Abstract
Nocardiopsis sp. KNU was found to degrade various lignocellulosic waste materials, namely, sorghum husk, sugarcane tops and leaves, wheat straw, and rice husk very efficiently. The strain was found to produce high amounts of cellulase and hemicellulase. Augmentation of cotton seed cake as an organic nitrogen source revealed inductions in activities of endoglucanase, glucoamylase, and xylanase up to 70.03, 447.89, and 275.10 U/ml, respectively. Nonionic surfactant Tween-80 addition was found to enhance the activity of endoglucanase enzyme. Cellulase produced by Nocardiopsis sp. KNU utilizing sorghum husk as a substrate was found to retain its stability in various surfactants up to 90%. The produced enzyme was further tested for saccharification of mild alkali pretreated rice husk. The changes in morphology and functional group were analyzed using scanning electron microscopy and Fourier transform infrared spectroscopy. Enzymatic saccharification confirmed the hydrolytic potential of crude cellulase. The hydrolysate products were analyzed by high-performance thin layer chromatography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alternatives to the existing sources of energy generation are rapidly being explored to meet the ever increasing energy requirements of the society. Utilization of regenerative lignocelluloses for energy production has, therefore, rapidly increased in the last few decades. Agricultural waste biomasses such as wheat straw, rice straw, and sugarcane bagasse have been reported to be utilized for production of second generation bioethanol (Cheng et al. 2008; Sun and Cheng 2002). Cellulose being the main component of plant biomasses, they are considered to be rich sources of lignocellulosic biomass in the bio-fuel industry. Lignocellulosic waste biomasses bear the advantages of lower cost and high natural abundance. However, the complex nature of lignocellulosic biomass is a major problem in its utilization as a substrate for enzyme production and hydrolysis. The problems associated with the degradation of lignocellulosic biomasses can be solved using various strategies such as microbial exploration, cellulolytic enzymes production, and saccharification by cellulase (Lynd et al. 2002). In addition, use of inexpensive lignocellulosic biomasses for cellulase production can certainly prove to be cost effective and environmental friendly.
Abundantly available low-cost lignocellulosic biomasses have been used as attractive sources for production of cellulolytic enzymes and thereafter bioethanol (Cheng et al. 2008). Lignocellulosic biomass is composed of cellulose, hemicelluloses, lignin, and pectin complex which are heterogeneously interlinked polysaccharide chains with different degrees of crystallinity. There are various microorganisms which degrade the biomass and produce multiple cellulolytic and hemicellulolytic enzymes called “enzyme systems” (Lynd et al. 2002). The enzyme system is responsible for active degradation of the plant cell wall components (cellulose and hemicelluloses). Classification of enzymes of the cellulase system is based on properties of the enzyme and their mode of degradation mechanism (Lynd et al. 2002). Production of ethanol from lignocellulosic biomass involves three major steps such as pretreatment, enzymatic hydrolysis, and fermentation (Rubin 2008). After pretreatment, enzymatic saccharification is the key step for improvement in sugar liberation from lignocellulosic biomass for further ethanol production. Before the enzymatic saccharification, pretreatment of lignocellulosic materials should be carried out for breakdown of the lignin locking pattern and other hemicellulosic component. The opened lignin moieties and cellulose enhance the accessibility for enzyme attack and to release sugars (Yang et al. 2011). Utilization of the low-cost lignocellulosic biomass for enzyme production and crude enzyme application for biomass hydrolysis significant affects the cost and efficiency of bio-fuel production process (Saratale et al. 2012). Use of low-cost substrate, instead of highly expensive commercial enzymes, is an important factor in cellulase production from economical point of view (Saratale and Oh 2015). Sorghum straw (Sorghum bicolor (L.) Moench) is the single finest lignocellulosic material used for bioethanol production. It grows easily in hot, subtropical, and barren regions of India, United States, Mexico, China, Southern Africa, and other developing countries, where the agricultural crop production faces common stress conditions (Tsujibo et al. 2003).
Biodegradation of lignocellulosic material and production of cellulase enzymes by microorganisms has been reported. Several bacteria and actinobacteria have been reported to exhibit multiple cellulolytic and hemicellulolytic enzyme activities at moderate temperature and pH (Stamford et al. 2001). Microorganisms hydrolyze the insoluble cellulose and produce free reducing sugar moeties using the free or cell-associated extracellular cellulases. Actinomycetes are soil microbes which have potential to produce various enzymes (cellulase, chitinase, and alkaline protease) by hydrolyzing different polysaccharides such as cellulose and chitin (Rawat et al. 2014; Rodhe et al. 2011).
