Abstract
Rhizoremediation processes are based on plant-bacteria interactions and can be effectively used for cleaning many pollutants from the environment to overcome the constraints of individual phytoremediation. Here, 1 mM and 1.5 mM concentrations of 2,4-dinitrotoluene (2,4-DNT) degrading Pseudomonas putida (P. putida) strain KT.DNT and various growth stages of Nicotiana tabacum (N. tabacum) were initially assayed in in vitro tissue culture system and the best conditions for the association of plant-rhizobacterium were ascertained to remediation of the soil contaminated with 2,4-DNT. 5-days old N. tabacum plants inoculated with 2 × 106 cfu/mL bacterial inoculum for 3 weeks were preferred for rhizoremediation experiments as they showed a nearly threefold increase in the fresh and dry biomass in comparison to noninoculated ones. When these seedlings were planted either alone or together with P. putida KT2440 or P. putida KT.DNT in soils contaminated with 1 mM and 1.5 mM of 2,4-DNT, the maximum degradation rate of 98% and ~ 93% were determined at the end of 14 days by KT.DNT inoculated tobacco plants. Our results indicate that it would be advantageous to use the 2,4-DNT-degrading bacterium inoculated with N. tabacum plants to accelerate and enhance the cleanup of soil contaminated with 2,4-DNT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4-DNT, is a typical soil contaminant at ammunition manufacturing sites, derived mainly from the synthesis of the explosive 2,4,6-trinitrotoluene (TNT) but also from the production of polyurethane foams and dyes (Nishino et al. 2000; Serrano-González et al. 2018). This mutagenic and carcinogenic substance is listed on the Hazardous Air Pollutants List ruled by the U.S. Environmental Protection Agency (EPA) (Lent et al. 2012; Xu and Jing 2012) and also on Resource Conservation and Recovery Act (RCRA) Toxicity Characteristic Leaching Procedure (TCLP) organics list. According to EPA (2006), soils and wastes that contain 2,4-DNT are classified as RCRA characteristic hazardous waste and a special treatment will be needed, if they produce leachate comprising 2,4-DNT equal or above the TCLP threshold concentration (0.13 mg/L). The concentration of 2,4-DNT at firing points was determined above the detection limit (0.01 mg/kg (ppm)) and even higher at several propellant disposal sites (Jenkins et al. 2006; Walsh et al. 2007). Since its accumulation in soil and groundwater at military areas is a serious obstacle to be solved (Dodard et al. 1999; Xu and Jing 2012), remediation of 2,4-DNT-contaminated sites has received great interest in recent years to ensure a safe environment.
There are a few technologies that can be adapted for the treatment of soil and groundwater polluted with dinitrotoluenes (DNTs) such as photolysis with surfactants, alkaline hydrolysis and incineration. However, those technologies require labor force and are highly expensive (Kuiper et al. 2004; Ma et al. 2017). Therefore, using low-cost and environmentally friendly in situ approaches to the clean the 2,4-DNT contaminated media has become a priority. Although phytoremediation is an eco-friendly application for the cleaning of the environment contaminated with persistent pollutants (Khan et al. 2015), the presence of these contaminants including DNTs in soil and water negatively affects plant growth and phytoremediation efficiency (Arslan et al. 2015). Besides, plants have certain degradation limits due to their not only slow removal capabilities but also high sensitivity to oxidative stress and less knowledge about their phytoremediation mechanisms (Carvalho et al. 2014).
An alternative approach for the mineralization of recalcitrant nitroaromatic compounds is to use bacterial strains capable of the 2,4-DNT degradation (Aburto-Medina et al. 2017). Genetic characterization of the 2,4-DNT catabolic pathway of Burkholderia sp. strain DNT and Burkholderia cepacia R34 was reported in detail. They can catalyze the oxidation of 2,4‐DNT through a series of steps ultimately resulting in the release of nitrite, pyruvate and propionyl‐coenzyme A (CoA) (Johnson et al. 2002; Karthikeyan and Spain 2016; Wang et al. 2011). However, both strains are not preferred for biodegradation studies since they both slowly degrade the complex compounds and have opportunistic pathogen properties for humans and plants (Coenye and Vandamme 2003; Eberl and Vandamme 2016; Suen and Spain 1993). Thus, the introduction of the dnt gene cluster, which does not encode toxic products for most microorganisms, into the nonpathogenic strains has been the favored approach in many biodegradation studies rather than using these bacteria for the 2,4-DNT degradation (Akkaya and Arslan 2019; Akkaya et al. 2018; Dutta et al. 2003; Monti et al. 2005; Nasr et al. 2001; Patel et al. 2000).
However, in the absence of plants, although a considerable number of organic pollutants are catabolized by bacteria, this process is sometimes inefficient due to the relatively few microbial populations in bulk soil (Gkorezis et al. 2016). The combined use of plants and the associated bacteria enhances the role of each partner as the plant-rhizobacteria interactions increase the proliferation and degradation potential of applied bacteria through the rhizosphere environment of the growing plants. In return, inoculation of rhizobacteria enhances plant resistance to the pollutant stress and increases their adaptation rate and biomass formation (Correa-García et al. 2018; Khan et al. 2013).
