Abstract
In this study, the controlled synthesis of microcarriers and CpE loading were aimed to achieve by microfluidic system. The effect of temperature on chitosan in the stabilization of silver nanoparticles, microcarrier size and the encapsulation efficiency was measured. Encapsulation was achieved by the controlled rapid mixing in polymethyl-methacrylate (PMMA) microfluidic chip. The CpE was found to contain primary and secondary phenols in the phytochemical screening. The capping property of chitosan was achieved by heating reaction mixture in polytetrafluoroethylene (PTFE) tube to get the stabilized silver nanoparticles which were found to be embedded in chitosan microspheres of size 23.7 µm on average. Fourier-transform infrared (FTIR) and ultraviolet–visible (UV–Vis) spectroscopy results indicated the successful encapsulation of CpE in microcarriers. The average percentage encapsulation efficiency (% EE) of CsAg was found to be 77.125 ± 6.9%. The CpE-loaded microcarriers had shown significant anti-oxidant activity (p < 0.01). CpE-loaded microcarriers were evaluated to have robust cytotoxicity against 4T1 breast cancer cells at very low dose (IC50 = 42.53 µg/mL), which inhibited 95% of cancer cells viability. These results confer that microfluidic system plays an important role in the formation of CpE-loaded CsAg microcarriers that could effectively (p < 0.0001) kill the breast cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive research has been conducted to prepare different types of nanoparticles, such as metallic, polymeric and ceramic nanoparticles. Owing to unique physical and chemical properties, they are playing a vital role in the materials technology, such as manufacturing of nanodevices (Hoseinzadeh et al. 2017a; Najafi-Ashtiani et al. 2018; Hoseinzadeh and Ramezani 2019), nanofluids (Sarafraz et al. 2014; Salari et al. 2016, 2017; Yousefzadeh et al. 2019; Ellahi et al. 2020), thin films (Hoseinzadeh et al. 2017b, 2018) and in biomedical applications particularly drug delivery (Jacob et al. 2020). The uniform dispersion of nanoparticles in different solvents form nanosuspension, which has diverse applications (Sarafraz and Arjomandi 2018a, b). It has been well established that the pharmaceutical nanosuspensions consist of uniformly distributed nanoparticles which act as nano-carriers to encapsulate the drug in order to provide a long shelf life and a safe targeted delivery of the drug in human body for cancer therapy (Jacob et al. 2020).
The recent research not only focuses on nano-scale carriers for the encapsulation of natural drugs but also demonstrates a wide range of applications for microcarriers or microspheres as drug carriers (Medina-Sánchez et al. 2018; Zugic et al. 2020). These microscale carriers have also been studied to give robust anti-cancer properties by showing the enhanced cell penetration and cellular uptake through endocytosis at the targeted cell site just like nano-carriers (Huang et al. 2010; Munin and Edwards-Lévy 2011; Adhikari and Yadav 2018). Among these microcarriers, polymeric particles are the best studied carriers of drug due to their biocompatible properties and controllable drug delivery system (Kong et al. 2013). The biodegradable polymeric microspheres prepared by poly DL-lactide-co-glycolide (PLGA) and poly vinyl alcohol (PVA) have been reported for a localized delivery for breast cancer therapy (Obayemi et al. 2014). The chitosan, for example, has been working efficiently as nano and microcarrier of hydrophilic drug from ages (Nair et al. 2009; Li et al. 2018).
Chitosan, a naturally occurring polysaccharide has gained keen interest in medicine because of its desirable features including biodegradability, biocompatibility and non-toxicity (Stoica et al. 2013; Kummari et al. 2015; Hussein-Al-Ali et al. 2018). In biomedical fields, it has been used for wound healing, tissue engineering and drug delivery (Hussein-Al-Ali et al. 2018). In combination with silver nanoparticles is emerging as an efficient composite for diverse medical applications (Yang et al. 2016; Palem et al. 2018; Nate et al. 2018). The chitosan and its derivatives have shown excellent anti-tumour effects due to their anti-oxidative properties and scavenging of cancer causing free radicals (Adhikari and Yadav 2018).
The uncontrolled division of abnormal cells in breast tissues is called the breast cancer which is spreading in women worldwide (American Cancer Society 2019). Surgery, chemotherapy, targeted therapy and hormone therapy are the unaffordable treatment options for breast cancer with the survival rate of just up to 5 years (American Cancer Society 2019). This leads the world of pharmacopoeia to develop the affordable, efficient and precise medicinal plants to fight against the complex disease, such as cancer. One remedy lies in Calotropis procera which is a xerophytic, evergreen, erect, perennial shrub and a flowering plant. It grows widely in the tropical and sub-tropical areas of Africa and Asia including different areas of Pakistan like Balochistan, Sindh and Northern areas (Khanzada et al. 2008). Calotropis procera extract (CpE) produces many bioactive constituents such as phenolic acids, flavonoids, alkaloids, calotropin, uskerin and calotaxein. These plant extracted constitutes have been reported to possess biological properties like anti-cancer, anti-oxidant, anti-apoptotic and anti-microbial activities (Farouk et al. 2016; Alzahrani et al. 2019). Novel Calotropis procera protein has been reported to suppress the tumour growth in the breast cancer through the downregulation of NF-κB pathway (Samy et al. 2012a). However, the bioavailability of pharmacologic phytochemicals present in the plant extract (including CpE) is very important for its efficacy. The availability of phytochemicals can be addressed to meet the clinical importance by developing a carrier system with the best bioefficacy, pharmacokinetics and bioavailability (Munin and Edwards-Lévy 2011). An efficient carrier system could be a nano or microsized biomaterial which will contribute towards the stability and controlled release of bioactive nutrients and non-nutrients in the diverse biological environment. Therefore, the chitosan microcarriers can be used as CpE-loading material. This edible microcarriers system not only provides a safe delivery of drug, but also constitute a targeted delivery in the complex human body (Kesente et al. 2017; Chandirika et al. 2018).
