Abstract
The synthesis and characterization of an aggregate of AgNPs coated with plant extract (PE) from Alphonsea sclerocarpa and its significant antimicrobial activity and inhibition on K562 (blood cancer) cells have been appended in the article. Synthesis of aggregate [(AgNPs)-(PE)] has been followed by a facile eco-friendly approach without using any harmful chemicals. The morphology of an aggregate [(AgNPs)-(PE)] was confirmed by TEM and SEM microscopic characterizations. Properties like solid state, the presence of functional groups, and elemental composition have been characterized through the XRD, FTIR, and EDAX. The biocompatibility of synthesized aggregate of [(AgNPs)-(PE)] was confirmed by the MTT assay. An in vitro cell (HEK293)-based studies were performed for the biocompatibility tests and it is found that the aggregate [(AgNPs)-(PE)] is not harmful to normal/healthy cells. Even though A. sclerocarpa show the antimicrobial (antibacterial and antifungal) activity, it has been further enhanced with the developed aggregate of [(AgNPs)-(PE)]. Furthermore, it has been extended to examine the cellular inhibition on K562 cells and obtained > 75% cell inhibition for 24 h treated cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are the man’s primary source of drugs for various diseases from the ancient times. Medicines derived from plants have played a pivotal role in health care of ancient and modern cultures (Petrovska 2012). Plants contain a plethora of compounds, including antioxidants, immune stimulants, cell proliferation stimulators, anti-inflammatory agents, anticancer components, and antimicrobial agents. A large number of plants with potential therapeutic capabilities to cure a large number of ailments caused to man are identified and recognized globally. Plant-based materials have been demonstrated as excellent drug stores for therapeutic implants. However, Green tea, Cabbage, Holy basil leaves, Beets, Aloe vera, etc. are having potential and active functional groups which show antimicrobial activity and to cure several cancers. Likely, Alphonsea sclerocarpa is widely distributed in the state of Andhra Pradesh (India) and well known for its medicinal importance towards cancer therapy and antimicrobial usage. Every part of the A. sclerocarpa such as branches, bark, leaves, fruits, and flowers are extensively useful (Prasad 2009; Suman Joshi et al. 2017; Tacic et al. 1987). The plant extracts with the combination of metal nanoparticles such as silver (Ag), gold (Au), and iron oxide (FeO3), Zinc (Zn), and cobalt (Co) show the enhanced properties towards the antimicrobial activity. Similarly, silver (Ag) and silver nanoparticles are well known for their oligodynamic property which means the capability to show biocidal activity even at low concentration (Siim et al. 2016). As per the literature, silver in its nanoscale size is exhibiting strong antimicrobial activity; it is further used as primary source for production of wide range of active functional materials. As per the global statistics, ~ 500 tons of silver nanoparticles were produced annually for the use of biomedical applications (Silver and Phung 1996; Morones et al. 2005; Mueller and Nowack 2008). Scientists and researchers are interested in curing diseases by the application of nanotechnology and nanobiotechnology through the development of plant-mediated metal nanoparticles (Iravani 2011). There are capsules, particles, and composite materials which have medicinal usages, but metal nanoparticles with the combination of plant extract are more efficient than their counterparts. After detailed literature survey on varieties of composite materials with the metal nanoparticles; we come across and decided to develop an aggregate with the help of silver nanoparticles and A. sclerocarpa extract. It is having a potential role towards antimicrobial and anticancer activity (Korbekandi et al. 2009; Iravani and Zolfaghari 2013). Therefore, we focused on the extraction of phytochemicals from A. sclerocarpa and it is further used for the reduction of AgNO3 to AgNPs through the in situ (single step) reduction reaction. The A. sclerocarpa extract has the affinity to interact with metal ions such as Ag+, Au+, Fe+, Zn+, etc. for the formation of composite materials and aggregates. The active functional groups which are present in the plant extract directly associated or interacted with the metal mentioned above ions and formed aggregates show significant and novel benefits towards for the usage of mankind. These are remarkable and effective reservoirs with antimicrobial activity and with sustained inhibition on various cancers (Morones et al. 2005). However, the effect of A. sclerocarpa alone shows the limited effect, but in combination with AgNPs, it shows enhanced and significant activity on bacterial and fungal species; it was chosen as a coating material for the AgNPs (El-Chaghaby and Ahmad 2011; Veerasamy et al. 2011) (Scheme 1).