Characteristics like its adaptable transport and metabolic systems and the ability to function as a chassis in biocatalysis and biodegradation make the soil bacterium P. putida KT2440 a model strain. The efficient biotransformation of 2,4-DNT in contaminated soil using P. putida KT.DNT, which has all necessary genes for 2,4-DNT degradation from Bulkholderia sp. R34, was previously reported by Akkaya and Arslan (2019). Thus, it has been hypothesized that this genetically stable rhizobacterium could be a good candidate for rhizoremediation. Due to the complex dynamics of plant-bacteria interactions in rhizophere, plant tissue culture allows the simple and controlled system for testing bacteria on plant performance, determination of the best inoculum concentration and inoculation time for culture utilization (Nowak 1998). Hence, the present study aims (i) to investigate the optimal bacterial inoculum density and the inoculation time of N. tabacum plants in in vitro conditions; (ii) to test the effect of high concentrations of 2,4-DNT on plant growth and development in this partnership and (iii) to analyze the biotransformation efficiency of 2,4-DNT-contaminated soils on the basis of pot assays using N. tabacum-P.putida interactions. Tobacco was chosen due to its previous reveal of nitroaromatic compound uptake (Hannink et al. 2007).
Materials and methods
Chemical products
2,4-DNT was ordered from Fluca (97%, Sigma-Aldrich Co. (St. Louis, USA)). The solvents and other chemicals used during this study were analytical grade and purchased from commercial sources as follows: MgSO4 (Sigma-Aldrich), acetonitrile (Merck Millipore).
GFP tagged P. putida KT.DNT
The construction process of GFP (Green Fluorescent Protein) tagged P. putida KT.DNT (Akkaya and Arslan 2019) cells and imaging bacteria by confocal laser scanning microscopy were the same as P. putida KT2440-G (GFP-labeled KT2440; Arslan and Akkaya 2020). Tobacco seedlings grown for 5 days in in vitro conditions were transferred to Petri dishes where GFP tagged KT.DNT (2 × 106 cfu/mL) was spreaded and then incubated for 14 days to visualize the colonization of the bacterium on plant roots.
Preparation of bacterial inoculum
P. putida KT.DNT cells were grown 16 h at 30 °C in M9 minimal medium (Abril et al. 1989) amended with citrate (0.4%, w/v). Then, cells were collected by centrifugation at 8000 rpm for 10 min, dissolved twice in MgSO4 (10 mM) and finally suspended at a density of 2 × 103–2 × 106–2 × 108 cfu/mL for the treatment with the N. tabacum seedlings.
Cultivation of plants and bacterial treatment
In vitro tissue culture experiments were conducted in a plant growth chamber to obtain the best inoculum density of P. putida KT.DNT and the best growth stage of tobacco plants for inoculation experiments. Then, the same inoculum density was used for the inoculation of the plants with P. putida KT2440 before soil experiments. Soil experiments were performed in a greenhouse with P. putida KT.DNT and wild-type P. putida KT2440 as a control to ascertain the biodegradation ability of root-associated KT.DNT in 2,4-DNT-polluted soil.
Seeds of the N. tabacum (Nicotiana tabacum cv Samsun) were sterilized by a sterilization protocol by which modified from Kavas et al. (2016). First, tobacco seeds were submerged for 20 min in 30% sodium hypochlorite, then washed three times for 5 min in sterile dH2O. Subsequently, the seeds were planted on 10 square Petri dishes (8 seeds/Petri plate) with half-strength MS agar (0.8%, Murashige and Skoog, Duchefa) and placed vertically into plant growth chamber (at 22 ± 2 °C, 70% humidity, 300 μmol m−2s−1 fluorescent light) with a photoperiod of 16/8 h.
After 5-, 10- 14-days, plantlets were transferred to new Petri dishes with or without P. putida KT.DNT. Different suspensions of P. putida KT.DNT (2 × 103–2 × 106–2 × 108 cfu/mL) were spreaded on Petri dishes and tested. Then, plates continued to be cultured in a plant growth chamber, and at day 21, tobacco plants were harvested to assay the effect of bacteria on plant growth properties.
Measurement of growth parameters
The morphologic data including root length (RL), shoot length (SL), fresh and dry root weights (RFW, RDW), fresh and dry shoot weights (SFW, SDW), total fresh and dry weights (FW, DW), chlorophyll (chl a, chl b), and carotenoid content were measured on each of the individual 5-, 10-, 14-days old plantlets according to the procedure reported by Akkaya and Arslan (2019). To obtain the dry weights of the whole plant, root and shoot systems, each plant was dried in the oven at 65 °C for 24 h. Leaf chlorophyll concentration was detected by extraction of chlorophyll in acetone for 24 h at 4 °C in dark followed by measurements in a spectrophotometer (Lichtenthaler and Buschmann 2001).
Experimental design for soil trials
Two weeks after seedling, plantlets were transplanted into the soil in pots. Pot trial experiments were done to obtain the efficiency of the plant-bacteria association to remove 2,4-DNT from the nonsterile rhizospheric soil of plant N. tabacum and compared with the degradation in the soil which has P. putida (KT2440 or KT.DNT) inoculated (2 × 106 cfu/mL) or non-inoculated N. tabacum. Experiments were conducted in a controlled greenhouse (under 16/8 h light/dark periods and corresponding 27°/20 °C thermoperiods) at Gebze Technical University, Department of Molecular Biology and Genetics to determine the effects of bacterial treatment on plant growth and 2,4-DNT degradation. Prior to use the nonsterile soil [matured manure, sand, peat and perlite (1:1:1:1)] was air-dried, homogenized through a 30 mesh sieve. Then the 21-day-old plant seedlings were carefully removed from the MS agar and plants were placed in pots (Round black, 70 mm deep × 65 mm diameter) of different sets, which were filled with 140 g of soil. Soil water content was adjusted to a 60% capacity in all pots of different sets: N. tabacum (Nt); 2,4-DNT + N. tabacum (Nt); 2,4-DNT + KT2440 + Nt; 2,4-DNT + KT.DNT + Nt. Two different 2,4-DNT concentrations (1 mM and 1.5 mM) and 2x106 cfu/mL of bacteria (P. putida KT2440 and KT.DNT) were used for pot experiments. 0.5 M of 2,4-DNT stock solution was diluted into the dH2O added to the soils to get final concentrations of 1 mM or 1.5 mM and mixed thoroughly before diving to the pots. The pots were kept covered until seedlings were placed into soil. During the first 5 days, seedlings were covered with plastic bags to avoid dehydration. They were incubated in greenhouse conditions at 22 °C and daily irrigated with 15 ml of water to compensate for water loss. The growth parameters (SL, SFW, and SDW) of inoculated and control plants were measured and compared at the end of the 14th day. The experiment was designed with one plant per pot with eight replicates and it was repeated for twice. 0, 6, 10, 14 and 21-days after transplanting, 1 gr of soil samples in the rhizospheric ecosystem were collected to determine if the 2,4-DNT reduction by the roots-attached bacteria using HPLC (high-performance liquid chromatography) and 2 gr soil sample at day 14 was taken to analyze the survival of bacteria in the soil.