Numerous research studies have proposed different techniques for synthesis of chitosan–silver (CsAg) particles, such as the sol–gel reaction method, gamma irradiation synthesis and chemical or green synthesis. However, the fabrication of uniform particles in nano or microsize remains a challenge. Microfluidic system is an emerging technology for precise controlling and manipulating fluids to generate the monodispersed and size-controlled droplets (Yang et al. 2016; Amoyav and Benny 2018; Wang et al. 2018; Maslanka Figueroa et al. 2020). This system provides a microemulsion reactor which can be used to achieve the monodispersity of CsAg particles by the application of high-speed mixing (Amoyav and Benny 2018).The CsAg nanoparticles have been widely used for anti-tumour and anti-microbial effects. These nanocomposite films, modified with PVA, have been previously used for the encapsulation of curcumin for improved anti-bacterial activity (Vimala et al. 2011). To the best of our knowledge, no research has been performed on encapsulation of the Calotropis procera extract (CpE) in CsAg microcarriers using microfluidic system. The current study focuses on the one-step encapsulation of CpE in monodispersed CsAg particles and achievement of robust cytotoxicity against breast cancer cells.
Methodology
This study consists of preparation of plant extract, its analysis and designing a microfluidic setup for the encapsulation of plant extract in CsAg microcarriers. Furthermore, the anti-oxidant and anti-breast cancer activities of CpE-loaded CsAg microcarriers were carried out. The flowchart of the synthesis route is illustrated in Fig. 1.
Chemicals
Chitosan (low molecular weight, Sigma), silver nitrate (AgNO3, Duksan), acetic acid, ethanol, de-ionized water (d.H2O), canola oil (Sigma), hydrogen peroxide (H2O2), ascorbic acid, phosphate buffer (pH = 7.4), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulpho oxide (DMSO) and sodium hydroxide (NaOH). All these chemicals were of analytical grade.
Plant extract preparation and analysis
Medicinal plant collection
The Calotropis procera (NCBI: txid141467), was collected from Nilore, Islamabad, Pakistan owing to its importance as ethno botanical medicinal plant. The verification of the plant sample was definite by the Taxonomist and Herbarium of National Agriculture Research Council (NARC), Islamabad, Pakistan.
Preparation of extract
CpE was prepared using a combination of maceration and percolation extraction methods described before (Kaur 2018) with little modifications. The plant was washed with distilled water and dried under shade. The leaves of plant were separated and frozen overnight at − 20 °C. The leaves were immediately lyophilized and grinded afterwards to form a powder. The active components present in leaves were extracted with hydro-alcoholic solvent (menstruum), i.e. mixture of ethanol and d.H2O (3:1). 50 g of plant powder was mixed in 100 mL of menstruum in an airtight container for 12 h using the shaker (Gallenkamp Model BKS-350) at 120 rpm and room temperature. Afterwards, the mixture was packed in percolator (having the opening at both ends) and menstruum was added to be passed through the plant material at a very low speed. Now this menstruum containing dissolved phytochemicals was collected from the outlet of percolator and more menstruum was poured from top until the collected sample was clear in colour. The mixture was subjected for filtration using Whatman filter paper (Grade 1 qualitative, 125 mm Ø) and filtrate was dried using the rotatory evaporator (SENCO) at 30 °C. This dried ethanolic CpE was stored at 4 °C for further experimentations.
Preliminary qualitative phytochemical analysis
The phytochemical screening was executed to determine the active components extracted in ethanolic CpE. The qualitative biochemical tests were performed for analysis of terpenoids, alkaloids, flavonoids, tannins and phenols following the previous methodology (Gul et al. 2017). After confirmation of presence of essential phytochemicals, the extract was further processed for encapsulation and anti-cancer activity.
Microfluidic reactor for microcarriers synthesis and encapsulation
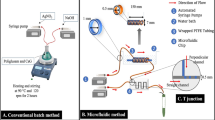
The experimental set-up for microfluidic synthesis is illustrated in Fig. 2. The T-shaped microfluidic reactor was fabricated on polymethyl-methacrylate (PMMA) using CNC machine (Shahzadi et al. 2018). The continuous oil phase (canola oil) was fed through the straight channel and the dispersed phase, consisting a mixture of silver nitrate solution, chitosan solution and CpE, was fed through the perpendicular channel.