Steps involved in the synthesis of aggregate of AgNPs coated with Alphonsea sclerocarpa plant extract [(AgNPs)-(PE)]. Photo a for Alphonsea sclerocarpa leaves, b for dry leaves, c for grinded fine powder from leaves, d for reaction set-up, e plant extract, f AgNO3 solution, g for e, f of synthesized [(AgNPs)-(PE)] in the liquid form, h for [(AgNPs)-(PE)] in the powder form, and i for possible reduction reaction
Materials and methods
Collection of plant leaves
The leaves of plant A. sclerocarpa were collected from Seshachalam forest region of Andhra Pradesh, India identified with taxonomist, and a voucher specimen was deposited at Herbarium of Sri Venkateswara University with a voucher number 871. The leaves of the plant were collected, cleaned thrice with rinsing tap water to remove dirt, and allowed to dry at room temperature for a couple of weeks. The well-dried leaves were grinded into fine powder. The obtained fine powder was labelled and used for further experiments.
Synthesis of aggregate of [(AgNPs)-(PE)]
An amount of ~ 1.6 mg (0.0016 g) of silver nitrate (AgNO3) was dissolved in ~ 250 mL of dd-water (H2O) and stored as the stock solution for further use. Then, ~ 3.0 g of fine A. sclerocarpa powder was taken in a ~ 250 mL of round bottom (RB) flask and added with ~ 100 mL of dd-H2O. The solution of AgNO3 was added to RB flask and allowed to boil at ~ 65 °C for 1 h. However, boiling has been carried out with continuous stirring at 500 RPM on a magnetic hot plate. The colour of the mixture turned into light brown to dark brown after 1 h stirring at ~ 65 °C. After 1 h stirring, the mixture of A. sclerocarpa extract with AgNPs was filtered with Whatman filter paper, and it was recrystallized thrice. After a notable colour change upon incubation for 24 h, the [(AgNPs)-(PE)] was centrifuged at 8000 RPM for 20 min. The supernatant was discarded, and the pellet was collected and air dried.
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) was defined as the minimum concentration required to inhibit the growth of the microorganisms by synthesized nanoparticles against both Gram-positive and Gram-negative bacteria, viz., Bacillus subtilis, Lactobacillus acidophilus, Staphylococcus aureus, Streptococcus mutans, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, and Proteus vulgaris. The MIC of AgNPs was determined using serial dilution microplate method (Eloff 1998; Andrews 2001). The bacterial inoculum was adjusted to match the tube of 0.5 McFarland turbidity standard using the spectrophotometer at 600 nm (Wavelength), which equals to ~ 1.5 × 108 colony-forming units (CFU)/mL (McFarland 1907). Different aliquots of AgNPs were mixed with the inoculums in test tubes and incubated at ~ 37 °C for 24 h. After incubation, MIC value of AgNPs against tested bacteria was calculated by taking the optical density of the samples (Krishnan et al. 2015). All experiments were performed in triplicates and meant values along with standard deviation are represented. Nanoparticles dilution without inoculums was used as blank control, whereas Streptomycin was used as positive control and DMSO was used as negative control.
Antifungal activity
The antifungal activity was carried out using different fungal strains such as Aspergillus brasiliensis, Aspergillus flavus, Aspergillus niger, Aspergillus solani, and Candida albicans. Into the sterile Petri plates, 1 mL of the fungal suspension was taken followed by addition of molten state potato dextrose agar medium and mixed well. After complete solidification, wells were bored with sterile cork borer of 6 mm diameter followed by filling with ~ 100 µL of ~ 50 mg concentrated synthesized A. sclerocarpa-AgNPs which was dissolved in DMSO. The drug Fluconazole with the concentration of 10 µg/100 μL was used as the standard antifungal agent (positive control). The Petri plates were incubated at ~ 25 °C for 72 h (Narayanan and Park 2014; Mallmann et al. 2015).