Determination of tolerance index
The effect of 2,4-DNT on growth parameters (SL, SFW and SDW) was evaluated using a tolerance index (TI) which is a percentage of treated plants to the untreated ones and calculated according to the following formula: TI = (2,4-DNT treated plants/untreated plants) × 100 as described by (Balint et al. 2002). Leaf area index was also measured by Adobe Photoshop 5.0 Software.
Microbial enumeration
P. putida KT.DNT grown on M9 medium were recognized according to their colony morphology and GFP signal under the blue light. The bacterial (P. putida KT2440 and P. putida KT.DNT) colony number (cfu g−1) was calculated before the inoculation of seedlings into soil and 14-days after the inoculation. Soil (2 g) was suspended in 5 mL of 10 mM MgSO4 and bacterial dilutions were prepared according to Akkaya and Arslan (2019) and then they were plated on minimal medium (M9) with Km for selecting GFP tagged KT.DNT colonies. The number of P. putida colonies/g was calculated. Attachment of KT.DNT to roots was also demonstrated by confocal microscopy.
Quantitative analysis of biotransformation of 2,4-DNT in soil
To clarify the effect of bioavailability of the contaminant on degradation efficiency of root-associated P. putida KT.DNT in soil, two different concentrations of 2,4-DNT were examined in the present study. The total residual amount of the 2,4-DNT in soil was measured by HPLC (Shimadzu, Colombia, MD). Soil samples on days 0, 6, 10, and 14 were taken and were extracted according to Method 8330 (Jenkins and Walsh 1991; Walsh et al. 2007) with slight modifications (Akkaya and Arslan 2019). Each analysis was repeated three times with the samples taken from each pot and the results were given as the averages of three values. The supernatant was collected and filtered by 0.45 μm Millipore membrane before separation by Shimadzu Inertsil ODS-3 V column with UV–VIS detector (λ = 254 nm) using a flow rate of 0.5 mL/min and a mobile phase of 80:20 acetonitrile–water at 30 °C. Unknown concentrations were computed from the calibration curve of the prepared standards.
Statistical analysis
All treatments were performed in this work are mean ± SE (Standard Error) of a minimum of eight replicates. To test the significance of experiments, a one-way ANOVA with post hoc test for multiple comparisons was performed with GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA). Differences between groups proved to be significant at a P value of ≤ 0.05.
Results
Evaluation of the effects of bacterial inoculation on the plant in vitro growth characteristics
The influences of the various concentrations (2 × 103–2 × 106–2 × 108 cfu/mL) of P. putida KT.DNT on plant growth characteristics were evaluated in the different developmental stage of tobacco seedlings (5, 10 and 14 days old), after in vitro seed germination. The beneficial effects of the bacterium upon plant characteristics and pigment contents were detected relative to control when treated to 5-days-old seedlings at a concentration of 2 × 106 cfu/mL and harvested at the end of the 14th day. The measured parameters including SFW, SDW, RFW, RDW, FW, DW were found to be increased more than half by 2.45-fold, 2.74-fold, 2.84-fold, 2.55-fold, 2.85-fold and 2.98-fold, respectively, compared with their controls (non-inoculated, NI) (Table 1). In all developmental stages of the tobacco, as the bacterial concentration was increased to 2 × 108 cfu/mL, total biomass was progressively inhibited as presented in Table 1. Inoculation of tobacco plants with KT.DNT at concentrations higher than 2 × 106 cfu/mL reduced the shoot and root weight, eventually leading to a 50% decline in whole plant weight. Moreover, after the seedlings were incubated longer with P. putida KT.DNT at 2 × 106 cfu/mL, twofold reduction in dry weight was observed for the 10th and 14th days.
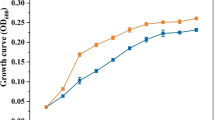
Furthermore, treatment of P. putida KT.DNT with the same concentration (2 × 106 cfu/mL) resulted in the greater accumulation of chlorophyll and carotenoid in the leaves of the N. tabacum seedlings (Fig. 1). After 5 days of treatment with 2 × 106 cfu/mL inoculum density, Chl a ranged from 0.49 to 0.67 mg/gFW, chl b ranged from 0.21 to 0.25 mg/gFW and the content of total carotenoid ranged from 0.12 to 0.14 mg/gFW in comparison to controls. Therefore, further research for 2,4-DNT degradation was conducted using 2 × 106 cfu/mL concentration of P. putida inoculum.
