Abstract
In the present study, we aimed to extract, purify, analyze monosaccharide composition of exopolysaccharide (EPS) produced by Halorubrum sp. TBZ112 (KCTC 4203 and IBRC-M 10773) and also to evaluate its possible antiproliferative activity against human gastric cancer (MKN-45) cell line and its biocompatibility effect on normal cells using human dermal fibroblast (HDF) cell line. Average molecular weight and monosaccharide composition were determined by high-pressure size exclusion chromatography (HPSEC) with multi-angle laser light scattering (MALLS) and high-pressure anion exchange chromatography (HPAEC), respectively. Fourier transform infrared (FTIR) spectroscopy was used for the partial characterization of the EPS. The EPS effect on the cell proliferation and viability of MKN-45 and HDF cells was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and trypan blue dye exclusion, respectively. Strain TBZ112 excreted 480 mg.l−1 of the EPS under optimal growth conditions. The EPS had a molecular weight of 5.052 kDa and was a heteropolysaccharide containing ten moieties mainly composed of mannose (19.95%), glucosamine (15.55%), galacturonic acid (15.43%), arabinose (12.24%), and glucuronic acid (12.05%). No significant difference of the EPS treatments on the proliferation activity of MKN-45 and HDF cells were observed (P > 0.05). For the first time, the EPS from Halorubrum sp. TBZ112, an extremely halophilic archaeon related to Halorubrum genus, was isolated and chemically characterized. The EPS from Halorubrum sp. TBZ112 possesses a relatively low molecular weight and might be applied as a biocompatible compound. More investigations are needed to determine other biological activities of the EPS along with further details of its chemical structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exopolysaccharides (EPSs) secreted by microorganisms are linear and branched polymers mainly composed of sugar residues which are linked by glycosidic bonds (Moscovici 2015; Hussain et al. 2017). In addition to sugar residues, the organic and inorganic components might be present in their structures such as acetate, succinate, phosphate, and sulphate (Sutherland 1994). The microbial EPSs derived from different origins have different structures, compositions, physical, and chemical characteristics leading to their unique properties (Moscovici 2015). Several previous studies have shown some beneficial effects of EPSs such as excellent biodegradability and biocompatibility (Ates 2015), nanodrug carriers (Banerjee and Bandopadhyay 2016), immunomodulatory and antioxidant properties (Sun et al. 2014; Hussain et al. 2017; Banerjee et al. 2018), anti-viral effects (Arena et al. 2009), and anti-tumor activities (Chen et al. 2013; Queiroz et al. 2017). These properties have resulted in application of EPSs in various fields like pharmaceutical, cosmeceutical, biomedical, and nutraceutical industries (Hussain et al. 2017). Among various microbial EPSs, the biocompatible and non-toxic EPSs can be used for medical and pharmaceutical applications.
On the basis of the above features for EPSs, there has been an increasing interest regarding biopolymers like EPSs for therapeutic and industrial uses. Although various EPSs derived from different origins have been identified in the recent years, nevertheless, assessing novel EPSs with improved characteristics, especially those produced under extreme conditions could be worthy of research (Mishra and Jha 2013).
The extremely halophilic archaea are microorganisms adapted to living at high salt concentrations (1.7 M Na+ or greater) (Oren 2002; Bowers et al. 2009). Various microorganisms like extremely halophilic archaea have been isolated from Urmia Lake (the largest saltwater lake in the Middle East and the second largest saline lake in the world) (Fazeli et al. 2006). Unfortunately, this lake is gradually drying and needs to be revived (its reserves need to be preserved and restored). Halorubrum sp. TBZ112 (KCTC 4203 and IBRC-M 10773) is an extremely halophilic archaeon isolated and identified recently from Urmia Lake and has been reported as a potent carotenoid pigments producer (Hamidi et al. 2017).
Considering the limited knowledge on the EPS production by Halorubrum genus and its related species, the aim of the present study was to isolate and chemically identify a new EPS produced by Halorubrum sp. TBZ112 and also to evaluate its possible antiproliferative effect on human gastric cancer (MKN-45) cell line and its biocompatibility effect on human dermal fibroblast (HDF) cell line as normal cells.