Cell inhibition assay (MTT assay)
The anticancer activity or inhibition capacity of a developed aggregate of [(A. sclerocarpa)-(AgNPs)] used to evaluate based on different cell lines. However, HEK293 (human embryonic kidney) are non-cancerous, and K562 (leukaemia blood) cancer cells were chosen for the cell inhibition assay (Amgoth et al. 2016). Well-cultured cell lines, ~ 4 × 105 cells/well were poured in the wells of 96-well plate in two series of sets with control and blank wells followed by the wells with [(AgNPs)-(PE)]. Then, wells containing ~ 4x105 cells/well were added with six different concentrations (1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 µg/mL) of aggregate of developed [(AgNPs)-(PE)]. Furthermore, it was allowed to incubate for 24 h in a CO2 incubator at ~ 37 °C. The purple formazan crystal formation was observed upon addition of MTT chemical which was dissolved in 100 μL dimethyl sulfoxide (DMSO). After 24 h incubation or treatment, 96-well plates were taken out from the incubator and used to analyze cell inhibition assay using BioTeck Synergy H4 model multiple readers (Jang et al. 2016). The obtained or recorded data have been collected from the instrument and used to plot a bar graph as represented in the results and discussion section (Fig. 5).
Results and discussion
Morphology of [(AgNPs)-(PE)]
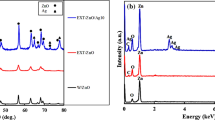
The morphology of developed [(AgNPs)-(PE)] was characterized by the TEM (Transmission Electron Microscopy) imaging. From the TEM image studies, it is concluded that AgNPs are coated with A. sclerocarpa extract, and it appears as an aggregate of [(AgNPs)-(PE)]. The synthesized nanoscale AgNPs are confirmed through the TEM imaging and reported in Fig. 1. Figure 1 a, b corresponds to silver nanoparticles coated with A. sclerocarpa extract which shows the distribution of AgNPs and dispersion of A. sclerocarpa extract at lower magnification. The size of the AgNPs ranges from ~ 20 to 40 nm (in diameter) with an average size of 33 nm. The shape of the silver nanoparticles is irregular and inconsistent with a random distribution. The functional groups present in the phytochemicals (plant extract) are responsible for reduction and determination of AgNPs size and shape. The aggregate of AgNPs coated with A. sclerocarpa extract has shown in Fig. 1c, d at higher magnifications. It is clearly evidenced that nanoscale silver particles have been coated with A. sclerocarpa extract. The yellow arrows in the image (c) (in Fig. 1) illustrate the distribution of metal (silver) nanoparticles bonded with PE. Since plant extract is sensitive at higher accelerating voltages (200 kV), samples for TEM imaging were added with phospho tungstic acid (PTA) to avoid the burning/charge affect at such high accelerating voltage (Protima et al. 2015). However, PTA forms a thin layer on the surface of the samples, and it resists/avoids the charge effect during imaging. Furthermore, the morphology of [(AgNPs)-(PE)] was confirmed by the SEM (Scanning Electron Microscopy) imaging. The SEM images in Fig. 2a–d corroborate the morphology of [(AgNPs)-(PE)]. From SEM, the clumsy aggregates of the plant extract with silver nanoparticles have been observed. Silver particles were surrounded by plant extract to form aggregates. Notably, silver nanoparticles are positively charged (Ag+) and this could be the main reason for the formation of aggregates with plant extract. An aggregation between AgNPs and plant extract can be avoided with sonication methods based on our applications. The figure S1 c–d, (supporting information), shows the morphology of the aggregates of the [(AgNPs)-(PE)] at higher magnifications such as 500 nm. However, a non-conducting plant extract with AgNPs was sputter coated with conducting (Au) material to get the conductivity throughput the sample surface during SEM imaging (see supporting files). Furthermore, [(AgNPs)-(PE)] aggregate was characterized by FTIR (Fourier Transform Infrared), to know about the presence of functional groups. However, Fig. 3a illustrates the significant peaks for active functional groups. The peaks showed at ~ 3304 cm−1 (± 10) corresponds to the amide (− NH) group of the primary aromatic amine (Sastry et al. 1998), ~ 2950 cm−1 (± 10) corresponds to an asymmetric stretch of − CH of the methyl group with SP3 hybridization. The peak at ~ 2428 cm−1 (± 10) corresponds to an asymmetric stretch of – CH and ~ 1634 cm−1 (± 10) peak corresponds to − NH adjacent to methyl functional groups (Sastry et al. 1997; Biswajit Dey et al. 2013). The peak at ~ 1384, 1246, and 1086 cm−1 corresponds to other functional groups present in the plant extract. Powder X-ray diffraction (XRD) has been performed to evaluate the solid state