Effect of three inoculum concentrations (2 × 103–2 × 108 cfu/ml) of P. putida KT.DNT on the chlorophyll a (a), chlorophyll b (b) and carotenoid (c) contents of tobacco seedlings under in vitro tissue culture conditions. On day 21, carotenoid and chlorophyll contents were taken for 5, 10, 14-days-old tobacco seedlings inoculated with 2 × 103–2 × 108 cfu of the bacterium. Chlorophyll contents were recorded using green leaves
For this purpose, plants inoculated with the 2 × 106 cfu/mL of P. putida KT.DNT and P. putida KT2440 before soil trials grew to a significantly greater extent than plants that were not treated with the bacterial inoculum (Fig. 2a, b). Moreover, inoculation of plants with 2 × 106 cfu/mL concentration of the bacteria resulted in a decline in primary root lengths for KT2440 and KT.DNT-inoculated plants (compare 7.2 to 3.4 and 7.2 to 2.9, respectively) and a ~ 1.5-fold increase in shoot length was detected as a result of treatment with 2 × 106 cfu/mL of P. putida KT2440 or P. putida KT.DNT inoculation in comparison to N. tabacum plants (Fig. 2b).
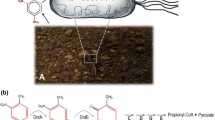
Inoculation with bacteria decreased primary root length and increased lateral roots and root hairs. Vertical agar plate with 21-days-old non-inoculated plants (left) and inoculated plants with 2 × 106 cfu/ml of P. putida KT.DNT (right) (a), Primary root length and shoot length of non-inoculated plants (Nt), KT2440 inoculated plants (Nt-KT) and KT.DNT inoculated plants (Nt_KT.DNT) at day 21 (b), Photograph of confocal microscopic image of fluorescent gfp-tagged Pseudomonas putida KT.DNT colonizing the root surface of a 14-day-old seedling of N. tabacum (c)
Detection of rhizospheric localization of P. putida in N. tabacum
Presence of root-attached bacteria was verified by examining the bacteria-root interaction under confocal microscopy. Thus, GFP-tagged KT.DNT promoted the following bacterial localization on plant roots. Successive transformation of the plasmid pSEVA237-G into P. putida KT.DNT was verified by PCR detection of the gfp gene (data not shown) and colonization of the bacteria onto plant roots was approved by confocal microscopy (Carl Zeiss, Jena, Germany). Green fluorescence was visualized around the roots inoculated with the bacteria (Fig. 2c) but not in non-inoculated plants (data not shown).
Impact of the 2,4-DNT treatment on the morphology of seedlings and tolerance index
Two different 2.4-DNT concentrations (1 mM and 1.5 mM) were chosen to determine the reduction efficiency of root-associated KT.DNT in degradation experiments. At higher concentration of 2,4-DNT, SDW is a more sensitive factor to 2,4-DNT treatment than SFW and SL (Fig. 3a, b). Furthermore, the toxicity of 2,4-DNT with a concentration of 1.5 mM was observed by stunted development of shoots and leaves in non-inoculated or KT2440-inoculated N. tabacum plants (Fig. 3b and Supplementary Fig. SF1). The plant results indicated that in the presence of 1 mM 2,4-DNT in soil, KT.DNT was able to increase shoot length by 1.7-fold, and fresh and dry shoot weights by more than twofold (Fig. 3a). In the case where 2,4-DNT concentration is 1.5 mM, KT.DNT inoculated plants showed maximum shoot biomass production with an increase in 6.5-fold and 7.2-fold for SFW and SDW, respectively, as well as 3.1-fold increase in SL compared to non-inoculated 2,4-DNT exposed plants (Fig. 3b). Moreover, the leaf area increased in the presence of 1.5 mM concentration of 2,4-DNT by eightfold as compared to non-inoculated tobacco plants (Supplementary Fig. SF1).
The growth parameters (SL, SFW, and SDW) of KT2440-inoculated, KT.DNT-inoculated and non-inoculated tobacco seedlings exposed to 1 mM (a) and 1.5 mM (b) of 2,4-DNT were measured and compared at the end of the 14th day. The experiment was designed with one plant per pot with eight replicates and it was repeated twice. Different lowercase letters above the bars indicate significant differences (P ≤ 0.05)
The calculation of the SL, SW and SDW tolerance index (TI) revealed a high degree of performance for KT.DNT-inoculated tobacco plants. Plants inoculated with KT.DNT in soil containing 1 mM 2,4-DNT appear to be more tolerant as means of SL, SW and SDW (132%, 222% and 370%, respectively) whereas plants inoculated with KT.DNT in soil containing 1.5 mM 2,4-DNT were the most sensitive for SL, SW and SDW (78%, 120% and 113%, respectively). Noninoculated and KT2440-inoculated plants showed nearly the same behavior for both in concentrations of 2,4-DNT (Fig. 4).
Shoot length, shoot weight and shoot dry weight for untreated and 2,4-DNT-treated seedlings after 14 days of exposure to 2,4-DNT. Grey bars are for non-inoculated N. tabacum (Nt) plants, dark grey bars are for KT2440-inoculated Nt and black bars for KT.DNT-inoculated Nt. The results showed average of the measurements made on 8 individual seedlings, and the shoot length tolerance index (shoot length of 2,4-DNT-treated seedlings/shoot length of untreated control ×100) (a), shoot weight tolerance index (shoot weight of 2,4-DNT-treated seedlings/shoot weight of untreated control ×100) (b) and shoot dry weight index (shoot dry weight of 2,4-DNT-treated seedlings/shoot dry weight of untreated control ×100) (c) were calculated for untreated and treated seedlings at each 2,4-DNT concentration (1 mM or 1.5 mM) tested. ‘*’ indicate differences among the Nt_KT.DNT and Nt_KT as well as Nt
Bacterial survival in soil
After 3 weeks, the survival of P. putida cells (P. putida KT2440 and P. putida KT.DNT) in the rhizosphere of plant was 2 × 106 cfu/g soil and 1 × 106 cfu/g soil contaminated with 1 mM 2,4-DNT and 1.5 mM 2,4-DNT, respectively.