Materials and methods
Culture of Halorubrum sp. TBZ112
The Gram-negative, aerobic, and rod-shaped archaeon (Halorubrum sp. TBZ112) with circle, mucous, and red colonies was isolated from Urmia Lake (Northwest Iran), and used in this study. The phenotypic characterization and molecular identification of the isolate were done before (Hamidi et al. 2017). All culturing procedures were performed aseptically. Halorubrum sp. TBZ112 was cultured in modified marine broth medium containing (per liter) glucose, 10 g; MgCl2•7H2O, 5.9 g; MgSO4, 3.24 g; CaCl2, 1.8 g; KCl, 0.55 g; NaHCO3, 0.16 g; KBr, 0.08 g; SrCl2, 34.0 mg; H3BO3, 22.0 mg; Na2O3Si, 4.0 mg; NaF, 2.4 mg; NH4 NO3, 1.6 mg; Na2HPO4, 8.0 mg; peptone, 5 g and yeast extract 1 g under optimum conditions of temperature (32 °C), pH(8), and NaCl concentration (20%) as previously described (Hamidi et al. 2014).
EPS extraction and purification
The 7-day culture (at the logarithmic stage of growth) was used as the inoculum. This culture was inoculated at 10% (v/v) for our experiments and then incubated for 9 days using the shaking incubator at 32 °C and 150 rpm. At the next stage, the supernatant was separated from the culture by high-speed centrifugation (Sigma 3K30, USA) at 20,000×g for 1 h at 4 °C and then the supernatant was collected in a sterile bottle. The cell-free supernatant containing the released EPS was precipitated by adding three volumes of absolute cold ethanol drop by drop with stirring and then incubated at 4 °C for overnight. Then the upper phase was removed and the precipitated phase was washed twice by adding cold absolute ethanol and centrifugation (3000×g, 12 min, 4 °C). The pellet was dissolved in deionized distilled water, the obtained solution was then removed from protein with Sevag method (Staub 1965) and dialyzed (cut off: 3.5 kDa) against deionized distilled water for 5 days at 4 °C. The dialyzed EPS solution was lyophilized (Freeze Dryer Christ Alpha 1–2/ LD Plus, Nemacka, Germany) at − 56 °C for 24 h, weighed, and stored at ambient temperature. Total carbohydrate of the EPS was measured by the phenol sulphuric acid method (DuBois et al. 1956) with glucose as standard. The uronic acid content was analyzed by the carbazole-borate test (Bitter and Muir 1962), and the protein content was determined by the Bradford method (Bradford 1976) with bovine serum albumin (BSA) as standard. In addition to the above-mentioned colorimetric methods, the contamination amount with nucleic acid and protein was monitored by measuring the OD at 260 and 280 nm using NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific Inc., USA).
Determination of molecular weight
According to the method previously described by (Goncalves Mdos et al. 2014) “average molecular weight and molecular weight distribution were determined by high-pressure size exclusion chromatography (HPSEC) with on-line multi-angle laser light scattering (MALLS) filled with a K5 cell (50 µl) and two detectors: a He–Ne laser (λ = 690 nm) and a differential refractive index (DRI). Columns [OHPAK SB-G guard column, OHPAK SB806, 804 and 803 HQ columns (Shodex)] were eluted with NaNO3 0.1 M at 0.7 ml/min. The solvent was filtered through 0.1-µm filter unit (Millipore), degassed and filtered through a 0.45-µm filter upstream column. The sample was injected at 5 g/l through a 100-µl full loop. The collected data were analyzed using the Astra 4.50 software package and a dn/dc of 0.15”. Calibration of SEC/ MALLS was obtained with Dextran standard (from 10 to 500 kDa).
Monosaccharide composition evaluation
The EPS (5 mg) was dissolved in 2 M TFA (1 ml) and heated at 120 °C for 90 min. It was then neutralized with 4 M NH4OH solution. According to the method previously described by Goncalves Mdos et al. (2014) “monosaccharide composition was evaluated by high-pressure anion exchange chromatography (HPAEC) on an ICS 3000 (Dionex, USA) equipped with pulsed amperometric detection and AS 50 autosampler. It was assembled with a guard CarboPac™ PA1-column (4 × 50 mm) and analytical CarboPacTMPA1-column (4 × 250 mm). Samples (10 mg/ml) were filtered using 0.2 µm membrane filter and the injection volume was fixed at 25 µl. Before each injection, columns were equilibrated by running buffer for 15 min with 18 mM NaOH. Samples were eluted isocratically with 18 mM NaOH for 30 min, followed by a linear gradient between 0 to 1 M sodium acetate in 200 mM NaOH for 20 min to elute acidic monosaccharides. Further characterization of EPS was followed by 15 min washing with 200 mM NaOH with the eluent flow rate of 1 ml/min. Columns were thermostated at 25 °C. Data were collected and analyzed with Dionex Chromeleon 6.80 software (Sunnyvale, USA)”.
