Abstract
The present study aims to explore the effects of oral astaxanthin administration on the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). Juvenile crucian carps with a bodyweight of 40.06 ± 2.17 g were randomly assigned to four groups, i.e., one control group fed with a basic diet and three treatment groups fed with a diet that contains 200, 400 and 800 mg/kg astaxanthin. After 60 days of feeding, the groups fed with astaxanthin-containing diets had improved body weight gain rate; feed conversion ratio; intestinal digestive protease, lipase and amylase levels; serum superoxide dismutase, catalase, acid phosphatase, alkaline phosphatase and lysozyme activities; complement 3 and complement 4 levels; interleukin (IL)-10 and resistance to Aeromonas hydrophilia and reduced serum aspartate aminotransferase and alanine aminotransferase activities and tumour necrosis factor-α, IL-1β and IL-8 levels compared with those of the control group. Based on the efficiency of the oral administration of astaxanthin on the growth performance of juvenile crucian carps, the optimum dose of astaxanthin was 400 mg/kg. Results indicated that astaxanthin may be used as a dietary supplement for the crucian carp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astaxanthin, a keto-carotenoid (3,3′-dihydroxy-β,β-carotene-4,4′-dione), is mainly isolated from Haematococcus pluvialis (Martínez et al. 2019), which is responsible for the pinkish or reddish colour of certain plants, crustaceans and fish. This material possesses many biological activities, such as antioxidant, immunomodulatory, hypolipidemic and hypoglycemic activities (Eren et al. 2019; Kumar et al. 2017; Sifi et al. 2016; Wang et al. 2012). Recently, astaxanthin has been applied to improve the growth of many animals, such as giant tiger shrimp (Penaeus monodon), long-snout seahorse (Hippocampus guttulatus) juveniles, postlarval kuruma shrimp (Marsupenaeus japonicus), white shrimp (litopenaeus vannamei), pufferfish (Takifugu obscurus) and red swamp crayfish (Procambarus clarkia) (Cheng and Wu 2019; Cheng et al. 2018; Liu et al. 2018; Palma et al. 2017; Wang et al. 2018; Wade et al. 2017).
Crucian carp (Carassius auratus) lives in major water systems outside the Qinghai–Tibet Plateau and is one of the most common freshwater fishes in China. This species has a wide range of feeding habits, strong adaptability, strong reproductive power, strong disease resistance, fast growth, low water temperature requirements and easy breeding (Wu 2020). In recent years, the rapid expansion of crucian carp culture has led to infection outbreaks, resulting in huge yield loss. In recent years, the aquaculture farmers have been compelled to use antibiotics to control infectious diseases. However, the long-term use of antibiotics has harmful influences on environmental and consumer health (Romano et al. 2015). Thus, safe native resources with high efficiency should be developed to control infectious diseases in aquaculture. Recently, many native resources, such as curcumin, purple coneflower (Echinacea purpurea), selenium nanoparticle, selenomethionine, Chlorella meal, phosphorus, cellulase and red swamp crayfish (Procambarus clarkia), have been applied to improve crucian carp growth (Cheng and Wu 2019; Chen et al. 2017; Jiang et al. 2016; Shi et al. 2017a, b; Tang et al. 2016; Zhou et al. 2009). However, the data on the effects of dietary astaxanthin on the growth and innate immunity of the crucian carp are limited.
This study aims to explore the effects of dietary astaxanthin on the growth performance and innate immunity of the crucian carp.
Materials and methods
Materials
Astaxanthin was purchased from Fujian Kangshimei Biotechnology Co., Ltd. (Fuzhou, China). The microenzyme-linked immunosorbent assay (ELISA) strip plate provided in the kit was precoated with an antibody specific to the target enzyme. All other chemicals were reagent grade.
Diet preparation
The proximate composition of the basal diet for crucian carp are presented in Table 1. Astaxanthin was added to the basal diet to replace wheat flour and obtain 0.0, 200, 400 and 800 mg/kg astaxanthin. All ingredients were pulverised and sifted through a 100-mesh sieve, fully mixed with an appropriate amount of water, coldly extruded using an extruder (Polylab, Thermo Fisher Scientific Company, USA), cut into particles, air dried and stored at − 20 °C until use.