The present work deals with the investigation of production of several synergistically produced cellulases using different inexpensive lignocellulosic materials. Various strategies such as addition of organic nitrogen sources and surfactants were tried for enhancement of cellulase activity. The production of cellulolytic enzymes was carried out using sorghum husk biomass which is an agricultural waste material produced at enormous scales annually (Wiselogel et al. 1996). The enzymes were further explored for saccharification of rice husk for bioethanol production.
Materials and methods
Materials
All lignocellulosic substrates used in this study were collected from agricultural fields surrounding Kolhapur, India. Sorghum husk (SH), wheat straw, sugarcane tops and leaves, dried grass, and rice husk were selected as carbon sources. Commercial animal feed was procured from Pranav Agro Industries Ltd, Sangli, Maharashtra, India. Carboxymethyl cellulose and birch wood xylan were obtained from Hi-Media (Mumbai, India) Avicel PH-101 and p-nitrophenyl-β-glucoside (PNPG) was procured from Sigma Aldrich (USA). The lignocellulosic wastes were dried in sunlight, milled using pulverizer, sieved through a 0.2 mm sieves, and stored at room temperature.
Pretreatment of rice husk
The alkaline pretreatment of rice husk biomass was performed with different NaOH concentration as 0.025, 0.05, and 0.1 N and the pretreatment reaction conditions were 121 °C for 60 min (Table 1).
Microorganism and culture conditions
Nocardiopsis sp. KNU culture was grown on the modified Dubos salt medium consisting of (g/l): CMC, 10; NaNO3 0.5; MgSO4 7H2O, 0.5; KH2PO4, 1; KCl, 0.5; and FeSO4, 0.001. The most favorable environmental conditions were standardized such as initial pH of the media (6.5), growing temperature (30 °C), and medium for improved cellulolytic and xylanolytic enzymes production by Nocardiopsis sp. KNU.
Preparation of enzyme source
Static condition studies were carried out in 250 ml Erlenmeyer flask using 100 ml of Dubos salt medium with sorghum husk (1% w/v) as the exclusive carbon source. Nocardiopsis sp. KNU culture (approximately 107 cells) was inoculated and growth conditions were maintained at temperature 30 °C for up to 7 days. The crude extract was centrifuged at 4000×g for 10 min. The supernatant (free of cell biomass) obtained after centrifugation was directly used as an extracellular source of enzymes for the determination of enzyme activities.
Enzyme assay
The activities of endoglucanases and exoglucanases were determined as described earlier (Nitisinprasert and Temmes 1991). Endoglucanase reaction mixture contained 1 ml of enzyme along with 1 ml of 1% carboxymethyl cellulose (CMC) in McIlvaine’s buffer (0.1 mol/l citric acid–0.2 mol/l phosphate buffer; pH 5) which was incubated at 50 °C for 30 min. Exoglucanase enzyme assay mixture contained 1 ml enzyme source and 0.5 ml of 1% Avicel cellulose in McIlvaine’s buffer and was incubated at 50 °C for 2 h, followed by the addition of 1 ml of 2, 5 dinitrosalicylic acid (DNSA) reagent. The β-glucosidase enzyme activity was determined by measuring the amount of p-nitrophenol from PNPG released. In this assay mixture, 200 μl of enzyme and 5 mM PNPG were added to 1 ml of citrate buffer (50 mM), and incubated at pH 4.5, 50 °C for 10 min. The reaction was terminated by the addition of 2 ml of 1 M sodium carbonate. One unit of β-glucosidase activity is defined as the amount of enzyme required to hydrolyze 5 mM of PNPG per min (Waghmare et al. 2014b). Filter paper unit activity was estimated by quantifying the reducing sugars produced from Whatman no. 1 filter paper (50 mg, 1 × 6 cm) (Ghose 1987). Total cellulase activity [filter paperase (FPU)] was assayed according to IUPAC recommendations using filter paper as the substrate. Filter paperase (FPase) activity was calculated according to the International Union of Pure and Applied Chemistry (IUPAC).
The xylanase activity was determined as described earlier Saratale et al. (2010); the reaction mixture contained 1 ml of enzyme in McIlvaine’s buffer and 1 ml of xylan (1%) and was incubated at 50 °C for 10 min. One unit (U) of xylanase activity is defined as the amount of enzyme that releases one micromole of xylose from the substrate per minute under the above-mentioned conditions. Glucoamylase enzyme assay was carried out as per the procedure reported by Swain and Dekker (1966) with slight modification. In this assay, 1 ml of enzyme in McIlvaine’s buffer and 1 ml soluble starch (1%) were incubated at 50 °C for 10 min. The reaction was terminated by adding 1 ml of DNSA reagent and then kept in boiling water bath for 10 min (Miller 1959). One unit of enzyme activity is defined as the amount of enzyme that produces one microgram of reducing sugar from the substrate per min.