Fourier transform infrared (FTIR) spectroscopy
FTIR is widely used for the partial characterization of polysaccharides. EPS from Halorubrum sp. TBZ112 was analyzed using a Perkin Elmer spectrum two FT-IR system (USA) and the characteristic absorption bands were assigned. The EPS was dispersed on the universal attenuated total reflectance (UATR). The IR spectra (50 scans) were recorded at room temperature (referenced against air) with the wave number range of 450–4000 cm−1. Spectra were analyzed with spectrum ES software.
Cell culture
Human poorly differentiated gastric cancer (MKN-45) cell line was used to evaluate the possible antiproliferative effect of the EPS extracted from Halorubrum sp. TBZ112. HDF cell line as normal cells was used to evaluate the possible side effects of the EPS (Gatea et al. 2017). These cell lines were originally obtained from Pasture Institute (Tehran, Iran). MKN45 cells and HDF cells were cultured in RPMI-1640 medium (Gibco, Karlsruhe, Germany) and Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Karlsruhe, Germany), respectively, at 37 °C in humidified air containing 5% CO2. The culture media were supplemented with 10% fetal bovine serum (FBS) (Gibco, Karlsruhe, Germany), 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich, Stockholm, Sweden). The cells were split for additional culture since the cell density was around 80%. The cells were regularly harvested by treating with trypsin–EDTA (0.25% trypsin, 1 mM EDTA.4Na) (Gibco, Karlsruhe, Germany).
Cell proliferation assay and trypan blue exclusion test
The effect of the EPS on the cell proliferation was assessed by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MKN-45 and HDF cells were seeded (10,000 cells/well) onto a 96-well plate. After 24 h of seeding, the EPS at four concentrations of 100, 250, 500, and 1000 µg/ml was added and incubated for 24 and 48 h separately. MTT solution (100 µl/well, 5 mg/ml) (Sigma–Aldrich, Stockholm, Sweden) was added to each well and then incubated for 4 h at 37 °C. After removing the medium, 100 µl of DMSO (Sigma–Aldrich, Stockholm, Sweden) was added in each well and the plate was shaken for 10 min at 100 rpm for dissolving the formazan crystals. The absorbance of each well was measured by an ELISA reader (Stat Fax 2100, Awareness, USA). The experiments were performed in triplicate. The results of each of the four EPS treatments were normalized with the control medium and relative proliferation activity was calculated as a percentage of the control (activity of control was considered as 100). Similarly, cell viability was assessed using trypan blue staining.
Statistical analyses
Data are presented as mean ± SD. To compare proliferation activity among different concentrations of the EPS, one-way ANOVA followed by the Tukey’s post hoc test was used. Paired t test was used to compare between 24 and 48 h in each concentration of the EPS. Significance difference was accepted at P < 0.05. Analysis was performed using SPSS software version 16.
Results
EPS production and the purity analysis
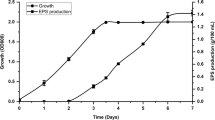
The yield of the EPS produced by Halorubrum sp. TBZ112 was around 480 mg.l−1 under optimal conditions for growth. The EPS was mainly composed of carbohydrate (70%) with uronic acid (8.3%), a low percentage of nucleic acid (2.3%) and protein (0.8%). OD at 260 and 280 nm confirmed the low amount of contamination with protein and nucleic acid. The remaining 18.6% of the EPS was not identified.
Molecular weight and monosaccharide composition analysis
The molecular mass of the purified EPS was approximately 5.052 kDa with an index of polydispersity estimated at 1.45 which corresponded with the heteropolysaccharides. The monosaccharide composition of the EPS is presented in Table 1. The extracted EPS was mainly composed of mannose, glucosamine, galacturonic acid, arabinose, and glucuronic acid.
FTIR
FTIR spectrum showed absorption bands at 3284.72 cm−1, 1635.37 cm−1, 1550.86 cm−1, 1405.86 −1 cm, and 1082 cm−1 (Fig. 1).