Experimental setup for synthesis of microcarriers and encapsulation of CpE. a The microfluidic system without heating. b T-junction image capture by camera-aided optical microscope during the formation of microemulsions. c PTFE tubular microfluidic reactor for heating the reaction mixture. d Cascaded tubular microreactor and the formation of CpE-loaded microcarriers. Red arrows showing the direction of flow of fluids, i.e. canola oil and reaction mixture consisted of AgNO3, CpE and chitosan. AgNO3 silver nitrate, CpEcalotropis procera extract, PTFE polytetrafluoroethylene
The effective capping efficiency of chitosan for stabilization of metallic nanoparticles is achieved when chitosan is heated up to 95 °C as stated in previous reports (Akmaz et al. 2013; Nate et al. 2018; Mohan et al. 2019). However, PMMA chip cannot resist the higher temperature (> 90 °C) due to the depolymerization of PMMA that causes the channel blockage (Tsao and DeVoe 2009). Therefore, in the current study, the dispersed phase was heated up in helically coiled polytetrafluoroethylene (PTFE) tubing, which has high heat resistance (up to 260 °C (Hintzer et al. 2016)). This tubular reactor was immersed in temperature-controlled water bath to heat the reaction mixture up to 95 °C and it was cascaded with perpendicular channel of microchip in order to feed the heated mixture of the dispersed phase into the microchip. This study focused to synthesize the chitosan-stabilized silver nanoparticles and encapsulation of CpE using the microfluidic system.
Synthesis of CsAg microcarriers
The reaction mixture for CsAg microcarriers was prepared by mixing 1.5% of chitosan solution and 4 mM of AgNO3 solution in volumetric ratio of 4:1, respectively. The CsAg microcarriers were prepared both at room temperature directly in microfluidic chip and also at 95 °C via a tubular reactor cascaded with microfluidic chip. The CsAg reaction mixture at room temperature as well as at elevated temperature (95 °C) was fed through a perpendicular channel and the continuous phase was fed through the straight channel of the microchip to form microemulsions as illustrated in Fig. 2. The flow rates of both dispersed and continuous phases were adjusted to form stable monodispersed microemulsions. The synthesized CsAg microcarriers were collected at the outlet of microfluidic chip in both cases and 10% of NaOH was added into the samples for further reduction. The samples were centrifuged at 10,000 rpm for 30 min to separate the microcarriers from their colloidal solution. The resulting microcarriers were lyophilized and stored at room temperature for further analysis.
Synthesis of CpE-loaded-CsAg (CpE–CsAg) microcarriers
The CpE solution was prepared by dissolving 40 mg of CpE in 20 mL of solvent (2:3, ethanol: d.H2O). The CpE was added into the reaction mixture of chitosan and AgNO3 in volumetric ratios of 1, 2, 4 and 6 respectively. The CpE was loaded into the mixture of CsAg in both aforementioned cases prior to the emulsion formation in order to encapsulate the CpE in CsAg microcarriers. The collected samples were split into two equal parts. 10% NaOH was added in one of the samples as a reducing agent. However, the other sample was left without NaOH in order to analyze the reducing property of CpE.
Encapsulation efficiency percentage (%EE)
The encapsulation efficiency of CsAg microcarriers was calculated by indirect method, i.e. by measuring the concentration of free CpE in samples. The samples were centrifuged at 10,000 rpm for 30 min and supernatant was subjected to ultraviolet–visible (UV–Vis) spectrophotometer (Shimadzu UV-1201) analysis. The absorption was measured at 340 nm for calculating the concentration of free CpE in supernatant. The %EE was then calculated using the Eq. 1:
where Cont is the concentration of total CpE added initially and Conf is the concentration of free/unloaded CpE in supernatant.
Characterization
The physical characteristics analysis of microcarriers was performed by UV–Vis spectrophotometer. The morphology analysis of microcarriers was performed by field-emission scanning electron microscope (FESEM) equipped with energy-dispersive X-ray (EDX) spectrometer (TESCAN MAIA3) having 5 kV accelerating voltage. Whereas, Fourier-transform infrared (FTIR) spectroscopy (Nicolet 6700, USA) was performed for functional group analysis.
Anti-oxidant activities
Anti-oxidant activity was measured to identify and compare the ability of CpE and CpE–CsAg microcarriers to scavenge H2O2. The assay was carried out by following the Ruch et al. (1989) method (Ruch et al. 1989) with little modifications. 4 mM of H2O2 solution was prepared in 50 mM of phosphate buffer (pH 7.4). CpE and CpE–CsAg microcarriers (10 mg/mL) were suspended again and diluted to the concentration of 60 µg/mL, 80 µg/mL, 100 µg/mL, 120 µg/mL, 140 µg/mL and 160 µg/mL. Ascorbic acid (10 mg/mL) was used as standard. A 20 mL of H2O2 was added in each dilution of test sample. Absorbance was measured at 230 nm using UV–Vis spectrophotometer after 10 min, against a blank. The percentage of radical scavenging capacity (%RSC) of test samples was calculated using Eq. 2:
where \({Abs }_{\mathrm{C}\mathrm{o}\mathrm{n}\mathrm{t}\mathrm{r}\mathrm{o}\mathrm{l}}\) is absorbance of blank and \({Abs}_{\mathrm{s}\mathrm{a}\mathrm{m}\mathrm{p}\mathrm{l}\mathrm{e}}\) is absorbance of test samples.
Cytotoxicity
The cytotoxic effect of CpE and CpE–CsAg microcarriers was evaluated. The 4T1 cells (5 × 103 per well) were seeded in 96 well plate and cultured for 24 h at 37 °C. Different formulations of microcarriers with increased concentration were added into the cells and incubated for next 24 h. Then, the medium was replaced with fresh medium and the cells were incubated for further 24 h. Afterwards, medium was removed, MTT (1 mg/mL) was added for 4 h and then DMSO (100 μL) was added to dissolve the formazan. Relative cell viabilities were measured by microplate reader (Model 680, Bio-Rad) to determine the absorbance of each well at 570 nm.
