed and written the manuscript. MG has conceptualized, supervised the study and edited the manuscript. GST has helped in conducting the experiments. RK has co-supervised the microbiological studies. RP has supervised the microscopic studies and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
On behalf of all authors, the corresponding author states that the presented manuscript does not contain any studies involving human participants and/or animals.
Informed consent
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
13205_2023_3503_MOESM2_ESM.tif
Supp. Fig. 2. Co-cultivation of Azospirillum and Bacillus strains; 3a: Mixed colonies of AIM57 and IMSB1; 3b: Azospirillum and Bacillus type colonies on Rojo congo agar medium (TIF 2512 kb)
13205_2023_3503_MOESM3_ESM.tif
Supp. Fig. 3. Seed germination and root traits as influenced by individual and combined bacterial treatments under 0% (a), 10% (b) and 20% (c) PEG6000 conditions (TIF 1133 kb)
13205_2023_3503_MOESM4_ESM.tif
Supp. Fig. 4. Phylogenetic relation of strain IMSBI with reference strains based on 16S rRNA gene sequenceanalysis (TIF 2734 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yaadesh, S., Tomar, G.s., Kaushik, R. et al. Azospirillum–Bacillus associations: synergistic effects on in vitro PGP traits and growth of pearl millet at early seedling stage under limited moisture conditions. 3 Biotech 13, 90 (2023). https://doi.org/10.1007/s13205-023-03503-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03503-4