FTIR spectrum of the EPS isolated from Halorubrum sp.TBZ112 (KCTC 4203 and IBRC-M 10773). The band at 3284.72 cm−1 might be attributed to the stretching of hydroxyl group as well as water adsorption. The band at 1635 cm−1 was a result of the C=O stretching. The ether group from the EPS was indicated at 1082.09 cm−1 absorption band. The absence of symmetric strong band in the frequency of 1150 cm−1 and asymmetric strong band in the frequency of 1200 cm−1 (Pavia et al. 2008) demonstrated that the EPS from Halorubrum sp. TBZ112 most likely did not include S=O group
Cell proliferation assay and trypan blue exclusion test
There were no significant differences in the proliferation activity of MKN-45 cell line among four concentrations of EPS treatments after 24 and 48 h separately (Fig. 2a). Also, proliferation (survival) activity in the MKN-45 cell line did not differ significantly between 24 and 48 h under any of the matched EPS treatments (Fig. 2a). The proliferation activity of HDF cell line was similar in control condition and each of the four EPS concentrations after 24 and 48 h separately (Fig. 2b). The proliferation activity in the HDF cell line was not significantly different between 24 and 48 h under each of the matched EPS treatments (Fig. 2b). The similar results were obtained by cell viability evaluation using trypan blue staining (data not shown).
Discussion
For the first time, we have isolated and chemically characterized the EPS produced by Halorubrum sp. TBZ112. Some previous studies have purified the EPSs from extreme halophilic archaea belonging to Haloferax, Haloarcula, Halococcus, Natronococcus, Halobacterium, and Haloterrigena genera (Antón et al. 1988; Paramonov et al. 1998; Nicolaus et al. 1999; Squillaci et al. 2016; Lu et al. 2017), and no haloarcheon related to Halorubrum genus has been reported as an EPS producer. Our results showed that the amount of the EPS produced by Halorubrum sp. TBZ112 was higher than that of Haloarcula japonica strain T5, Haloarcula sp. strain T7, Haloarcula sp.strain T6 (Nicolaus et al. 1999), Haloterrigena turkmenica (Squillaci et al. 2016), and Haloarcula hispanica (Lu et al. 2017), but it was less than Haloferax mediterranei (Antón et al. 1988) (Table 2).
In the present study, the molecular weight of the EPS from Halorubrum sp. TBZ112 was estimated at around 5.052 kDa, as indicated by HPSEC-MALLS. Some previous studies illustrated that the average molecular weight of most microbial EPSs from extreme marine habitats was between 100 and 300 kDa (Poli et al. 2010). Squillaci et al. reported that the EPS produced by Haloterrigena turkmenica (an extremely halophilic archaeon) was principally consisted of two polymers, one of 801.7 and another of 206.0 kDa (Squillaci et al. 2016). A recent study showed that an extremely halophilic archaeon (Haloarcula hispanica ATCC33960) excreted a 1.1 × 103 kDa acidic EPS (Lü et al. 2017). Also, Haloferax mediterranei produced an EPS with molecular weight larger than 100 kDa (Antón et al. 1988). The EPS from Halomonas almeriensis M8T was composed of two polymers with molecular weight of 6.3 × 106 and 1.5 × 104 Daltons (Llamas et al. 2012). The EPS from strain TBZ112 possesses a relatively low molecular weight in comparison with the above described EPSs isolated from extreme marine habitats. So, in the present study, a better purification index (low proteins amount) and a better polydispersity index are indicating the presence of only one polysaccharide. It has been suggested that culture condition and physiological factors could affect the monosaccharide composition and the molecular weight of the EPSs (Finore et al. 2014; Delbarre-Ladrat et al. 2014).
The present study indicated that the carbohydrate composition of the EPS (type of monomer and molar ratio) synthesized by Halorubrum sp. TBZ112 was different from other reported EPSs from extremely halophilic archaea (Lu et al. 2017; Nicolaus et al. 1999; Paramonov et al. 1998; Parolis et al. 1996; Squillaci et al. 2016). It has been reported that most of the microbial EPSs are usually heteropolymers containing two to four or five sugar components (Bhaskar and Bhosle 2005; Lu et al. 2017; Sutherland 1990). However, the EPS isolated from Halorubrum sp. TBZ112 seems to be a heteropolysaccharide composed of ten moieties including neutral, acid and amino sugars. Since the good hydration ability of acid sugars, the existence of two acid sugars (glucuronic acid and galacturonic acid) in the EPS might be desirable for its applications in the cosmetic products (Thibodeau 2005).