Effect of supplementation of organic nitrogen sources and surfactants on cellulase production performance
Various low-cost animal feed supplementations like ground nut cake, cotton seed cake, and commercial animal feed (1:1 concentration of organic nitrogen source and sorghum husk) were investigated for cellulase production in the presence of various surfactants. The surfactants used in this study were Tween-20, Tween-80, Triton X-100, PEG-4000, and PEG-6000 each at 1% concentration along with 1% (w/v) sorghum husk. Sorghum husk was taken as control and the effect of these surfactants was studied on cellulase production.
Effect of surfactants on cellulolytic and hemicellulolytic enzyme activity
The cellulase activities in the presence of various surfactants were investigated. The surfactants used in this study were PEG-4000, Tween-20, Tween-80, Triton X-100, and SDS at 1% concentration. Enzyme produced by Nocardiopsis sp. KNU was mixed with the surfactants solution (1:1 v/v). The enzyme activities were determined in the presence of surfactants at 50 °C and pH 5.0, while the control sample was without surfactant solution in the reaction mixture.
Effect of commercial detergents on cellulolytic enzyme activity
The stability of different commercial detergent solution on the crude cellulase enzyme was investigated. Various detergents employed for this study were Ariel (Procter & Gamble), Rin (Hindustan Unilever), Tide (Procter & Gamble), and Surf excels (Hindustan Unilever). The detergents used in this study were diluted in distilled water up to a final concentration of 7.0 mg/ml to simulate reaction conditions and were further boiled for 60 min, for inactivation of protease enzymes present in detergents (de Lima et al. 2005). To demonstrate the effect of detergent on the crude cellulase activity, equal volume of enzyme source was mixed with the detergent solutions (1:1 v/v). The enzyme activities were determined in the presence of detergents at 50 °C and pH 5.0, while the control sample was without detergent solution in the reaction assay mixture.
Enzymatic saccharification
Enzymatic saccharification of native and NaOH pretreated rice husk biomass was carried out using Nocardiopsis sp. KNU produced crude cellulase enzyme. 10% biomass loading was used for enzymatic saccharification as: A—native rice husk, B—0.025 N NaOH pretreated rice husk, C—0.05 N NaOH pretreated rice husk, D—0.1 N NaOH pretreated rice husk used with 10% of biomass loading, E—control rice husk, F—0.025 N NaOH pretreated rice husk, G—0.05 N NaOH pretreated rice husk, and H—0.1 N NaOH pretreated rice husk used with 15% of biomass loading. These combinations were incubated with crude cellulase (1.73 ± 0.11 FPU/ml) at pH 4.8 in 100 ml stopper bottle. 100 µl of sodium azide (2%) was added to avoid bacterial contamination, and the saccharification reaction bottles were incubated at 50 °C for 96 h under shaking conditions (120 rpm). Samples at different time intervals were collected from the enzymatic hydrolysate and centrifuged to remove the solid biomass. The supernatant aliquot was boiled for 5 min in boiling water bath for inactivation of cellulolytic enzymes present in hydrolysate. The reducing sugar was determined by DNSA method (Miller 1959).
Analytical methods
HPTLC analysis
The content of monosaccharide present in the enzymatic hydrolysate of rice husk was qualitatively determined by HPTLC. Standard sugars such as glucose, xylose, galactose, and cellobiose were used. All samples of concentrated hydrolysate supernatant (5 µl) were applied on HPTLC plate silica gel 60 F254 (Merck) by microsyringe using spray gas nitrogen sample applicator (Linomat V, CAMAG, Switzerland) with 8 mm band space and 14.5 mm track distance. The mobile phase used for HPTLC analysis was butanol:ethanol:water (5:5:3 v/v). The development of plate was carried out in the twin-trough chamber 10 × 10 cm (CAMAG) up to 85 mm from the bottom edge. The bands were visualized by a developing reagent 0.3% α-naphtol in 5% H2SO4 in methanol. The plates were dried in oven at 80 °C for 15 min. Data processing of all instrumentation was performed with the software platform winCATS 1.4.4.6337 (CAMAG, Switzerland).
Fourier transform infrared spectroscopy (FTIR) analysis
FTIR spectroscopy was used to investigate the changes in functional group of native and alkali pretreated rice husk biomass. The FTIR analysis was done using FTIR spectrometer (Perkin Elmer, Spectrum one B; Shelton, WA, USA). The native and alkali pretreated rice husk biomass were dried in oven at 60 °C for 12 h. The complete dried samples were equally mixed in KBr pellets with an approximate 1% KBr concentration. The background FTIR spectra were obtained using a pure KBr pellet without any rice husk biomass. FTIR spectra were recorded in the absorption band mode in the range of 4000–400 cm−1 with spectra resolution of 4 cm−1.