Reduction of 2,4-DNT from contaminated soil
Strain KT.DNT showed almost complete biotransformation for 1 mM and 1.5 mM concentration of 2,4-DNT (98.03% and 92.9%, respectively) in 14 days (Fig. 5). When the experiments were carried out with P. putida KT2440-inoculated or non-inculated tobacco plants, a slight decrease (1%) was detected in the rate of 2,4-DNT concentration in soil. HPLC results revealed that degradation occurs more rapidly in the first 6 days (more than 80% of both initial concentrations of 2,4-DNT) during 14 days of incubation. Especially after 6 days, the degradation rate of 2,4-DNT was slower in soil contaminated with 1.5 mM 2,4-DNT than in soil contaminated with 1 mM 2,4-DNT (Supplementary Fig. SF2). No intermediates accumulated due to the faster degradation rate was detected. Moreover, when the incubation time was extended for another week, approximately the same degradation rates were found (data not shown).
2,4-DNT degradation in the contaminated soil with 1 mM (a) and 1.5 mM (b) of 2,4-DNT. Non-inoculated (Nt), KT2440-inoculated (Nt_KT) and KT.DNT-inoculated (Nt_KT.DNT) 21-d-old plants transferred to contaminated soil and 14 days after inoculation, extracted soil samples were analyzed for 2,4-DNT reduction
Discussion
Harnessing plants and pollutant degrading bacteria beneficial associations in the field of remediation contaminated soil has a great biotechnological potential to clean-up polluted environments (Afzal et al. 2012; Gkorezis et al. 2016; Ma et al. 2011). Although several studies were conducted to understand the importance of rhizobacteria-plant interactions in enhancing soil remediation, usage of the engineered bacterium associated with plants to remove nitroaromatics polluted soils were rarely investigated (Dutta et al. 2003; Monti et al. 2005; Thijs et al. 2014). P. putida is referred to as safe strain for in situ bioremediation by Nelson et al. (2002) and previously constructed its 2,4-DNT degrading strain, KT.DNT, showed higher degradation capacity in 2,4-DNT contaminated soil (Akkaya and Arslan 2019). Several studies have reported the enhanced degradation ability of rhizobacteria in polluted environments when it is combined with plants (Arslan et al. 2015; Gkorezis et al. 2016). Hence, in the present study, KT.DNT was employed for rhizoremediation of 2,4-DNT contaminated soil in association with tobacco. It has already been known that the density of bacterial inoculum and developmental stage of plants affect the metabolic status and activity of the inoculated bacteria during the phytoremediation of the polluted soil (Shabir et al. 2016). Additionally, plant tissue culture systems for studying plant-bacteria interactions allow more easily controlled conditions than soil, especially with regard to testing single factors (Nowak 1998). The importance of timing and level of inoculation with rhizosphere bacteria on plants has been noted by Bashan (1986). Therefore, different concentrations of the bacteria (2 × 103–2 × 108 cfu/mL) at different time intervals were tested for the determination of the best inoculation density and time to inoculate tobacco plants in tissue culture conditions and apparently, 2 × 106 cfu of KT.DNT applied to plants, significantly enhanced shoot, root and total plant biomass together with increased shoot lengths. It was also reported that inoculation with a lower concentration of KT2440 strain (2 × 103 cfu/mL) resulted in the production of more plant biomass (A.thaliana) as compared to inoculum with higher cell density (Arslan and Akkaya 2020). On the other hand, in this study, inoculation of other dicot N. tabacum with higher inoculum concentration of the P. putida KT.DNT led to more plant growth promotion in in vitro tissue culture conditions. Therefore, optimization of inoculum density for each plant is an important parameter for evaluating the effects of bacteria. The increase in total chlorophyll and carotenoid contents recorded in plants inoculated with the same concentration of the bacterium could be explained by their increased leaf area which contributes to more photosynthesis, and hence more dry matter accumulation. It was known that the root hairs of P. putida KT2440 inoculated plants were significantly shorter and denser than the control plants (Arslan and Akkaya 2020; Molina et al. 2006). Dense root hairs and lateral roots that are colonized by P. putida KT.DNT can stimulate the degradation of 2,4-DNT in soil. In addition, KT.DNT strain showed the same positive effects on the growth of the tobacco plants under in vitro conditions as the parental strain KT2440 and it indicates that integration of the dnt pathway in the genome did not create any negative burden on interaction with its host.
At the end of the degradation assay, SDW and DW tolerance index were significantly enhanced (7.2-fold and 4.8-fold, respectively) together with higher leaf area index than the control plants in contaminated soil with 1.5 mM 2,4-DNT by inoculation with KT.DNT as compared to non-inoculated plants. In accordance with the results, high abundance (1 × 106 cfu/g soil) of bacterial populations in the rhizosphere were successfully recovered 14 days after inoculation. Although this rate of 2,4-DNT has a toxic effect on bacterial growth in liquid culture (Spain 1995), it is noteworthy that bacteria attached to plant roots can still survive and degrade the 2,4-DNT-contaminated soil. Previous research showed that bioengineered KT.DNT reduced 97.1% of 0.5 mM of 2,4-DNT in the polluted soil (Akkaya and Arslan 2019). In this study, using the same inoculation time reported in the literature (Akkaya and Arslan 2019), KT.DNT cells showed higher reduction rate at higher 2,4-DNT concentrations with the association of tobacco. It was reported that released organic compounds by plants can serve as sources of carbon and energy for bacteria (Rohrbacher and St-Arnaud 2016). The increased degradation potential of KT.DNT in combination with tobacco can be due to the higher metabolic activity of KT.DNT in the plant rhizosphere than in bulk soil. (Kowalchuk et al. 2002; Kuiper et al. 2004; Segura et al. 2009). Furthermore, the chemical compounds could facilitate the separation of organic pollutants from the organic matter in the soil and make them more useful to bacteria as previously reported (Chaudhry et al. 2005; Gao et al. 2010).