Information regarding the possible antiproliferative effect of the EPS from extremely halophilic archaea is lacking. In our study, the EPS from Halorubrum sp. TBZ112 did not show significant change against the proliferation of MKN-45 cells. It has been documented that the potential properties of EPSs could be different partly because of their substituent functional groups contents (Wang et al. 2016). The existence of sulphate functional groups is associated with the potential bioactivities of EPSs. Indeed, it has been previously suggested that natural polymers modification as oversulphated could be essential for EPSs biological activities or could enhance their activities (Haroun-Bouhedja et al. 2000; Koyanagi et al. 2003; Ruiz–Ruiz et al. 2011). It is likely that oversulphation via changing negative charges might cause fine structural alterations or folding of the EPSs for interacting with the cell targets (Ruiz–Ruiz et al. 2011). Our finding was partially similar to that of Ruiz–Ruiz et al. study (Ruiz–Ruiz et al. 2011) in which the desulphated EPS B100 from the new halophilic bacterium Halomonas stenophila exhibited a minor antiproliferative impact on Jurkat T cells and HEL erythroleukaemia cells. It was concluded that this outcome might be moderately related to the absence of sulfur in the EPS structure. Another study (Koyanagi et al. 2003) showed that natural polysaccharide at low concentrations compared with oversulfated one was not effective in inhibiting the cell proliferation. Therefore, the role of degree of sulfation in the antiproliferative potency was suggested. So, our finding might be moderately related to the absence of sulphate functional groups in our EPS, as indicated by the FTIR analysis. Previous studies revealed that the antiproliferative property of a polysaccharide depended partly on its molecular size (Haroun-Bouhedja et al. 2000). So, another possible reason for the present finding might be attributed to the low molecular weight of our EPS. More relevant research is encouraged to determine the potential effect of the chemically modified like oversulphated EPS on different cancer cell lines along with its in vivo activity and cytological analysis. Also, according to the no cytotoxic effect of the EPS on normal cell line of HDF, it appears that the EPS could have good biocompatibility property (Rani et al. 2017; Saber-Samandari and Saber-Samandari 2017); however, further detailed investigations such as surface morphology analysis are needed.
Conclusion
In this study, for the first time, the EPS from Halorubrum sp. TBZ112, an extremely halophilic archaeon related to Halorubrum genus, was isolated and chemically characterized. The EPS from Halorubrum sp. TBZ112 might be applied as a biocompatible compound. Further studies exploring more details of its chemical structure such as the glycosyl linkages, the main repetitive units, and the putative branching sugars as well as its other biological activities are warranted.
References
Antón J, Meseguer I, Rodriguez-Valera F (1988) Production of an extracellular polysaccharide by Haloferax mediterranei. Appl Environ Microbiol 54:2381–2386
Arena A, Gugliandolo C, Stassi G, Pavone B, Iannello D, Bisignano G, Maugeri TL (2009) An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: antiviral activity on immunocompetent cells. Immunol Lett 123:132–137. https://doi.org/10.1016/j.imlet.2009.03.001
Arias S, Del Moral A, Ferrer MR, Tallon R, Quesada E, Bejar V (2003) Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 7:319–326. https://doi.org/10.1007/s00792-003-0325-8
Ates O (2015) Systems biology of microbial exopolysaccharides production. Front Bioeng Biotechnol 3:200. https://doi.org/10.3389/fbioe.2015.00200
Banerjee A, Bandopadhyay R (2016) Use of dextran nanoparticle: a paradigm shift in bacterial exopolysaccharide based biomedical applications. Int J Biol Macromol 87:295–301. https://doi.org/10.1016/j.ijbiomac.2016.02.059