of developed [(AgNPs)-(PE)]-based aggregate and corresponding peaks for AgNPs were assigned in Fig. 3b. However, plant extract is amorphous in nature. It gives broad peak (noise) and crystalline nature of AgNPs was confirmed through the 2θ values on the x-axis; for example, ~ 38° for (111) planes, ~ 45° for (200) planes, ~ 64° for (220) planes, and ~ 78° for (311) planes. All together correspond to the FCC (face-centered cubic) crystal structure of silver nanoparticles (Ma et al. 2001). The XRD peaks at 28, 33, 47, 55, 58, and 82° (2θ) correspond to AgCl face-centered cubic structure. All the corresponding chlorine peaks were highlighted in the red circle in Fig. 3b, (Kumar et al. 2016). Furthermore, the synthesized [(AgNPs)-(PE)] has been used to analyze the elemental composition, and it has been achieved through the EDAX (Energy Dispersive X-ray Spectroscopy). The EDAX results in Fig. 4a, b shows weight and the atomic percentage of elements present in synthesized [(AgNPs)-(PE)] aggregate. The main peak is for source silver nanoparticles (AgNPs); C (Carbon) and O (Oxygen) are secondary source elements come from plant extract. However, the elements like Si (Silicon) and Au (Gold) are appeared in the sample because of contamination and as an impurity from sputter coating materials. The inset table in Fig. 4b shows the presence of all the elements in the developed aggregate of [(AgNPs)-(PE)]. From the EDAX, 57.23 weight (%) and 16.65 atomic (%) of Ag metal have been confirmed. The aggregated morphology of [(AgNPs)-(PE)] showed that, in TEM, images (Fig. 1) were evidenced/confirmed by the EDAX analysis. However, these silver nanoparticles were once again confirmed by using UV–visible spectrophotometric analysis (figure S2, Supporting information). The broad absorbance peak at ~ 437 nm corresponds to AgNPs. The appearance of the broad peak in AgNPs between ~ 400 and 500 nm is because of surface plasmon resonance (Sastry et al. 1998). The AgNPs from PE can be separated through the sonication methods. The optimization of sonication time and temperatures is key factors to consider separating the silver particles from PE.
TEM images of an aggregate of AgNPs coated with Alphonsea sclercarpa extract [(AgNPs)-(PE)]. Images from a to d are captured from lower to higher magnifications. Yellow arrows show the distribution of AgNPs coated with PE and dotted blue lines illustrate the dispersion of Alphonsea sclerocarpa extract
Minimum inhibitory concentration (MIC)
The MIC values of A. sclerocarpa-AgNPs were given in Table 1 and the results revealed that the MIC values ranged from 8.66 ± 1.15 to 44.33 ± 0.57 mg/100 μL against different bacteria tested. Lowest MIC value was observed against Streptococcus mutans, whereas higher MIC value was found to be against Pseudomonas aeruginosa. The difference between these MIC values among tested bacteria may be due to the structural difference between the cell wall of Gram-positive and Gram-negative bacteria. The reason for the antibacterial activity of AgNPs was reported earlier which may be due to the penetrating of silver ions into cells and intercalating between purine and pyrimidine bases leading to denaturation of DNA (Ahmed et al. 2016). It is also evident from earlier reports that the signal transduction in bacteria is regulated and determined by the phosphorylation of proteins. The AgNPs are reported to alter the peptide substrates leading to dephosphorylation of tyrosine residues in proteins, thus inhibiting the cell signalling and leads to cell death (Shrivastava et al. 2007; Nagababu and Uma Maheswara Rao 2017).
Antifungal activity
Similarly, to examine the antifungal activities, fungal inhibition with the developed [(AgNPs)-(PE)]-based aggregate has been evaluated using five different types of fungal species, as reported in Table 2. As per the results obtained, the high (19.5 ± 0.29) antifungal activity (fungal inhibition) with [(AgNPs)-(PE)] was observed on Aspergillus brasiliensis and least inhibition (10 ± 0.81) on Aspergillus flavus. The results compared with positive control are represented in Table 2 and Figure S3, (supporting information). However, AgNPs with plant extract show enhanced antifungal activity. It is attributed based on various factors such as particle size (typically particle size should be in the range of ~ 20–50 nm). The increase in specific surface area promotes better contact and interaction with bacterial and fungal species leading to reducing their further proliferation (Feng et al. 2000; Lok et al. 2006; Rai et al. 2009; Siim et al. 2016). However, penetrated AgNPs will induce free radicals and cause oxidative stress leading to inhibition of bacteria and fungi (Morones et al. 2005; Song et al. Song et al. 2006).