The degradation of pollutants was substantially affected by bacterial survival and colonization in the rhizosphere was reported by Afzal et al. (2013). Enhanced plant biomass production by bacterial inoculation would increase plant tolerance to phytotoxic contaminants leading to the plant stress resulting in soil health recovery (Gurska et al. 2009; Monti et al. 2005). These genus of P. putida KT2440 have already been described as ability to tolerate different types of aromatic compounds (Jiménez et al. 2010). This may explain how P. putida KT2440 can survive in 2,4-DNT-contaminated soil. In addition, plants inoculated with P. putida KT.DNT have a higher tolerance to 2,4-DNT-contaminated soils (1 mM and 1.5 mM) than non-inoculated or KT2440-inoculated ones due to the powerful degradation capacity of KT.DNT.
Although there are some examples where plants are used alone for bioremediation of 2,4-DNT-contaminated soil, one of the major obstacles arising from the adoption of removing 2,4-DNT from polluted soil is due to the sensitivity of many plant species to 2,4-DNT, i.e. while some crop plants are sensitive to 1 mg/mL 2,4-DNT (Podlipná et al. 2015), others are generally sensitive to lower concentrations such as 0.5 mg/mL (Remans et al. 2012). Here, the maximum 2,4-DNT reduction (98.03%) was achieved with inoculated plants for 14 days in 1 mM of 2,4-DNT contaminated soil with no accumulation of intermediates because of the almost complete biotransformation of 2,4-DNT. The slightly (1%) initial decrease in the concentration of 2.4-DNT in boxes containing N. tabacum and N. tabacum plants inoculated with KT2440 probably caused by abiotic factors, such as photolysis of 2,4-DNT with sunlight or absorption in the container walls (Gong et al. 2016; Podlipná et al. 2015). The fact that the rate of biotransformation did not change when the incubation time is extended to 3 weeks may be due to the adsorption of the remaining 2,4-DNT by the adventitious root system in soil (Pandey and Souza-Alonso 2019).
Previously, Dutta et al. (2003) applied an engineered strain DHK1 carrying PJS1 DNT-biodegradative plasmid, which enables alfalfa plants to grow two folds greater than the parent strain in 0.14 mM 2,4-DNT contaminated soil at the end of the 6 weeks. However, in the present study, KT.DNT strain is capable of faster and higher reduction in the 2,4-DNT-contaminated soil (98.03% in 14 days) than the ones reported by Dutta et al. (2003). Since this strain has chromosomal integration of the genes encoding the 2,4-DNT catabolic pathway, it can overcome the limitation associated with degradation ineffectiveness due to plasmid instability (Timmis and Pieper 1999). Thus, this bacterium has been considered promising for rhizoremediation of contaminated environments due to the high metabolic capacity and safety.
During bacterial inoculation and biodegradation experiments, the usage of GFP tagged KT.DNT cells has provided easy monitoring of the viability and metabolic activity of bioengineering bacterium. To sum up, the incorporation of the engineered bacterium in rhizoremediation studies is an effective and low-cost application for sustained cleaning of 2,4-DNT-contaminated soil.
Conclusion
In bacteria assisted phytoremediation of pollutants, rhizobacteria that possess appropriate genes for the degradation of the contaminants allows to alleviate toxicity to the plant. Although there are some studies reporting 2,4-DNT degradation through bacterial-assisted phytoremediation, the efficiency of degradation still needs to be improved. In this study, it is shown that plant-associated 2,4-DNT-degrading bacterium has a great potential for rapid degradation of 2,4-DNT in the contaminated soil. The efficient, economic, and sustainable green remediation technology reported in this paper can be easily used for the degradation of the toxic compound in environmental soil.