Banerjee A, Rudra SG, Mazumder K, Nigam V, Bandopadhyay R (2018) Structural and functional properties of exopolysaccharide excreted by a novel Bacillus anthracis (Strain PFAB2) of hot spring origin. Indian J Microbiol 58:39–50. https://doi.org/10.1007/s12088-017-0699-4
Bernardet A, Bowman J (2015) Bergey’s manual of systematics of archaea and bacteria. John Wiley & Sons, New York
Bhaskar P, Bhosle NB (2005) Microbial extracellular polymeric substances in marine biogeochemical processes. Curr Sci 88:45–53
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334. https://doi.org/10.1016/0003-2697(62)90095-7
Bowers KJ, Mesbah NM, Wiegel J (2009) Biodiversity of poly-extremophilic bacteria: does combining the extremes of high salt, alkaline pH and elevated temperature approach a physico-chemical boundary for life? Saline Syst 5:9. https://doi.org/10.1186/1746-1448-5-9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chen YT, Yuan Q, Shan LT, Lin MA, Cheng DQ, Li CY (2013) Antitumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp. isolated from Ophiopogon japonicus. Oncol Lett 5:1787–1792. https://doi.org/10.3892/ol.2013.1284
Delbarre-Ladrat C, Sinquin C, Lebellenger L, Zykwinska A, Colliec-Jouault S (2014) Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front Chem 2:85
DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Fazeli MR, Tofighi H, Samadi N, Jamalifar H (2006) Effects of salinity on beta-carotene production by Dunaliella tertiolecta DCCBC26 isolated from the Urmia salt lake, north of Iran. Bioresour Technol 97:2453–2456. https://doi.org/10.1016/j.biortech.2005.10.037
Finore I, Di Donato P, Mastascusa V, Nicolaus B, Poli A (2014) Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar Drugs 12:3005–3024
Gatea F, Teodor ED, Seciu AM, Radu LE (2017) Monosaccharides composition and cytostatic activity of polysaccharide fraction of Phemeranthus Confertiflorus L. Farmacia 65:796–801
Goncalves Mdos S et al (2014) Anti-biofilm activity: a function of Klebsiella pneumoniae capsular polysaccharide PLoS One 9:e99995. https://doi.org/10.1371/journal.pone.0099995
Hamidi M, Nazemyieh H, Hejazi MA, Hejazi MS (2014) Optimization of cell growth and carotenoid production of Halorubrum sp. TBZ112 through statistical experimental methods. Iran J Public Health 43:242
Hamidi M, Hejazi MS, Nazemyieh H, Hejazi MA, Naziri D (2017) Halorubrum Sp. TBZ112, an extremely halophilic carotenoid-producing archaeon isolated from Urmia Lake Pharm Sci 23:150–158. https://doi.org/10.15171/PS.2017.22
Haroun-Bouhedja F, Ellouali M, Sinquin C, Boisson-Vidal C (2000) Relationship between sulfate groups and biological activities of fucans. Thromb Res 100:453–459. https://doi.org/10.1016/S0049-3848(00)00338-8
Hussain A, Zia KM, Tabasum S, Noreen A, Ali M, Iqbal R, Zuber M (2017) Blends and composites of exopolysaccharides; properties and applications: a review. Int J Biol Macromol 94:10–27. https://doi.org/10.1016/j.ijbiomac.2016.09.104
Koyanagi S, Tanigawa N, Nakagawa H, Soeda S, Shimeno H (2003) Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem Pharmacol 65:173–179. https://doi.org/10.1016/S0006-2952(02)01478-8
Llamas I, Amjres H, Mata JA, Quesada E, Béjar V (2012) The potential biotechnological applications of the exopolysaccharide produced by the Halophilic Bacterium Halomonas almeriensis. Molecules 17:7103. https://doi.org/10.3390/molecules17067103
Lu Y, Lu H, Wang S, Han J, Xiang H, Jin C (2017) An Acidic Exopolysaccharide from Haloarcula hispanica ATCC33960 and two genes responsible for its synthesis. Archaea 2017:5842958. https://doi.org/10.1155/2017/5842958
Mishra A, Jha B (2013) Microbial exopolysaccharides. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The Prokaryotes. Springer, Berlin, Heidelberg, pp 179–192
Moscovici M (2015) Present and future medical applications of microbial exopolysaccharides. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01012
Nicolaus B et al (1999) Haloarcula spp able to biosynthesize exo-and endopolymers. J Ind Microbiol Biotechnol 23:489–496. https://doi.org/10.1038/sj.jim.2900738
Oren A (2002) Halophilic microorganisms and their environments. vol 5. Springer Science & Business Media, Washington