Scanning electron microscopy (SEM) analysis
Native rice husk biomass and mild alkali pretreated rice husk biomass were collected, washed with distilled water, filtered with muslin cloth, and then dried in oven at 60 °C for 24 h. All samples were fixed with glutaraldehyde (2.5%) solution at 4 °C for 12 h. Gradual dehydration of all samples was achieved using solutions of increasing ethanol concentrations after glutaraldehyde fixation. Samples were incubated at 50 °C for 24 h for complete drying. SEM images were obtained by the particles coated with platinum and recorded using a JEOL JSM-6360A microscope at operating voltage 20 kV.
Statistical analysis
Data analysis was carried out by one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparisons test.
Results and discussion
Cellulase and hemicellulase production performance
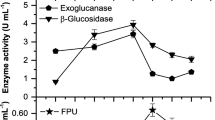
Cellulase enzyme production from commercial cellulose substrate is extremely expensive as compared to lignocellulosic waste biomass and hence the later strategy can be called an alternate strategy for bioethanol production. Potential of lignocellulosic biomass degradation of Nocardiopsis sp. KNU was determined by production of cellulase and hemicellulase enzymes. The Nocardiopsis sp. KNU is a potent strain which produces thermostable cellulolytic enzymes (Tsujibo et al. 2003). Enzyme production performance at different incubation periods is depicted in Fig. 1. It showed maximum enzyme activities after 7 days of incubation in case of sorghum husk as lignocellulosic substrate. Five different lignocellulosic substrates were used for cellulolytic and xylanolytic enzyme production using Nocardiopsis sp. KNU. Table 2 shows cellulolytic and hemicellulolytic enzyme activities after 7 days of incubation as follows: Filter paper unit activity using various substrates; sorghum husk (1.73 ± 0.11 U/ml), grass powder (1.37 ± 0.17 U/ml), sugar cane tops (1.53 ± 0.04 U/ml), wheat straw (1.58 ± 0.08 U/ml), and rice husk (1.33 ± 0.06 U/ml). Grass powder showed higher enzyme activities of glucoamylase and β-glucosidase (150 ± 3.23 and 6.87 ± 0.17 U/ml, respectively). In addition, the isolated KNU strain utilizes sorghum husk produces reducing sugar 1.0 mg/l with reducing sugar production rate of 0.14 mg/l/h (Fig. 2). Thus, sorghum husk was considered for further studies owing to the highest enzyme production. Nocardiopsis sp. KNU degraded the sorghum husk rapidly when compared to other substrates and was further used for the production of cellulase and xylanase. Walia et al. (2014) reported the alkalophilic Cellulosimicrobium cellulans CKMX1 isolate for production of thermostable cellulose-free xylanases. Very few reports are available on cellulolytic and hemicellulolytic enzymes production using low-cost substrate by Nocardiopsis sp. (Engelhardt et al. 2010; Sekurova et al. 1999). The comparative study of enzymes production using low-cost substrates by various microorganisms is shown in Table 3. In this study, Nocardiopsis sp. KNU produces endoglucance (23 U/ml) and 171 (U/ml) by utilizing sorghum husk as carbon source (Table 3).
Induction of cellulase and xylanase by organic nitrogen sources
The enzyme activities of endoglucanase, exoglucanase, filter paperase, xylanase, and glucoamylase were monitored after 7 days of incubation (Table 4) to understand the enzyme induction mechanism more specifically related to the composition of the lignocellulosic substrates. The cotton seed cake (containing 37% polysaccharides, 31% proteins, and 16% lignin) has been reported as a supplementation to the oil seed cake for the stimulation of cellulolytic, hemicellulolytic, and ligninolytic enzymes (Panagiotopoulos et al. 2013). The secretion of active cellulase results in the destruction of lignocellulosic complex constituents, and ultimately, more substrate becomes accessible for microorganism and brings out effective degradation of other cellulosic polymer. Cotton seed cake was also used as a substrate for glucoamylase production by Thermomucor indicae-seudaticae (Kumar and Satyanarayana 2004). Supplementation of cotton seed cake resulted in significant improvement in the enzyme activities, viz. endoglucanase, glucoamylase, and xylanase as 70, 447, and 275 U/ml, respectively. Oil seed cakes are mainly used for lipase enzyme production (Kamini et al. 1998). Only a few reports are available that represent the enhancement of enzyme activity by supplementation of various oil seed cakes. Enzyme activity induction profile after 7 days showed induction of almost all cellulase as well as hemicellulase enzymes (Table 4). Among them, endoglucanase and glucoamylase showed superior induction as compared to other enzymes. In case of cotton seed cake mixture, endoglucanase (292%), xylanase (257%), and glucoamylase (349%) were induced notably showing their synergistic role in the degradation process. Walia et al. (2015b) reported the applicability of cellulase-free, thermostable, and alkaline stabile xylanase produced by C. cellulans CKMX1 in pulp and paper industries.