Crucian carp rearing
The culture equipment was a 500 L cylindrical fiberglass tank provided with a continuous water flow (1.5 L min−1) and aeration with air-stones to supply enough dissolved oxygen (DO). The juvenile grass carps, which had an initial body weight of 10.03 ± 0.57 g, were purchased from a local aquaculture farm in Lianyungang, China. Prior to the feeding trial, the fish were fed with basal diet for two weeks and placed in clean aquaculture water without feeding for two days to acclimatise the fish to indoor recirculating aquaculture conditions. A total of 360 crucian carps were randomly distributed to 12 tanks. Notably, each tank was stocked with 30 crucian carps, and each diet was randomly assigned to triplicate tanks. The crucian carps were hand-fed twice a day (08:00 and 18:00), and the total feeding amount was 3% to 4% (w/w) of the body. During the trial period, the water temperature was maintained at 25 °C to 28 °C, and the water conditions were maintained as follows: pH, 7.3–7.6; DO, 6 mg/L; NH3–N < 0.05 mg/L and H2S < 0.01 mg/L. The culture period was 60 days.
Growth performance
At the end of the rearing experiment, the final bodyweight of the crucian carps in each group was weighed. The body weight gain rate (BWGR), feed conversion ratio (FCR) and survival rate were calculated using the following equations:
where W0 is the initial body weight, W1 is the final body weight, W2 is the total weight of the dried diet consumption, N0 is the initial number of survival crucian carps and N1 is the final number of crucian carps.
Sampling
At the end of the rearing trial, three crucian carps from each tank were randomly collected and anesthetised with 100 mg/L MS-222. Blood samples were collected from caudal venipuncture with 1 mL medical syringe, allowed to clot at 4 °C for 2 h and centrifuged at 3000 × g and 4 °C for 15 min. The resultant supernatant was collected and frozen at − 20 °C until use. The peritoneal cavity was aseptically opened using a sterile scalpel, and the part of the intestine between the pyloric caeca and ~ 1 cm anterior to the anus was excised. The intestinal wall was washed with sterile saline, and the faeces with mucus were stripped off using sterile forceps. The intestines of the crucian carps were homogenised in 10 volumes (v/w) of ice-cold phosphate-buffered saline and centrifuged at 5000 × g at 4 °C for 10 min. The resulting supernatant was stored in a refrigerator at − 20 °C and used to assay the intestinal digestive protease, lipase and amylase activities.
Biochemical measurement
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), protease, lipase, amylase, superoxide dismutase (SOD), catalase (CAT), acid phosphatase (ACP), alkaline phosphatase (AKP), lysozyme (LYZ), complement 3 (C3) and complement 4 (C4) activities were determined using ELISA kits in accordance with the methods of Gao et al. (2017). A unit of enzyme activity was defined as the amount of enzyme that decreased the absorbance by 0.001 min−1. The serum tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-8 and IL-10 of the crucian carps were determined using ELISA kits in accordance with the instructions described in the commercial kit.
Pathogen challenge test
At the end of the rearing trial, 12 crucian carps were randomly selected from each tank, raised in tanks for five days and intraperitoneally injected with 1.5 × 105 cfu per gram body weight of A. hydrophila based on our previous results. The challenge test period was conducted for 15 days. The culture conditions were the same as the aforementioned culture conditions. During the challenge test, the behaviour of the crucian carps was observed, and cumulative mortality was recorded.
Statistical analysis
All tests were performed in triplicate, and data were reported as means ± standard deviation. Variance and significant differences amongst the means were tested through one-way analysis of variance by using the SPSS software (version 17.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results
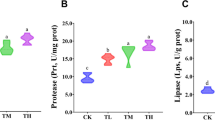
After 60 days of feeding experiments, the groups administered with oral astaxanthin had significantly improved BVGR; FCR (Table 2); intestinal digestive enzyme (protease, lipase and amylase; Table 3), AST, ALT, SOD, CAT, ACP, AKP and LYZ activities; and C3, C4 (Table 4), TNF-α, IL-1β, IL-8 and IL-10 levels (Table 5) compared with those in the control group (p < 0.05). In addition, oral astaxanthin administration decreased the cumulative mortality of crucian carp fed with astaxanthin diets after being challenged by the pathogen, A. hydrophila (Fig. 1; p < 0.05). However, a high dose of astaxanthin (800 mg/kg) did not further improve its efficiency. Moreover, no significant difference was noted in the survival rate of crucian carps amongst all groups (Table 2).