Cellular inhibition assay and imaging
From the obtained data, bar graph was plotted and it shows increased inhibition tendency as the increase of the concentration of aggregate of [(AgNPs)-(PE)]. The concentration of aggregate of [(AgNPs)-(PE)] with 1.0 µg (IC50) shows greater than 50% cell inhibition and for 3.5 µg. It is measured as greater than 75%, which is ever reported with such aggregate of [(AgNPs)-(PE)], (silver nanoparticles coated with A. sclerocarpa extract). Blank well is used as zero inhibition without any developed [(AgNPs)-(PE)] compounds. However, inhibition is observed on cancerous (K562) cells only; the inhibition on normal HEK293 cells is negligible which is less than 10%. With these results, it is evidenced as the synthesized aggregate of [(AgNPs)-(PE)] can be used to treat the cancer cells Fig. 5. The K562 cells used for cellular inhibition assay were once again added with different fluorescent dyes and used for morphological imaging. Figure 6a–d illustrates the morphology of K562 cells which are stained with FITC (green fluorescent dye) and Rhodamine-B (red fluorescent dye) which stains cytoplasm of cells and DAPI (blue fluorescent dye) which mainly stains genetic material of DNA/RNA. Specifically, it stains A-T, (adenine–thymine)-rich regions. Figure 6d depicts the merged images of cells with different fluorescent dyes. From these fluorescent images of K562 cells, it is confirmed that the cellular rupture and morphological distortions are occurred compared to optical microscopy images in figure S4, (Supporting information).
Conclusions
Herein, silver nanoparticles were successfully coated with A. sclerocarpa extract to develop an aggregate of [(AgNPs)-(PE)]. The A. sclerocarpa extract has been used as reducing agent as well as binding glue to silver nanoparticles. The developed [(AgNPs)-(PE)] was characterized using various microscopic techniques. [(AgNPs)-(PE)] is biocompatible for non-cancerous cells. It is a new approach to develop [(AgNPs)-(PE)] aggregate with enhanced antimicrobial and anticancer activities. It is a green synthesis followed by the facile eco-friendly approach. Through this atom economy reaction, we have developed [(AgNPs)-(PE)], and it shows enhanced anticancer activity upon 24 h treatment on leukaemia blood cancer (K562) cells. The cellular inhibition with [(AgNPs)-(PE)] is calculated as > 75%, which is ever reported with such type of materials. Furthermore, the bacterial inhibition was found to be highest with Pseudomonas aeruginosa with 44.33 ± 0.57 mg/100 μL, whereas for fungal species, highest inhibition is found for Aspergillus brasiliensis with an inhibition of 19.5 ± 0.29. Furthermore, these types of aggregates can be recommended for other biomedical and pharmaceutical applications.
References
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28
Amgoth C, Dharmapuri G, Kalle AM, Paik P (2016) Nanoporous capsules of block copolymers of [(MeO-PEG-NH)-b-(L-GluA)]-PCL for the controlled release of anticancer drugs for therapeutic applications. Nanotechnology 27(12):125101
Andrews JM (2001) Determination of minimum inhibitory concentration. J Antimicrob Chemother 48(1):5–16
Dey Biswajit, Saha Rajat, Mukherjee Priyanka (2013) A luminescent-water soluble inorganic co-crystal for a selective pico- molar range arsenic (III) sensor in a water medium. Chem Commun 63:7064–7066
El-Chaghaby GA, Ahmad AF (2011) Biosynthesis of silver nanoparticles using Pistacia lentiscus leaves extract and investigation of their antimicrobial effect. Orient J Chem 27(3):929–936
Eloff JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713. https://doi.org/10.1055/s-2006-957563
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on E. coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. https://doi.org/10.1039/c1gc15386b
Iravani S, Zolfaghari B (2013) Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int 2013:639725. https://doi.org/10.1155/2013/639725
Jang SJ, Yang IJ, Tettey CO, Kim KM, Shin HM (2016) In-vitro anticancer activity of green synthesised silver nanoparticles on MCF-7 human breast cancer cells. Mater Sci Eng 68:430–435. https://doi.org/10.1016/j.msec.2016.03.101
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms. Crit Rev Biotechnol 29:279–306. https://doi.org/10.3109/07388550903062462