Enhancements of cellulase production performance using nonionic surfactants
Studies on cellulase production using various surfactants indicated significant enhancement in all the enzyme activities. An increase in cellulase enzyme production was observed by addition of various surfactants (Fig. 3). Results showed that Tween-80 enhanced the cellulase enzyme activities. Similar enhancement of cellulase activities using 0.1% Tween-80 was reported by various researchers (Pardo 1996; Reese and Maguire 1969). Reese and Maguire (1969) reported a significant increase in cellulase activities using 0.1% Tween-80 with Trichoderma viride QM6a. The significant increased enzyme activities in the presence of Tween-80 were FPU (2.28 ± 0.04 U/ml), β-glucosidase (4.13 ± 0.27 U/ml), exoglcanase (21.56 ± 0.21 U/ml), endoglucanase (78.98 ± 0.55 U/ml), xylanase (323.43 ± 2.08 U/ml), and glucoamylase (261.44 ± 2.17 U/ml). Significant increase in cellulase production was also observed because of increase in permeability of cell membrane and release of cell bound enzymes. Figure 3 indicates the Tween-20, PEG-4000, and PEG-6000 showed slight increase in cellulolytic enzymes production. Triton X-100 showed much lower yield of all enzyme activities due to inhibition of the growth of Nocardiopsis sp. KNU. Walia et al. (2015a) showed the improvement of xylanase production from Cellulosimicrobium cellulans CKMX1 due to Tween-20 (0.02%) media using central composite design of response surface methodology. Triton X-100 also inhibits the growth of microorganism and thereby cellulase production (Pardo 1996).
Enzyme activity in the presence of non ionic surfactants and commercial detergents
The activity of cellulase produced by Nocardiopsis sp. KNU in the presence of nonionic surfactants is shown in Fig. S1. PEG 4000, Tween-20, Tween-80, Triton X-100, and SDS showed relative enzyme activity of endoglucanase enzyme as 77.81 ± 2.55, 92.64 ± 2.41, 66.38 ± 2.71, 61.63 ± 1.28, and 60.85 ± 1.45 and of xylanase enzyme as 89.45 ± 1.93, 66.57 ± 1.96, 79.38 ± 2.17, 55.66 ± 1.29, and 45.80 ± 1.67%, respectively ,of its initial 100% activity. SDS showed lower relative enzyme activities of all enzymes. Protease enzyme produced by Bacillus licheniformis KBDL4 showed 97% stability of enzymes after 1 h incubation at 30 °C, exhibited positive effect of surfactants on enzymes stabilization, and reduced the deactivation of cellulase (Pathak and Deshmukh 2012). In the presence of Tween-20, the β-glucosidase showed 100% enzyme activity after 96 h (Eriksson et al. 2002); under agitated conditions, Tween-80 stabilized the unstable components of the cellulase (Okino et al. 2013). Endoglucanase and glucoamylase showed maximum relative enzyme activities in the presence of all commercial detergents (Fig. S1). In the presence of Ariel, Surf excel, and Tide, the endoglucanase enzyme retaining 78.00 ± 2.00, 87.14 ± 2.21, and 84.29 ± 1.46% of its initial activity and glucoamylase showed 92.27 ± 2.47, 89.35 ± 3.11, and 70.60 ± 2.15%, respectively. The Bacillus licheniformis KBDL4 produced by protease enzymes were extremely stable in commercial detergents like Surf excel, Nirma, and Tides 97, 95 and 92%, respectively (Pathak and Deshmukh 2012).
Enzymatic saccharification experiments
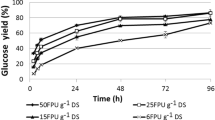
Enzymatic saccharification efficiency was monitored by employing Nocardiopsis sp. KNU produced crude cellulase enzyme on various mild alkali pretreated rice husk biomasses. The stepwise increase of reducing sugar up to 96 h incubation was observed (Fig. 4). The 10 and 15% biomass loadings were studied for enzymatic saccharification of native and mild alkali pretreated biomass. Significant improvements of release of reducing sugar were observed in mild alkali pretreated rice husk biomass than the native rice husk. The higher reducing sugar production was observed in 0.05 N NaOH (15% biomass loading) pretreated rice husk biomass (5.035 ± 0.18 mg/ml) and in native rice husk (1.48 ± 0.04 mg/ml) up to 96 h incubation. This confirmed the enhancement of enzymatic saccharification of lignocellulosic biomass due to pretreatment. Pretreatment of lignocellulosic biomass is the key step in lignocellulosic biofuels before enzymatic saccharification, as it increases accessibility of surface area of cellulose for reducing sugar production (Kshirsagar et al. 2015). Alkali pretreatment of rice husk biomass made the cellulose is highly accessible in enzymatic saccharification experiment. Ultimately, higher reducing sugars were released in pretreated rice husk biomass than native (Kshirsagar et al. 2015).