References
Abril MA, Michan C, Timmis KN, Ramos JL (1989) Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol 171:6782–6790
Aburto-Medina A, Taha M, Shahsavari E and Ball AS (2017) Degradation of the dinitrotoluene isomers 2,4- and 2,6-DNT: appraising the role of microorganisms. In enhancing cleanup of environmental pollutants. In: Anjum NA, Gill SS, Tuteja N (ed). Springer, Berlin, pp 5–20
Afzal M, Yousaf S, Reichenauer TG, Sessitsch A (2012) The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int J Phytorem 14:35–47
Afzal M, Khan S, Iqbal S, Mirza MS, Khan QM (2013) Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int Biodeterior Biodegr 85:331–336
Akkaya Ö, Arslan E (2019) Biotransformation of 2,4-dinitrotoluene by the beneficial association of engineered Pseudomonas putida with Arabidopsis thaliana. 3 Biotech 9:408
Akkaya Ö, Pérez-Pantoja DR, Calles B, Nikel PI, de Lorenzo V (2018) The metabolic redox regime of Pseudomonas putida tunes its evolvability towards novel xenobiotic substrates. MBio 9:e01512–e01518
Arslan E, Akkaya Ö (2020) Biotization of Arabidopsis thaliana with Pseudomonas putida and assessment of its positive effect on in vitro growth. In Vitro Cell Dev Biol Plant 34:1–9
Arslan M, Imran A, Khan QM, Afzal A (2015) Plant-bacteria partnerships for the remediation of persistent organic pollutants. Environ Sci Pollut Res 24:4322–4336
Balint AF, Kovács G, Sutka J (2002) Copper tolerance of Aegilops, Triticum, Secale and Triticale seedlings and copper and iron contents in their shoots Acta Biol. Szeged. 46:77–78
Bashan Y (1986) Significance of timing and level of inoculation with rhizosphere bacteria on wheat plants. Soil Biol Biochem 18:297–301
Carvalho P, Basto MC, Almeida CM, Brix H (2014) A review of plant-pharmaceutical interactions: from uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ Sci Pollut Res 21:11729–11763
Chaudhry Q, Blom-Zandstra M, Gupta S, Joner EJ (2005) Utilizing the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut Res 12:34–48
Coenye T, Vandamme P (2003) Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol 5:719–729
Correa-García S, Pande P, Séguin A, St-Arnaud M, Yergeau E (2018) Rhizoremediation of petroleum hydrocarbons: a model system for plant microbiome manipulation. Microb Biotechnol 11:819–832
Dodard SG, Renoux AY, Hawari J, Ampleman G, Thiboutot S, Sunahara GI (1999) Ecotoxicity characterization of dinitrotoluenes and some of their reduced metabolites. Chemosphere 38:2071–2079
Dutta SK, Hollowell GP, Hashem FM, Kuykendall DL (2003) Enhanced bioremediation of soil containing 2,4-dinitrotoluene by a genetically modified Sinorhizobium meliloti. Soil Biol Biochem 35:667–675
Eberl L and Vandamme P (2016) Members of the genus Burkholderia: good and bad guys. F1000Res: 5
EPA (2006) Characteristics of hazardous waste toxicity characteristic. Code of Federal Regulations (CFR). CFR Section 261.24
Gao YZ, Ren LL, Ling WT, Gong SS, Sun BQ, Zhang Y (2010) Desorption of phenanthrene and pyrene in soils by root exudates. Bioresour Technol 101:1159–1165
Gkorezis P, Daghio M, Franzetti A, Van Hamme JD, Sillen W, Vangronsveld J (2016) The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: an environmental perspective. Front Microbiol 7:1836
Gong T, Liu R, Che Y, Xu X, Zhao F, Yu H, Song C, Liu Y, Yang C (2016) Engineering Pseudomonas putida KT2440 for simultaneous degradation of carbofuran and chlorpyrifos. Microb Biotechnol 9:792–800
Gurska J, Wang W, Gerhardt KE, Khalid AM, Isherwood DM, Huang XD, Glick BR, Greenberg BM (2009) Three year field test of a plant growth promoting rhizobacteria enhanced phytoremediation system at a land farm for treatment of hydrocarbon waste. Environ Sci Technol 43:4472–4479
Hannink NK et al (2007) Enhanced transformation of TNT by tobacco plants expressing a bacterial nitroreductase. Int J Phytoremed 9:385–401
Jenkins TF, Walsh ME (1991) Field screening method for 2,4-dinitrotoluene in soil (No. CRREL-SR-91-17). Cold Regions Research and Engineer
Jenkins TF, Hewitt AD, Grant CL, Thiboutot S, Ampleman G, Walsh ME, Ranney TA, Ramsey CA, Palazzo A, Pennington JC (2006) Identity and distribution of residues of energetic compounds at army live-fire training ranges. Chemosphere 63:1280–1290
Jiménez JI, Nogales J, García JL, Díaz E (2010). A genomic view of the catabolism of aromatic compounds in Pseudomonas. In handbook of hydrocarbon and lipid microbiology, In: Timmis KN (ed). Springer: Berlin, pp 1297– 1325
Johnson GR, Jain RK, Spain JC (2002) Origins of the 2,4-dinitrotoluene pathway. J Bacteriol 184:4219–4232
Karthikeyan S, Spain JC (2016) Biodegradation of 2,4-dinitroanisole (DNAN) by Nocardioides sp. JS1661 in water, soil and bioreactors. J Hazard Mater 312:37–44
Kavas M, Baloğlu MC, Yücel AM, Öktem HA (2016) Enhanced salt tolerance of transgenic tobacco expressing a wheat salt tolerance gene. Turk J Biol 40:727–735
Khan S, Afzal M, Iqbal S, Khan QM (2013) Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90:1317–1332
Khan MU, Sessitsch A, Harris M, Fatima K, Imran A, Arslan M et al (2015) Cr-resistant rhizo- and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front Plant Sci 5:755
Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg BJJ (2004) Rhizoremediation: a beneficial plant-microbe interaction. Mol Plant Microbe Interact 17:6–15
Lent EM, Crouse LCB, QuinnJr MJ, Wallace SM (2012) Assessment of the in vivo genotoxicity of isomers of dinitrotoluene using the alkaline comet and peripheral blood micronucleus assays. Mutat Res 742:54–60