Statistical analysis
The results were stated as mean, standard deviation (SD), 95% confidence interval (CI) and inhibition coefficient (IC50). ANOVA test was also accessed. All the statistical analyses were performed using the GraphPad Prism V.6.01. Statistical significance was established when *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Results and discussion
CpE phytochemical screening
Figure 3 shows CpE prepared by percolation method. The qualitative phytochemical screening of CpE was found to contain alkaloids, flavonoids, terpenoids, phenols and tannins. Kar et al. (2018) and Morsy et al. (2016) have also reported the presense of those natural products in extract of Calotropis procera (Morsy et al. 2016; Kar et al. 2018). These phytochemicals have potent anti-cancer activities, but encapsuation of those phenolic compounds can aid their anti-cancer properties and safe delivery in complex human body (Huang et al. 2010; Samy et al. 2012a).
Emulsion dispersity analysis
For water in oil monodisperse microemulsion, the flow rate for the continuous phase was adjusted to 4.26 mL/h and for the dispersed phase was adjusted to 1.80 mL/h. The water in oil emulsion provides a chemical reaction to occur within the aqueous phase and acts as microreactor for nanoparticles synthesis. The factors, such as size and shape of microemulsions, influence the mixing speed and reaction in aqueous phase. The spherical and monodispersed microemulsions provide uniform distribution of microenvironment to a reaction mixture which ultimately contributes significantly in the formation of high-encapsulation efficiency drug carriers (Damiati et al. 2018). Therefore, the monodispersed emulsions (0.49 mm ± 0.02) formed in this research by microfluidic system were proposed to provide microcarriers with uniform size distribution and high-encapsulation efficiency of CpE.
Characterization of CpE–CsAg microcarriers
UV-spectrum analysis
The samples collected from microchip at 95 °C did not show any change in colour. However, with the addition of 10% NaOH, the colour changed from light green to reddish brown. This change in colour was quite visible after every 30 s; however, the reddish brown colour became stable after 90 s and no change was observed afterwards (Fig. 4). The change in colour and the intensity of reddish brown colour is due to the excitation of AgNO3 surface plasmon resonance, confirming the formation of silver nanoparticles (Kalaivani et al. 2018). The UV–Vis spectrum of samples without NaOH had not shown the silver nanoparticle characteristic peak around 400 nm and peak of CpE was quite visible at 340 nm (Electronic supplementary material figure ESM_1). This indicates that CpE was unable to reduce AgNO3 and NaOH was required as reducing agent for synthesis of CsAg. This might be because of the difference in the preparation of plant extract in the current research as compared to the earlier reported studies in which the leaves of Calotropis procera were just cut, boiled and filtered and the filtrate was then used for reduction of silver nanoparticles (Dada et al. 2017; Mutluq Alkammash 2017). Although, in this research, the proficient maceration and percolation methods had been used for the extraction of pharmacological active phytochemicals as described in Sect. 2.2.
It was observed that there was no peak of silver nanoparticles in UV–Vis spectrum when analysis was performed at room temperature as shown in Fig. 5a. This is because precipitates of Ag2O were formed after 30–40 min by adding NaOH. Therefore, it was inferred that in order to obtain the adequate capping property of chitosan for stabilization of silver nanoparticles, the chitosan is needed to be heated up to 95 °C. After heating the reaction mixture at 95 °C, the resulting CsAg were found to be more stable and had shown quite visible peak around 400 nm as indicated in Fig. 5b. These results justified that the chitosan capping ability increases as it is heated to 95 °C which is in agreement with the previous reported studies (Akmaz et al. 2013; Nate et al. 2018). In UV–Vis spectrometry of CpE, a wide peak at 340 nm corresponds to the unsaturated groups (Jain et al. 2016). This peak was observed in every sample of CsAg which is justifying the encapsulation of CpE in microspheres. The fluctuation in the temperature of water bath was noticed to be 95 ± 5 °C.
SEM analysis and %EE calculation
The samples prepared at room temperature had revealed the flower-like appearance consisting of interconnected nano-needles of Ag2O with mean diameter of 161 ± 25 nm as shown in (ESM_2). These results were in agreement with the literature (Wei et al. 2011; Zhou et al. 2014). The SEM image confirmed that the silver nanoparticles were not formed at room temperature as justified by the absence of characteristic peak of silver in UV–Vis graph.
Figure 6 illustrates the SEM images of microcarriers and silver nanoparticles synthesized at 95 °C using PTFE tubular reactor. The SEM analysis of chitosan raw powder revealed its non-porous and smooth surface which was similar to the CpE surface morphology with only difference of cake-like appearance of chitosan as shown in Fig. 6a, b, respectively. The unloaded CsAg revealed the irregular shaped particles with rough surface morphology which might be due to some agglomeration of silver particles. While on loading the CpE in CsAg, a significantly different morphology of microcarriers was noticed. The spherical silver nanoparticles became quite visible on smooth surface of chitosan and CpE at magnification scale of 5 µm and 10 µm shown in Fig. 6c–g. It can be interpreted that CpE might also play a role in the surface morphology and stability of silver nanoparticles to some extent. Therefore, the difference in size of silver nanoparticles in different samples is significant and can be related to the role of drug in stabilization of particles. In this research, smooth circular chitosan microspheres were also visible with size distribution of 23.7 ± 14 µm at a magnification of 200 µm. Therefore, this could be claimed that silver nanoparticles were found embedded in chitosan microspheres.