Enzymatic saccharification of native, mild acidic and alkali pretreated biomass by sorghum husk crude cellulase (1.73 ± 0.11 FPU/ml) produced by Nocardiopsis sp. KNU A Native rice husk, B 0.025 N NaOH pretreated rice husk, C 0.05 N NaOH pretreated rice husk, D 0.1 N NaOH pretreated rice husk (10% biomass loading) and E Native rice husk, F 0.025 N NaOH pretreated rice husk, G 0.05 N NaOH pretreated rice husk, H 0.1 N NaOH pretreated rice husk (15% of biomass loading)
Analytical methods
HPTLC analysis
Reducing sugars produced after the enzymatic saccharification experiments were qualitatively determined using HPTLC analysis. The HPTLC profile showed the production of glucose as a major endproduct and less amount of xylose in all the enzymatic hydrolysate samples (Fig. 5). Waghmare et al. (2014a) reported the detection of glucose and xylose produced after enzymatic saccharification using HPTLC method. The HPTLC results also confirmed the absence of cellobiose accumulation after enzymatic saccharification indicating a synergistic action of enzymes as cocktail. The reducing sugars (glucose and xylose) produced after enzymatic saccharification can be further use for bioethanol generation using the co-fermentation strategy.
FTIR analysis
FTIR spectroscopy was used for the analysis of structural changes in lignocellulosic material during alkali pretreatment. The alkali treatment removes the lignin and hemicelluloses from the lignocellulosic biomass. Figure S2 represents FTIR spectra of native rice husk 0.025 N NaOH, 0.05 N NaOH, and 0.1 N NaOH. 3450–3350 cm−1 was assigned for the –OH stretching, and the partial hydrogen bonding destroyed might be due to more accessibility of cellulose after alkali pretreatment (He et al. 2008; Nelson and O’Connor 1964). 2500–2325 cm−1 was assigned for the NH+ stretching that is observed in 0.025 N NaOH and 0.1 N NaOH pretreated rice husk biomass at 2346 and 2361 positions, respectively. 1470–1430 cm−1 was assigned for the C–H deformation and the peaks observed in between these spectra were of different absorption bands (Sun et al. 2004). 3595–3425 cm−1 indicates –OH stretching in the intermolecular hydrogen bonds (Wang et al. 2010). The decrease in peak intensities in the pretreated samples as compared to the native rice husk confirmed the partial removal of hemicellulose and lignin from the biomass; hence, cellulose becomes more inaccessible for enzymatic saccharification.
SEM analysis
The SEM images of native and pretreated rice husk taken at 300× and 1000× magnification clearly show the effect of pretreatment on rice husk. Figure S3 shows variable topology differences among the alkali pretreated biomass as compared to the native rice husk biomass. The SEM images showed prominent change like higher localized damage in alkali pretreated biomass as compared to native. The damage occurred in biomass during alkali pretreatment is directly proportional to sugar release from enzymatic saccharification. The destruction of lignocellulosic material by pretreatment is showed by Wang et al. (2010). NaOH (0.1 N) pretreated biomass showed highly disrupted lignocellulose fibers, and the disruption of lignocellulose fibrous material followed the order: 0.1 N NaOH > 0.05 N NaOH > 0.025 N NaOH > Native rice husk. Native rice husk showed intact plant cell wall and complex structure of fibers in SEM [Fig. S3 (A, B)]. The native rice husk had no cracks but an increase in alkali concentration of pretreatments serially increased significant number of cracks.
Conclusion
This report suggests that sorghum husk biomass proves to be a potential substrate for in-house cellulolytic and hemicellulolytic enzymes production using Nocardiopsis sp. KNU. This strain utilizes different lignocellulosic materials as substrates for production of cellulase enzymes. Supplementation of the organic nitrogen source and surfactants to the substrate resulted in more suitable and significant induction of cellulase enzyme activities. The stability of cellulase in the presence of various surfactants and commercial detergents suggests its potential application for textile industry and various biotechnological processes. Various pretreatments of biomass could render substrates favorable for cellulase and hemicellulase production. The sorghum husk cellulase can be used as a preferential substrate for enzymatic saccharification and production of reducing sugar for bioethanol production. The analytical studies such as FTIR and SEM confirm the cellulosic biomass degradation. The studies on batch reactor development using Nocardiopsis sp. KNU for cellulase production are underway.