Krishnan R, Arumugam V, Vasaviah SK (2015) The MIC and MBC of silver nanoparticles against Enterococcus faecalis—a facultative anaerobe. J Nanomed Nanotechnol 6(3):285. https://doi.org/10.4172/2157-7439.1000285
Kumar VA, Uchida T, Mizuki T, Nakajima Y, Katsube Y, Hanajiri T, Maekawa T (2016) Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from a weed Solidago altissima (goldenrod). Adv Nat Sci 7(1):015002
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5(4):916–924. https://doi.org/10.1021/pr0504079
Ma CY, Rout MK, Mock WY (2001) Study of oat globulin conformation by Fourier transform infrared spectroscopy. J Agric Food Chem 49:3328–3334
Mallmann EJ, Cunha FA, Castro BN, Maciel AM, Menezes EA, Fechine PB (2015) Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop Sao Paulo 57(2):165–177. https://doi.org/10.1590/S0036-46652015000200011
McFarland J (1907) Nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc 14:1176–1178. https://doi.org/10.1001/jama.1907.25320140022001f
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bacterial effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. https://doi.org/10.1088/0957-4484/16/10/059
Mueller NC, Nowack B (2008) Exposure modelling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Nagababu P, Uma Maheswara Rao V (2017) Pharmacological assessment, green synthesis and characterization of silver nanoparticles of sonneratia apetala Buch.-Ham. leaves. J App Pharm Sci 7(8):175–182
Narayanan KB, Park HH (2014) Antifungal activity of silver nanoparticles synthesised using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur J Plant Pathol 140:185–192. https://doi.org/10.1007/s10658-014-0399-4
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6(11):1–5. https://doi.org/10.4103/0973-7847.95849
Prasad DN (2009) Antioxidant activity of Alphonsea sclerocarpa bark. Res J Pharmacol Pharmacodynamics 1(2):66–69
Protima R, Siim K, Stanislav F, Erwan R (2015) A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mat Sci Eng. https://doi.org/10.1155/2015/682749
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Sastry M, Mayya KS, Bandyopadhyay K (1997) pH-dependent changes in the optical properties of carboxylic acid derivatised silver colloidal particles. Colloids Surf A 127(1–3):221–228. https://doi.org/10.1016/S0927-7757(97)00087-3
Sastry M, Patil V, Sainkar SR (1998) Electrostatically controlled diffusion of carboxylic acid derivatised silver colloidal particles in thermally evaporated fatty amine films. J Phy Chem B 102(8):1404–1410
Shrivastava S, Bera T, Roy A, Singh G, Rama Chandra Rao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18:1–9
Siim K, Sander K, Protima R, Mithu G, David W, Erwan R (2016) Biocidal properties study of silver nanoparticles used for application in green housing. Int Nano Lett 6(3):191–197. https://doi.org/10.1007/s40089-016-0186-7
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789
Song HY, Ko KK, Oh IH, Lee BT (2006) Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cell Mater 11(1):58
Suman Joshi DSD, Venkata Rao G, Satya Prasad M, Kishore Babu M, Surya Narayana S, Krishna Satya A (2017) Phytochemical screening and evaluation of antioxidant, antibacterial and antifungal activity of medicinal plant Alphonsea sclerocarpa Thaw. J Pharmacogn Phytochem 6(4):1280–1286
Tacic D, Wannigama GP, Cassels BK, Cave A (1987) Alkaloids of Alphonsea sclerocarpa. J Nat Prod 50(3):518–519. https://doi.org/10.1021/np50051a036
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120. https://doi.org/10.1016/j.jscs.2010.06.004
Acknowledgements
The authors would like to acknowledge DST-FIST, Department of Physics, Acharya Nagarjuna University for providing IR facility, University of Hyderabad, for providing SEM with EDAX and XRD facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Both the first and second authors have equal contribution to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doddapaneni, S.J.D.S., Amgoth, C., Kalle, A.M. et al. Antimicrobial and anticancer activity of AgNPs coated with Alphonsea sclerocarpa extract. 3 Biotech 8, 156 (2018). https://doi.org/10.1007/s13205-018-1155-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1155-9