The CpE–CsAg microcarriers formed by heating were found to have higher % EE (average 77.125%) as compared to the ones found for non-heated samples (average % EE = 57.195%). The difference in % EE may be due to the role of CsAg microcarriers which appears as significant encapsulating agent for drug as compared to the Ag2O nano-needles. It was also found that the CsAg loaded with CpE of volumetric ratio 2 showed adequate % EE (77.25%) with even sized distribution of silver nanoparticles (97.5 ± 8.8 nm). The SEM images and high % EE are confirming the significant role of monodispersed microemulsions in the formation of evenly sized distributed chitosan microcarriers embedding the silver nanoparticles and CpE.
The silver nanoparticles shows an optical absorption typical band peak for metallic silver nano-crystallites (Murugan et al. 2016) as evident by the EDX results (Fig. 7a). The Fe peak might be due to its presence in CpE (Verma 2014) and the peaks of oxygen and carbon were due to polymeric chitosan layer on stable silver nanoparticles. The peak of Al came from the substrate used to analyze the EDX of sample. Along with the qualitative analysis, the quantitative measurement in % weight was also performed for silver nanoparticles (4.9%) as shown in Fig. 7b. EDX analysis confirmed the presence of silver nanoparticles in the sample capped with polymeric chitosan with no evidence for Ag2O (Murugan et al. 2016; Yang et al. 2016).
FTIR analysis confirming the CpE–CsAg microcarriers formation
The absorbance of IR by various functional groups of CpE and CsAg microcarriers is given in Table 1 and spectrum is illustrated in Fig. 8. The CpE displayed the peaks at various intensities which represents the presence of multifaceted functional groups. The absorbance at 1304 cm−1, 1277 cm−1 and 1043 cm−1 confirming the presence of primary and secondary alcohols, flavonoids and tertiary alcohol, respectively (Oliveira et al. 2016). The FTIR peaks verified the phytochemicals screening results for the presence of phenols and flavonoids. Furthermore, the appearance of CpE functional groups in CpE–CsAg microcarriers indicated the successful encapsulation of CpE in CsAg.
The shifting and disappearance of chitosan peaks in CsAg and control samples were noticeable that might be due to the formation of composites as reported earlier (Govindan et al. 2012). The peak of chitosan seen at wavelength 1636 cm−1 indicated the amino group of amide bonds which confirmed presence of acetyl group of deacetylated chitin and chitosan –CONH2 bond (Venkatesham et al. 2014). The disappearance of chitosan peak at 1636 cm−1 and appearance of small band of N-acetyl group (amide II) at 1557 cm−1 justified the stabilization of silver nanoparticles by chitosan amino groups (Queiroz et al. 2015). Furthermore, the slight broadening and elevation of peak around 3280 cm−1 in CsAg and control samples indicated the role of amino and hydroxyl groups of chitosan in chelation and stabilization of silver ions (Chen et al. 2014; Kalaivani et al. 2018; Nate et al. 2018).
Anti-oxidant activity
H2O2 is sometimes toxic to the cells since it gives rise to high concentration of hydroxyl radicals. Therefore, the anti-oxidant activity of extract and microcarriers can be evaluated by H2O2 anti-oxidant assay. The research results indicated that the H2O2 scavenging ability of CpE and CpE–CsAg2 were dependent on their concentration as shown in Fig. 9. The scavenging ability of loaded microcarriers (CpE–CsAg2, IC50 = 88.78 ± 15 µg/mL) was found to be higher as compared to the CpE (IC50 = 110.4 ± 18 µg/mL) at low concentration. The highest anti-oxidant activity of CpE was 71% which was similar to the previous reports (Rani et al. 2018). The radical scavenging ability of CpE and CpE–CsAg2 was significantly different (p < 0.01) from ascorbic acid. These results confirmed that the CsAg microcarriers play a vital role in increasing the radical scavenging proficiency of CpE. Hence, CpE-loaded CsAg microcarriers were predicted to have powerful combinational effect in anti-breast cancer activity by arresting the high-level reactive oxygen species (ROS). After validation of H2O2 scavenging ability, the loaded microcarriers were evaluated for their anti-tumour activity.
In vitro anti-breast cancer activity
The aforementioned analysis confirmed that the CpE had been successfully encapsulated into CsAg microcarriers. Therefore, it could be easily utilized to achieve a combinational therapy approach to halt the growth of breast cancer cells. Figure 10 demonstrates that the CsAg microspheres enhanced the cytotoxicity of CpE significantly, that is stronger than CpE alone. After 24 h of incubation, CpE reduced 9% of cells viability at concentration of 80 µg/mL, while CpE.CsAg6 could reduce 52.33% cells viability at concentration of 100 µg/mL. Moreover, CpE–CsAg2 showed the most significant anti-cancer cytotoxicity with reduced cells viability up to 6.34% and 4.63% at concentrations of 80 µg/mL and 100 µg/mL respectively. These results confirmed that the CpE-loaded CsAg microcarriers could effectively kill the 4T1 breast cancer cells on concentration-dependent manner. When the concentration of CpE–CsAg2 was more than 80 µg/mL, it could inhibit the growth of cancer cells above 95%.
In vitro cytotoxicity of CpE, CpE–CsAg2 and CpE–CsAg6 against 4T1 cells by MTT assay. Each value represents the mean (n = 8). Statistical analysis was performed using ANOVA followed by Tukey’s multiple comparison test (*p < 0.5, **p < 0.01, ***p < 0.001 and ****p < 0.0001) which is evaluating the results as highly significant
The IC50 values were measured to quantitatively characterize the cytotoxicity of CpE and CpE–CsAg2 in order to estimate the concentration of drug required for 50% of cell death. The IC50 value of CpE–CsAg2 was 42.53 µg/mL which was much lower than the values obtained for CpE (~ 60.54 µg/mL). The increased cytotoxicity of CpE-loaded CsAg against the 4T1 cells might be because of improvement in endocytosis uptake of the drug due to chitosan microcarriers which eventually increases the intracellular CpE concentration and leads to cell death.