References
Cheng KK, Cai BY, Zhang JA, Ling HZ, Zhou YJ, Ge JP, Xu JM (2008) Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem Eng J 38(1):105–109. doi:10.1016/j.bej.2007.07.012
Chiranjeevi T, Baby Rani G, Chandel AK, Satish Sekhar PV, Prakasham RS, Addepally U (2012) Optimization of holocellulolytic enzymes production by Cladosporium cladosporioides using Taguchi-L’16 orthogonal array. J Biobased Mater Bio. doi:10.1166/jbmb.2012.1201
Coman G, Bahrim G (2011) Optimization of xylanase production by Streptomyces sp. P12-137 using response surface methodology and central composite design. Ann Microbiol 61(4):773–779. doi:10.1007/s13213-010-0195-0
de Lima ALG, do Nascimento RP, da Silva Bon EP, Coelho RRR (2005) Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzyme Microb Technol 37:272–277. doi:10.1016/j.enzmictec.2005.03.016
Engelhardt K, Degnes KF, Kemmler M, Bredholt H, Fjaervik E, Klinkenberg G, Sletta H, Ellingsen TE, Zotchev SB (2010) Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl Environ Microbiol 76(15):4969–4976. doi:10.1128/AEM.00741-10
Eriksson T, Börjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 31(3):353–364. doi:10.1016/s0141-0229(02)00134-5
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. doi:10.1351/pac198759020257
Goluguri BR, Thulluri C, Addepally U, Shetty PR (2016) Novel alkali-thermostable xylanase from Thielaviopsis basicola (MTCC 1467): purification and kinetic characterization. Int J Biol Macromol 82:823–829. doi:10.1016/j.ijbiomac.2015.10.055
He YF, Pang YZ, Liu YP, Li XJ, Wang KS (2008) Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 22(4):2775–2781. doi:10.1021/ef8000967
Kamini NR, Mala JGS, Puvanakrishnan R (1998) Lipase production from Aspergillus niger by solid-state fermentation using gingelly oil cake. Process Biochem 33(5):505–511. doi:10.1016/s0032-9592(98)00005-3
Kshirsagar SD, Waghmare PR, Loni PC, Patil SA, Govindwar SP (2015) Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology. RSC Adv 5(58):46525–46533. doi:10.1039/c5ra04430h
Kumar S, Satyanarayana T (2004) Statistical optimization of a thermostable and neutral glucoamylase production by a thermophilic mold Thermomucor indicae-seudaticae in solid-state fermentation. World J Microbiol Biotechnol 20(9):895–902. doi:10.1007/s11274-004-2891-z
Liang YL, Zhang Z, Wu M, Wu Y, Feng JX (2014) Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. Biomed Res Int 2014:512497. doi:10.1155/2014/512497
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. doi:10.1128/MMBR.66.3.506-577.2002
Miller GL (1959) Use of dinitrosalicyclic reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Nathan VK, Rani ME, Rathinasamy G, Dhiraviam KN, Jayavel S (2014) Process optimization and production kinetics for cellulase production by Trichoderma viride VKF3. Springerplus 3:92. doi:10.1186/2193-1801-3-92
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J Appl Poly Sci 8:1311–1324. doi:10.1002/app.1964.070080322
Nitisinprasert S, Temmes A (1991) The characteristics of a new non-spore-forming cellulolytic mesophilic anaerobe strain CM126 isolated from municipal sewage sludge. J Appl Bacteriol 71:154–161
Okino S, Ikeo M, Ueno Y, Taneda D (2013) Effects of Tween 80 on cellulase stability under agitated conditions. Bioresour Technol 142:535–539. doi:10.1016/j.biortech.2013.05.078
Panagiotopoulos IA, Pasias S, Bakker RR, de Vrije T, Papayannakos N, Claassen PA, Koukios EG (2013) Biodiesel and biohydrogen production from cotton-seed cake in a biorefinery concept. Bioresour Technol 136:78–86. doi:10.1016/j.biortech.2013.02.061
Pardo AG (1996) Effect of surfactants on cellulase production by Nectria catalinensis. Curr Microbiol 33(4):275–278. doi:10.1007/s002849900113
Pathak AP, Deshmukh KB (2012) Alkaline protease production, extraction and characterization from alkaliphilic Bacillus licheniformis KBDL4: a Lonar soda lake isolate. Indian J Exp Biol 50(8):569–576
Rawat R, Srivastava N, Chadha BS, Oberoi HS (2014) Generating fermentable sugars from rice straw using functionally active cellulolytic enzymes from Aspergillus niger HO. Energy Fuels 28(8):5067–5075. doi:10.1021/ef500891g
Reese ET, Maguire A (1969) Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol 17(2):242–245
Rocha VAL, Maeda RN, Anna LMMS, Pereira N (2013) Sugarcane bagasse as feedstock for cellulase production by Trichoderma harzianum in optimized culture medium. Electron J Biotechnol. doi:10.2225/vol16issue5fulltext1