Discussion
In recent years, astaxanthin has been reported to promote the growth performance of many animals, such as giant tiger shrimp (Penaeus monodon), long-snout seahorse (Hippocampus guttulatus) juveniles, postlarval kuruma shrimp, white shrimp, pufferfish and red swamp crayfish (Procambarus clarkia) (Cheng and Wu 2019; Cheng et al. 2018; Liu et al. 2018; Palma et al. 2017; Wang et al. 2018; Wade et al. 2017). In the present study, the oral astaxanthin administration increased the BWGR and FCR of crucian carp. The biosyntheses of protein, lipid and carbohydrate need adequate reducing power, e.g., nicotinamide adenine dinucleotide phosphoric acid (NADPH). Astaxanthin has super antioxidant activity and can inhibit NADPH reductase activity and increase NADPH level, thus promoting biosynthesis (Ohno et al. 2011). At the same time, astaxanthin has hypolipidemic activity, which offsets its biosynthesis effects at a high dose (800 mg kg−1).
Intestinal digestive enzymes, such as protease, lipase and amylase, are responsible for the enzymatic hydrolysis of protein, lipid and starch and play a vital role in animal growth (Zahran et al. 2014). In the present study, the groups in administered with oral astaxanthin had significantly increased protease, lipase and amylase activities compared with those in the control group. This finding indicated that astaxanthin can promote the expression of these enzymes. This phenomenon can also be due to the biosynthesis effect of astaxanthin. Moreover, the super antioxidant activity of astaxanthin maintained a reducing environment in the intestine, inhibited the growth of aerobic bacteria, decreased the digestive enzymes secreted by these aerobic bacteria and induced the expression of these enzymes.
Blood biomarkers are vital pathological, physiological and toxicological indices, and changes in the blood parameters are widely applied to analyse the nutritional and health status of animals (Zhou et al. 2001). Pathological or physiological changes in crucian carp due to outside influences are inevitably reflected in blood indicators. AST and ALT are indicators of a healthy status of livers. Hepatocyte membrane permeability increases when the liver is damaged, and great quantities of AST and ALT transfer to the blood, thus reducing the functions of AST and ALT in the liver (Yang et al. 2014). Results indicated that oral astaxanthin administration decreased the activities of AST and ALT in the blood of the crucian carp. This finding can be due to the antioxidant and immunomodulatory activities of astaxanthin (Eren et al. 2019; Sifi et al. 2016). A system with antioxidant capacity can scavenge excess free radicals and prevent itself from oxidative damage. SOD and CAT are important indices for the evaluation of the antioxidant activities in animals (Rahman et al. 2006). Results indicated that the serum SOD and CAT activities of crucian carp fed with astaxanthin-containing diets significantly increased. Hence, oral astaxanthin administration can increase the antioxidant capacity of the crucian carp. ACP and AKP are vital metabolic regulatory enzymes in organisms and participate in the transfer reaction of phosphoric groups and metabolism of phosphorus. These enzymes play a key role in the nutrient utilisation of aquatic animals and ameliorate their disease resistance (Zhang et al. 2011). Results indicated that astaxanthin can improve the nutrient utilisation of crucian carps, increase protein biosynthesis and promote crucian carp growth. LYZ is an important innate specific immune marker of animals and can strongly kill Gram-positive bacteria (Mck and Peters 1990). The antibacterial effect of LYZ in animals is directly related to the immune and health status of fish (Li et al., 2013). The LYZ activity in the serum of crucian carp increased significantly compared with that of the control group. Fish complements can lyse and opsonise foreign cells (Holland and Lambris 2002), whereas C3 and C4 play an important role in the activation reaction of the complement system (Kuroda et al. 2000). Serum C3 and C4 levels were significantly increased with astaxanthin doses increasing up to 400 mg per kg diet, but higher astaxanthin doses did not further increase the serum C3 and C4 levels. Results indicated that an appropriate astaxanthin supplement can increase the innate immunity of crucian carp.