Previously, CpE has been reported to show significant results in supressing the growth of tumour cells (Alzahrani et al. 2017). The root extract of Calotropis procera has known to contain biological active protein that matches apolipoprotein A-I protein which acts in the suppression of breast cancer via the NF-kB pathway (Samy et al. 2012b). Likewise, chitosan and silver composites anti-breast cancer activity has been very well characterized in the previous reported studies (Nayak et al. 2016; Abaza et al. 2018; Bilal et al. 2019). However, the capability of CpE-loaded CsAg microcarriers synthesized in this research to inhibit the breast cancer proliferation with above 95% efficacy has not been reported previously.
Conclusion
In this research, the CsAg composites were synthesized as microcarriers for encapsulation of CpE to increase its bioavailability. The microcarriers synthesized at room temperature in microchip were found to precipitate out as Ag2O because of the instability of silver nanoparticles. On the other hand, stabilized silver nanoparticles were formed upon heating the reaction mixture at 95 °C in PTFE microfluidic reactor. These results showed the effect of heating on chitosan as an essential parameter to act as an efficient stabilizing agent. The characterization of these microcarriers had justified the formation of CsAg microcarriers and encapsulation of CpE with high % EE 77.125 ± 6.9%. Microfluidic system was found to be an efficient method for one-step encapsulation of anti-oxidant with uniform sized distribution of particles. Moreover, these loaded microcarriers had shown a remarkable activity to inhibit the 4T1 cell proliferation (p < 0.0001). This work has emphasized on the combinational therapeutic applications of microfluidic system. It can also be concluded that the lack of any noticeable toxicity of CsAg provides safe application of these microspheres in anti-cancer therapy. The in vivo studies in future can further validate the synergistic combination of CpE, chitosan and silver to supress the breast cancer cell growth.
References
Abaza A, Mahmoud GA, Hegazy EA et al (2018) Cytotoxic effect of chitosan based nanocomposite synthesized by radiation: in vitro liver and breast cancer cell line. J Pharm Pharmacol. https://doi.org/10.17265/2328-2150/2018.04.002
Adhikari HS, Yadav PN (2018) Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int J Biomater 2018:1–29. https://doi.org/10.1155/2018/2952085
Akmaz S, Dilaver Adgüzel E, Yasar M, Erguven O (2013) The effect of ag content of the chitosan-silver nanoparticle composite material on the structure and antibacterial activity. Adv Mater Sci Eng 2013:6. https://doi.org/10.1155/2013/690918
Alzahrani HS, Swed Alzahrani H, Mutwakil M et al (2017) Anticancer and antibacterial activity of Calotropis procera leaf extract. J Basic Appl Sci Res 7:18–25
Alzahrani HS, Almalki SA, Rizgallah MR (2019) Study of the cytotoxic effect of Calotropis procera on breast cancer cell line T47D in vitro: a traditional remedy. Int J Sci Innov 7:107–112. https://doi.org/10.32594/IJSI
American Cancer Society (2019) Cancer facts & figures 2019: Atlanta. Am cancer Soc 1–76
Amoyav B, Benny O (2018) Controlled and tunable polymer particles’ production using a single microfluidic device. Appl Nanosci 8:905–914. https://doi.org/10.1007/s13204-018-0790-0
Bilal M, Zhao Y, Rasheed T et al (2019) Biogenic nanoparticle–chitosan conjugates with antimicrobial, antibiofilm, and anticancer potentialities: development and characterization. Int J Environ Res Public Health 16:598–612. https://doi.org/10.3390/ijerph16040598
Chandirika JU, Sindhu R, Selvakumar S, Annadurai G (2018) Herbal extract encapsulated in chitosan nanoparticles: a novel strategy for the treatment of urolithiasis. INDO Am J Pharm Sci 1:1955–1961. https://doi.org/10.5281/zenodo.1212445
Chen Q, Jiang H, Ye H et al (2014) Preparation, antibacterial, and antioxidant activities of silver/chitosan composites. J Carbohydr Chem 33:298–312. https://doi.org/10.1080/07328303.2014.931962
Dada AO, Ojediran OJ, Dada FE et al (2017) Green synthesis and characterization of silver nanoparticles using Calotropis procera extract. Orig Res Artic J Appl Chem Sci Int 8:137–143
Damiati S, Kompella UB, Damiati SA, Kodzius R (2018) Microfluidic devices for drug delivery systems and drug screening. Genes (Basel) 9:103. https://doi.org/10.3390/genes9020103
Ellahi R, Hussain F, Asad Abbas S et al (2020) Study of two-phase newtonian nanofluid flow hybrid with hafnium particles under the effects of slip. Inventions 5:6. https://doi.org/10.3390/inventions5010006
Farouk AEA, Ahamed NT, Alzahrani O et al (2016) Antimicrobial activities evaluation from the extracts of leaves, flowers, fruits and latex of calotropis procera from Taif. Int J Curr Microbiol Appl Sci 5:240–256. https://doi.org/10.20546/ijcmas.2016.511.026