Paramonov NA, Parolis LA, Parolis H, Boán IF, Antón J, Rodríguez-Valera F (1998) The structure of the exocellular polysaccharide produced by the Archaeon Haloferax gibbonsii (ATCC 33959). Carbohydr Res 309:89–94. https://doi.org/10.1016/S0008-6215(98)00102-5
Parolis H et al (1996) The structure of the exopolysaccharide produced by the halophilic Archaeon Haloferax mediterranei strain R4 (ATCC 33500). Carbohydr Res 295:147–156. https://doi.org/10.1016/S0008-6215(96)90134-2
Parolis LA, Parolis H, Paramonov NA, Boán IF, Antón J, Rodríguez-Valera F (1999) Structural studies on the acidic exopolysaccharide from Haloferax denitrificans ATCC 35960. Carbohydr Res 319:133–140. https://doi.org/10.1016/S0008-6215(99)00111-1
Pavia DL, Lampman GM, Kriz GS, Vyvyan JA (2008) Introduction to spectroscopy, 4 ed. Cengage Learning, New York
Poli A, Anzelmo G, Nicolaus B (2010) Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Mar Drugs 8:1779–1802. https://doi.org/10.3390/md8061779
Queiroz E et al (2017) Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int J Biol Macromol 102:565–570. https://doi.org/10.1016/j.ijbiomac.2017.04.035
Rani A, Baruah R, Goyal A (2017) Physicochemical, antioxidant and biocompatible properties of chondroitin sulphate isolated from chicken keel bone for potential biomedical applications. Carbohydr Polym 159:11–19. https://doi.org/10.1016/j.carbpol.2016.12.015
Ruiz-Ruiz C et al (2011) An exopolysaccharide produced by the novel halophilic bacterium Halomonas stenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl Microbiol Biotechnol 89:345–355. https://doi.org/10.1007/s00253-010-2886-7
Saber-Samandari S, Saber-Samandari S (2017) Biocompatible nanocomposite scaffolds based on copolymer-grafted chitosan for bone tissue engineering with drug delivery capability. Mater Sci Eng C Mater Biol Appl 75:721–732. https://doi.org/10.1016/j.msec.2017.02.112
Squillaci G et al (2016) Production and properties of an exopolysaccharide synthesized by the extreme halophilic archaeon Haloterrigena turkmenica. Appl Microbiol Biotechnol 100:613–623. https://doi.org/10.1007/s00253-015-6991-5
Staub A (1965) Removal of protein-Sevag method. In: Whistler RL (ed) Methods in carbohydrate chemistry, vol 5. Academic Press Inc., New York, pp 5–6
Sun ML et al (2014) A novel exopolysaccharide from deep-sea bacterium Zunongwangia profunda SM-A87: low-cost fermentation, moisture retention, and antioxidant activities. Appl Microbiol Biotechnol 98:7437–7445. https://doi.org/10.1007/s00253-014-5839-8
Sutherland IW (1990) Biotechnology of microbial exopolysaccharides. vol 9. Cambridge University Press, Cambridge
Sutherland IW (1994) Structure-function relationships in microbial exopolysaccharides. Biotechnol Adv 12:393–448. https://doi.org/10.1016/0734-9750(94)90018-3
Thibodeau A (2005) Protecting the skin from environmental stresses with an exopolysaccharide formulation. Cosmet Toilet 120:81–90
Wang J, Hu S, Nie S, Yu Q, Xie M (2016) Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev 2016:5692852. https://doi.org/10.1155/2016/5692852
Acknowledgements
This research was performed as an MSc thesis (Number 13) at School of Paramedicene, Guilan University of Medical Sciences (Rasht, Iran). This work was financially supported by the Research Deputy of Guilan University of Medical Sciences (Rasht, Iran).
Author information
Authors and Affiliations
Contributions
MH designed the research. RM, GP, CG, EP, and SF performed the research. M.H., RM, CD, KK, and FK wrote the paper. MH, CD, and KK performed analysis and interpretation of data. All authors discussed the results and participated in the manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hamidi, M., Mirzaei, R., Delattre, C. et al. Characterization of a new exopolysaccharide produced by Halorubrum sp. TBZ112 and evaluation of its anti-proliferative effect on gastric cancer cells. 3 Biotech 9, 1 (2019). https://doi.org/10.1007/s13205-018-1515-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1515-5