The inflammatory cytokines found in fish can be divided into species, namely, anti-inflammatory cytokines (such as IL-10) and proinflammatory cytokines, (such as TNF-α, IL-1β and IL-8). The inflammation of fish can be reduced via the upregulation of anti-inflammatory cytokines and the downregulation of proinflammatory cytokines (Chen et al. 2015; Costa et al. 2011; Ottinger et al. 2016). In this study, the oral astaxanthin administration increased the IL-10 level and decreased the IL-1β and IL-8 levels and TNF-α mRNA levels, which indicated that oral astaxanthin administration can attenuate the inflammation in crucian carp.
During the challenge test against A. hydrophila, the oral astaxanthin administration groups showed significantly less cumulative mortality compared with the control group, and this result can be due to the immunomodulatory activity of astaxanthin (Sifi et al. 2016).
In conclusion, the groups administered with oral astaxanthin had increased BVGR; FCR; intestinal digestive protease, lipase and amylase levels; serum SOD, CAT, ACP, AKP and LYZ activities; C3 and C4 levels; IL-10 and resistance to Aeromonas hydrophilia and reduced serum AST and AST activities and TNF-α and IL-1β levels compared with those of the control group. On the basis of the efficiency of oral astaxanthin administration in the promotion of the growth performance of crucian carp, the optimal dose of astaxanthin was 400 mg/kg. Results indicated that astaxanthin can be used as an immunostimulant for the enhancement of the growth and immunity of the crucian carp.
References
Cheng YX, Wu SJ (2019) Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia. Aquaculture. https://doi.org/10.1016/j.aquaculture.2019.734341
Cheng CH, Guo ZX, Ye CX et al (2018) Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol Biochem 44:209–218
Chen MH, Sun YY, Kong CM et al (2017) Effect of dietary phosphorus levels on growth and body composition of crucian carp, Carassius auratus under indoor and outdoor experiments. Aquac Nutr 23:702–709
Chen L, Feng L, Jiang WD et al (2015) Dietary riboflavin deficiency decreases immunity and antioxidant capacity, and changes tight junction proteins and related signaling molecules mRNA expression in the gills of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 45:307–320
Costa MM, Maehr T, Diaz-Rosales P et al (2011) Bioactivity studies of rainbow trout (Oncorhynchus mykiss) interleukin-6: effects on macrophage growth and antimicrobial peptide gene expression. Mol Immunol 48:1903–1916
Eren B, Tanrıverdi ST, Köse FA et al (2019) Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J Cosmet Dermatol 18:242–250
Gao Y, He J, He ZL et al (2017) Effects of fulvic acid on growth performance and intestinal health of juvenile loach Paramisgurnus dabryanus (Sauvage). Fish Shellfish Immunol 62:47–56
Holland MC, Lambris JD (2002) The complement system in teleosts. Fish Shellfish Immunol 12:399–420
Jiang J, Wu XY, Zhou XQ et al (2016) Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture 463:174–180
Kumar R, Salwe K, Kumarappan M (2017) Evaluation of antioxidant, hypolipidemic, and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn Res 9:161–167
Kuroda N, Naruse K, Shima A et al (2000) Molecular cloning and linkage analysis of complement C3 and C4 genes of the Japanese medaka fish. Immunogenetics 51:117–128
Liu X, Wang B, Li Y et al (2018) Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, litopenaeus vannamei in the nursery phase. Invert Surv J 15:131–140
Li H, Zhang TE, Li Q (2013) Influence of compound Chinese herbal medicine on nonspecific immunity of turbot Scophthalmus maximus. J Dalian Ocean Univ 28:115–120
Martínez JM, Gojkovic Z, Ferro L et al (2019) Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga haematococcus pluvialis. Bioresource Technol 289:121694
Mck A, Peters G (1990) Lysozyme activity in rainbow trout, Oncorhynchus mykiss (Walbaum), stressed by handling, transport and waterpollution. J Fish Biol 37:873–885