Govindan S, Nivethaa EAK, Saravanan R et al (2012) Synthesis and characterization of chitosan–silver nanocomposite. Appl Nanosci. https://doi.org/10.1007/s13204-012-0109-5
Gul R, Jan SU, Faridullah S et al (2017) Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from ephedra intermedia indigenous to balochistan. Sci World J. https://doi.org/10.1155/2017/5873648
Hintzer K, Zipplies T, Carlson DP, Schmiegel W (2016) Fluoropolymers, organic. Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–55
Hoseinzadeh S, Ramezani AH (2019) Investigation of Ta/NII-WO 3 /FTO structures as a semiconductor for the future of nanodevices. J Nanoelectron Optoelectron 14:1413–1419. https://doi.org/10.1166/jno.2019.2564
Hoseinzadeh S, Ghasemiasl R, Bahari A, Ramezani AH (2017a) n-type WO3 semiconductor as a cathode electrochromic material for ECD devices. J Mater Sci Mater Electron 28:14446–14452. https://doi.org/10.1007/s10854-017-7306-7
Hoseinzadeh S, Ghasemiasl R, Bahari A, Ramezani AH (2017b) The injection of Ag nanoparticles on surface of WO3 thin film: enhanced electrochromic coloration efficiency and switching response. J Mater Sci Mater Electron 28:14855–14863. https://doi.org/10.1007/s10854-017-7357-9
Hoseinzadeh S, Ghasemiasl R, Bahari A, Ramezani AH (2018) Effect of post-annealing on the electrochromic properties of layer-by-layer arrangement FTO-WO3-Ag-WO3-Ag. J Electron Mater 47:3552–3559. https://doi.org/10.1007/s11664-018-6199-4
Huang WY, Cai YZ, Zhang Y (2010) Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 62:1–20. https://doi.org/10.1080/01635580903191585
Hussein-Al-Ali SH, Kura A, Hussein MZ, Fakurazi S (2018) Preparation of chitosan nanoparticles as a drug delivery system for perindopril erbumine. Polym Compos 39:544–552. https://doi.org/10.1002/pc.23967
Jacob S, Nair AB, Shah J (2020) Emerging role of nanosuspensions in drug delivery systems. Biomater Res 24
Jain PK, Soni A, Jain P, Bhawsar J (2016) Phytochemical analysis of Mentha spicata plant extract using UV–VIS, FTIR and GC/MS technique. J Chem Pharm Res 8:1–6
Kalaivani R, Maruthupandy M, Muneeswaran T et al (2018) Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front Lab Med 2:30–35. https://doi.org/10.1016/j.flm.2018.04.002
Kar D, Pattnaik PK, Pattnaik B, Kuanar A (2018) Antimicrobial analysis of different parts extract in different solvent system of a waste weed-Calotropis procera. Asian J Pharm Clin Res 11:227–231. https://doi.org/10.22159/ajpcr.2018.v11i2.21081
Kaur H (2018) Review on the extraction methods used in medicinal plants. Int J Adv Manag Technol Eng Sci
Kesente M, Kavetsou E, Roussaki M et al (2017) Encapsulation of olive leaves extracts in biodegradable PLA nanoparticles for use in cosmetic formulation. Bioengineering 4:1–14. https://doi.org/10.3390/bioengineering4030075
Khanzada SK, Shaikh W, Kazi TG et al (2008) Analysis of fatty acid, elemental and total protein of Calotropis procera medicinal plant from Sindh, Pakistan. Pakistan J Bot 40:1913–1921
Kong T, Wu J, Yeung KWK et al (2013) Microfluidic fabrication of polymeric core–shell microspheres for controlled release applications. Biomicrofluidics. https://doi.org/10.1063/1.4819274
Kummari SVKR, Kummara MR, Palem RR et al (2015) Chitosan-poly(aminopropyl/phenylsilsesquioxane) hybrid nanocomposite membranes for antibacterial and drug delivery applications. Polym Int 64:293–302. https://doi.org/10.1002/pi.4789
Li J, Cai C, Li J et al (2018) Chitosan-based nanomaterials for drug delivery. Molecules 23:1–26. https://doi.org/10.3390/molecules23102661
Maslanka Figueroa S, Fleischmann D, Beck S, Goepferich A (2020) Thermodynamic, spatial and methodological considerations for the manufacturing of therapeutic polymer nanoparticles. Pharm Res 37:59. https://doi.org/10.1007/s11095-020-2783-4
Medina-Sánchez M, Xu H, Schmidt OG (2018) Micro- and nano-motors: the new generation of drug carriers. Ther Deliv 9:303–316. https://doi.org/10.4155/tde-2017-0113
Mohan CO, Gunasekaran S, Ravishankar CN (2019) Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. NPJ Sci Food 3:2. https://doi.org/10.1038/s41538-019-0034-z
Morsy N, Al Sherif EA, Abdel-Rassol TMA (2016) Phytochemical analysis of Calotropis procera with antimicrobial activity investigation. Main Gr Chem 15:267–273. https://doi.org/10.3233/MGC-160206
Munin A, Edwards-Lévy F (2011) Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 3:793–829. https://doi.org/10.3390/pharmaceutics3040793
Murugan K, Anitha J, Dinesh D et al (2016) Fabrication of nano-mosquitocides using chitosan from crab shells: Impact on non-target organisms in the aquatic environment. Ecotoxicol Environ Saf 132:318–328. https://doi.org/10.1016/j.ecoenv.2016.06.021