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Prot Food Anal Chem 1:F4
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
Ma Z, Yang Y, Jiang Y, Xi B, Yang T, Peng X, Lian X, Yan K, Liu H (2017) Enhanced degradation of 2,4-dinitrotoluene in groundwater by persulfate activated using iron–carbon micro-electrolysis. Chem Eng J 311:183–190
Molina MA, Ramos JL, Espinosa-Urgel M (2006) A two-partner secretion system is involved in seed and root colonization and iron uptake by Pseudomonas putida KT2440. Environ Microbiol 8:639–647
Monti MR, Smania AM, Fabro G, Alvarez ME, Argarana CE (2005) Engineering Pseudomonas fluorescens for biodegradation of 2,4-dinitrotoluene. Appl Environ Microbiol 71:8864–8872
Nasr MA, Hwang K-W, Akbas M, Webster DA, Stark BC (2001) Effects of culture conditions on enhancement of 2,4-dinitrotoluene degradation by Burkholderia engineered with the Vitreoscilla hemoglobin gene. Biotechnol Prog 17:359–361
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Santos VAPM et al (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4:799–808
Nishino SF, Paoli G, Spain JC (2000) Aerobic degradation of dinitrotoluenes and pathway for bacterial degradation of 2,6-dinitrotoluene. Appl Environ Microbiol 66:2139–2147
Nowak J (1998) Benefits of in vitro “biotization” of plant tissue cultures with microbial inoculants. Vitro Cell Dev Biol Plant 34:122–130
Pandey VC, Souza-Alonso P (2019) Market opportunities: in sustainable phytoremediation. In phytomanagement of polluted sites. Elsevier, 51–82
Patel SM, Stark BC, Hwang K-W, Dikshit KL, Webster DA (2000) Cloning and expression of Vitreoscilla hemoglobin gene in Burkholderia sp. strain DNT for enhancement of 2,4-dinitrotoluene degradation. Biotechnol Prog 16:26–30
Podlipná R, Pospíšilová B, Vaněk T (2015) Biodegradation of 2,4-dinitrotoluene by different plant species. Ecotoxicol Environ Saf 112:54–59
Remans T, Thijs S, Truyens S, Weyens N, Schellingen K, Keunen E, Gielen H, Cuypers A, Vangronsveld J (2012) Understanding the development of roots exposed to contaminants and the potential of plant-associated bacteria for optimization of growth. Ann Bot 110:239–252
Rohrbacher F, St-Arnaud M (2016) Root exudation: the ecological driver of hydrocarbon rhizoremediation. Agronomy 6:19
Segura A, Rodríguez-Conde S, Ramos C, Ramos JL (2009) Bacterial responses and interactions with plants during rhizoremediation. Microb Biotechnol 4:452–464
Serrano-González MY, Chandra R, Castillo-Zacarias C, Robledo-Padilla F, Rostro-Alanis MDJ, Parra-Saldivar R (2018) Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction. Def Technol 14:151–164
Shabir G, Arslan M, Fatima K, Imran AMIN, Khan QM, Afzal M (2016) Effects of inoculum density on plant growth and hydrocarbon degradation. Pedosphere 26:774–778
Spain JC (1995) Biodegradation of nitroaromatic compounds. Annu Rev Microbiol 49:523–555
Suen WC, Spain JC (1993) Cloning and characterization of Pseudomonas sp. strain DNT genes for 2,4-dinitrotoluene degradation. J Bacteriol 175:1831–1837
Thijs S, Weyens N, Sillen W, Gkorezis P, Carleer R, Vangronsveld J (2014) Potential for plant growth promotion by a consortium of stress-tolerant 2,4-dinitrotoluene-degrading bacteria: isolation and characterization of a military soil. J Microbial Biotechnol 7:294–306
Timmis KN, Pieper DH (1999) Bacteria designed for bioremediation. Trends Biotechnol 17:201–204
Walsh ME, Collins CM, Ramsey CA, Douglas TA, Bailey RN, Walsh MR, Hewitt AD, Clausen JL (2007) Energetic residues on alaskan training ranges. US Army Engineer Research and Development Center, Cold Regions Research and Engineering Laboratory Report ERDC/CRREL TR-07-9. Hanover, NH
Wang ZY, Ye ZF, Zhang MH (2011) Bioremediation of 2,4-dinitrotoluene (2,4-DNT) in immobilized micro-organism biological filter. J Appl Microbiol 110:1476–1484
Xu J, Jing N (2012) Effects of 2,4-dinitrotoluene exposure on enzyme activity, energy reserves and condition factors in common carp (Cyprinus carpio). J Hazard Mater 203:299–307
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2020_2395_MOESM1_ESM.docx
Supplementary material 1 (DOCX 375 kb) Supplementary Fig. SF1 Effect of 1.5 mM concentration of 2,4-DNT on morphology of tobacco seedlings. The photographs were taken after 14th day (a) N. tabacum plant (non-exposed to 2,4-DNT) (b) N. tabacum plant exposed to 2,4-DNT, (c) KT2440-inoculated N. tabacum plant exposed to 2,4-DNT, and (d) KT.DNT-inoculated N. tabacum plant exposed to 2,4-DNT, respectively. Supplementary Fig. SF2 Calibration curve used in determination of 2,4-DNT by HPLC. Calibration curve was constructed in acetonitrile by preparing a series of concentrations of the 2,4-DNT (6.25, 3.13, 1.56, 0.78, and 0 μg/mL). The linear regression of the calibration curve produced an equation of (y = 84517x+5772.1), with a correlation coefficient of 0.9998 (R2) (a), Biotransformation of 2,4-DNT by P. putida KT.DNT on N. tabacum root surfaces. Consumption of 1.5 mM of 2,4-DNT by P. putida KT.DNT on days 6 (a, black), 14 (b, pink) and reduction of 1 mM of 2,4-DNT on days 6 (c, blue), 14 (d, green) were determined by HPLC analysis (b).
Rights and permissions
About this article
Cite this article
Akkaya, Ö. Nicotiana tabacum-associated bioengineered Pseudomonas putida can enhance rhizoremediation of soil containing 2,4-dinitrotoluene. 3 Biotech 10, 398 (2020). https://doi.org/10.1007/s13205-020-02395-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02395-y