Ottinger CA, Densmore CL, Robertson LS et al (2016) Transforming growth factor-b1 expression in endangered age-0 shortnose suckers (Chasmistes brevirostris) from Upper Klamath Lake, OR relative to histopathology, meristic, spatial, and temporal data. Fish Shellfish Immunol 49:1–6
Ohno M, Darwish WS, Ikenaka Y et al (2011) Astaxanthin can alter CYP1A-dependent activities via two different mechanisms: Induction of protein expression and inhibition of NADPH P450 reductase dependent electron transfer. Food Chem Toxicol 49:1285–1291
Palma J, Andrade JP, Bureau DP (2017) The impact of dietary supplementation with astaxanthin on egg quality and growth of long snout seahorse (Hippocampus guttulatus) juveniles. Aquac Nutr 23:304–312
Romano N, Koh CB, Ng WK (2015) Dietary microencapsulated organic acids blend enhances growth, phosphorus utilization, immune response, hepatopancreatic integrity and resistance against Vibrio harveyi in Pacific white shrimps, Litopenaeus vannamei. Aquaculture 435:228–236
Rahman MM, Escobedo-Bonilla CM, Corteel M et al (2006) Effect of high water temperature (33 °C) on the clinical and virological outcome of experimental infections with white spot syndrome virus (WSSV) in specific pathogenfree (SPF) Litopenaeus vannamei. Aquaculture 261:842–849
Shi X, Luo Z, Chen F et al (2017a) Effect of fish meal replacement by Chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquac Res 48:3244–3256
Shi X, Luo Z, Chen F et al (2017b) Effects of dietary cellulase addition on growth performance, nutrient digestibility and digestive enzyme activities of juvenile crucian carp Carassius auratus. Aquac Nutr 23:618–628
Sifi N, Martin-Eauclaire MF, Laraba-Djebari F (2016) K+ channel blocker-induced neuroinflammatory response and neurological disorders: immunomodulatory effects of astaxanthin. Inflamm Res 65:623–634
Tang XL, Fu JH, Li ZH et al (2016) Effects of a dietary administration of purple coneflower (Echinacea purpurea) on growth, antioxidant activities and 8 miRNAs expressions in crucian carp (Carassius auratus). Aquac Res 47:1631–1638
Wu SJ (2020) Dietary Astragalus membranaceus polysaccharide ameliorates the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). Int J Biol Macromol 149:877–881
Wang W, Ishikawa M, Koshio S et al (2018) Effects of dietary astaxanthin supplementation on survival, growth and stress resistance in larval and post-larval kuruma shrimp, Marsupenaeus japonicus. Aquac Res 49:2225–2232
Wade NM, Cheers S, Bourne N et al (2017) Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the Giant Tiger Shrimp, Penaeus monodon. Aquac Res 48:395–406
Wang JJ, Chen ZQ, Lu WQ (2012) Hypoglycemic effect of astaxanthin from shrimp waste in alloxan-induced diabetic mice. Med Chem Res 21:2363–2367
Yang WW, Liu WB, Shen MF et al (2014) Effects of selenium on antioxidant enzymes and transaminases of liver in cadmium chronic toxic Tilapia nilotica. Chinese Vet Sci 29:40–44
Zahran E, Risha E, AbdelHamid F et al (2014) Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 38:149–157
Zhang HJ, Li ZJ, Zhang JS et al (2011) Changes in non-specific immune parameters and water quality in Pacific white leg shrimp Litopenaeus vannamei culture ponds. J Dalian Ocean Univ 26:356–361
Zhou X, Wang Y, Gu Q et al (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291:78–81
Zhou Y, Guo WC, Yang ZG et al (2001) Advances in the study of haemotological indices of fish. J Shanghai Fish Univ 10:163–165
Acknowledgements
This research was supported by A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
This study was approved by the ethics committee of Jiangsu Ocean University, China. All procedures were conducted in compliance with relevant laws and institutional guidelines.
Rights and permissions
About this article
Cite this article
Wu, S., Xu, B. Effect of dietary astaxanthin administration on the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). 3 Biotech 11, 151 (2021). https://doi.org/10.1007/s13205-021-02700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02700-3