Rodhe AV, Sateesh L, Sridevi J, Venkateswarlu B, Venkateswar Rao L (2011) Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. 3 Biotech 1(4):207–215. doi:10.1007/s13205-011-0024-6
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454(7206):841–845. doi:10.1038/nature07190
Saratale GD, Oh M-K (2015) Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int J Biol Macromol 80:627–635. doi:10.1016/j.ijbiomac.2015.07.034
Saratale GD, Saratale RG, Lo YC, Chang JS (2010) Multicomponent cellulase production by Cellulomonas biazotea NCIM-2550 and its applications for cellulosic biohydrogen production. Biotechnol Prog 26(2):406–416. doi:10.1002/btpr.342
Saratale GD, Saratale RG, Oh SE (2012) Production and characterization of multiple cellulolytic enzymes by isolated Streptomyces sp. MDS. Biomass Bioenergy 47:302–315. doi:10.1016/j.biombioe.2012.09.030
Sekurova O, Hv Sletta, Ellingsen TE, Valla S, Zotchev S (1999) Molecular cloning and analysis of a pleiotropic regulatory gene locus from the nystatin producer Streptomyces noursei ATCC11455. FEMS Microbiol Lett 177(2):297–304. doi:10.1111/j.1574-6968.1999.tb13746.x
Stamford TLM, Stamford NP, Coelho LCBB, Araújo JM (2001) Production and characterization of a thermostable α-amylase from Nocardiopsis sp. endophyte of yam bean. Bioresour Technol 76(2):137–141. doi:10.1016/s0960-8524(00)00089-4
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. doi:10.1016/S0960-8524(01)00212-7
Sun JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab 84(2):331–339. doi:10.1016/j.polymdegradstab.2004.02.008
Swain RR, Dekker EE (1966) Seed germination studies. I. Purification and properties of an alpha-amylase from the cotyledons of germinating peas. Biochim Biophys Acta 122(1):75–86
Tsujibo H, Kubota T, Yamamoto M, Miyamoto K, Inamori Y (2003) Characterization of chitinase genes from an alkaliphilic actinomycete, Nocardiopsis prasina OPC-131. Appl Environ Microbiol 69(2):894–900. doi:10.1128/aem.69.2.894-900.2003
Waghmare PR, Kadam AA, Saratale GD, Govindwar SP (2014a) Enzymatic hydrolysis and characterization of waste lignocellulosic biomass produced after dye bioremediation under solid state fermentation. Bioresour Technol 168:136–141. doi:10.1016/j.biortech.2014.02.099
Waghmare PR, Kshirsagar SD, Saratale RG, Govindwar SP, Saratale GD (2014b) Production and characterization of cellulolytic enzymes by isolated Klebsiella sp. PRW-1 using agricultural waste biomass. Emir J Food Agric 26(1):44–59. doi:10.9755/ejfa.v26i1.15296
Walia A, Mehta P, Chauhan A, Kulshrestha S, Shirkot CK (2014) Purification and characterization of cellulase-free low molecular weight endo beta-1,4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J Microbiol Biotechnol 30(10):2597–2608. doi:10.1007/s11274-014-1683-3
Walia A, Mehta P, Guleria S, Shirkot CK (2015a) Improvement for enhanced xylanase production by Cellulosimicrobium cellulans CKMX1 using central composite design of response surface methodology. 3 Biotech 5(6):1053–1066. doi:10.1007/s13205-015-0309-2
Walia A, Mehta P, Guleria S, Shirkot CK (2015b) Modification in the properties of paper by using cellulase-free xylanase produced from alkalophilic Cellulosimicrobium cellulans CKMX1 in biobleaching of wheat straw pulp. Can J Microbiol 61(9):671–681. doi:10.1139/cjm-2015-0178
Wang B, Wang X, Feng H (2010) Deconstructing recalcitrant Miscanthus with alkaline peroxide and electrolyzed water. Bioresour Technol 101(2):752–760. doi:10.1016/j.biortech.2009.08.063
Wiselogel A, Tyson J, Johnsson D (1996) Biomass feedstock resources and composition. In: Wyman CE (ed) Handbook of bioethanol: production and utilization. Applied energy technology series. CRC, Tayler & Francis Group, Washington, DC, pp 105–118
Yang H, Wu H, Wang X, Cui Z, Li Y (2011) Selection and characteristics of a switchgrass-colonizing microbial community to produce extracellular cellulases and xylanases. Bioresour Technol 102:3546–3550. doi:10.1016/j.biortech.2010.09.009
Acknowledgements
Siddheshwar D. Kshirsagar would like to thank UGC (University Grants Commission), New Delhi for providing UGC-JRF fellowship under UGC Major Research project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kshirsagar, S.D., Bhalkar, B.N., Waghmare, P.R. et al. Sorghum husk biomass as a potential substrate for production of cellulolytic and xylanolytic enzymes by Nocardiopsis sp. KNU. 3 Biotech 7, 163 (2017). https://doi.org/10.1007/s13205-017-0800-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0800-z