Mutluq Alkammash N (2017) Synthesis of silver nanoparticles from artemisia sieberiand calotropis procera medical plant extracts and their characterization using SEM analysis. Biosci Biotechnol Res Asia 14:521–526. https://doi.org/10.13005/bbra/2474
Nair R, Reddy BH, Kumar CKA, Kumar KJ (2009) Application of chitosan microspheres as drug carriers: a review. J Pharm Sci Res 1:1–12
Najafi-Ashtiani H, Bahari A, Gholipour S, Hoseinzadeh S (2018) Structural, optical and electrical properties of WO3–Ag nanocomposites for the electro-optical devices. Appl Phys A Mater Sci Process 124:1–9. https://doi.org/10.1007/s00339-017-1412-5
Nate Z, Moloto MJ, Mubiayi PK, Sibiya PN (2018) Green synthesis of chitosan capped silver nanoparticles and their antimicrobial activity. MRS Adv 3:2505–2517. https://doi.org/10.1557/adv.2018.368
Nayak D, Minz AP, Ashe S et al (2016) Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J Colloid Interface Sci 470:142–152. https://doi.org/10.1016/j.jcis.2016.02.043
Obayemi JD, Soboyejo WO, Odusanya OS, et al (2014) Abstract B40: Injectable multifunctional biodegradable polymeric microspheres for localized drug delivery in breast cancer treatment. In: Cancer Epidemiology and Prevention Biomarkers. American Association for Cancer Research (AACR), pp B40–B40
Oliveira RN, Mancini MC, de Oliveira FCS et al (2016) FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio Janeiro) 21:767–779. https://doi.org/10.1590/s1517-707620160003.0072
Palem RR, Saha N, Shimoga GD et al (2018) Chitosan–silver nanocomposites: new functional biomaterial for health-care applications. Int J Polym Mater Polym Biomater 67:1–10. https://doi.org/10.1080/00914037.2017.1291516
Queiroz MF, Melo KRT, Sabry DA et al (2015) Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs 13:141–158. https://doi.org/10.3390/md13010141
Rani R, Sharma D, Chaturvedi M, Yadav JP (2018) Phytochemical analysis, antibacterial and antioxidant activity of Calotropis procera and Calotropis gigantea. Nat Prod J. https://doi.org/10.2174/2210315508666180608081407
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from chinese green tea. Carcinogenesis 10:1003–1008. https://doi.org/10.1093/carcin/10.6.1003
Salari E, Peyghambarzadeh SM, Sarafraz MM, Hormozi F (2016) Boiling thermal performance of TiO2 aqueous nanofluids as a coolant on a disc copper block. Period Polytech Chem Eng 60:106–122. https://doi.org/10.3311/ppch.8262
Salari E, Peyghambarzadeh SM, Sarafraz MM et al (2017) Thermal behavior of aqueous iron oxide nano-fluid as a coolant on a flat disc heater under the pool boiling condition. Heat Mass Transf und Stoffuebertragung 53:265–275. https://doi.org/10.1007/s00231-016-1823-4
Samy RP, Rajendran P, Li F et al (2012a) Identification of a novel Calotropis procera protein that can suppress tumor growth in breast cancer through the suppression of NF-κB pathway. PLoS ONE 7:1–14. https://doi.org/10.1371/journal.pone.0048514
Samy RP, Rajendran P, Li F et al (2012b) Identification of a novel Calotropis procera protein that can suppress tumor growth in breast cancer through the suppression of NF-κB pathway. PLoS ONE. https://doi.org/10.1371/journal.pone.0048514
Sarafraz MM, Arjomandi M (2018a) Demonstration of plausible application of gallium nano-suspension in microchannel solar thermal receiver: experimental assessment of thermo-hydraulic performance of microchannel. Int Commun Heat Mass Transf 94:39–46. https://doi.org/10.1016/j.icheatmasstransfer.2018.03.013
Sarafraz MM, Arjomandi M (2018b) Thermal performance analysis of a microchannel heat sink cooling with copper oxide-indium (CuO/In) nano-suspensions at high-temperatures. Appl Therm Eng 137:700–709. https://doi.org/10.1016/j.applthermaleng.2018.04.024
Sarafraz MM, Hormozi F, Kamalgharibi M (2014) Sedimentation and convective boiling heat transfer of CuO–water/ethylene glycol nanofluids. Heat Mass Transf und Stoffuebertragung 50:1237–1249. https://doi.org/10.1007/s00231-014-1336-y
Shahzadi I, Bashir M, Bashir S, Inayat MH (2018) Thermally assisted coating of PVA for hydrophilic surface modification of PMMA microchannel for oil in water emulsion. In: Proceedings of 2018 15th International Bhurban Conference on Applied Sciences and Technology, IBCAST 2018. pp 51–54
Singh M, Sinha I, Mandal RK (2009) Role of pH in the green synthesis of silver nanoparticles. Mater Lett 63:425–427. https://doi.org/10.1016/j.matlet.2008.10.067
Stoica R, Şomoghi R, Ion RM (2013) Preparation of chitosan—tripolyphosphate nanoparticles for the encapsulation of polyphenols extracted from rose hips. Dig J Nanomater Biostruct 8:955–963
Tsao CW, DeVoe DL (2009) Bonding of thermoplastic polymer microfluidics. Microfluid Nanofluidics 6:1–6. https://doi.org/10.1007/s10404-008-0361-x
Van PhuD, Duy NN, Quoc LA, Hien NQ (2009) The effect of ph and molecular weight of chitosan on silver nanoparticles synthesized by irradiation. Res Dev Cent Radiat Technol 47:47–52
Venkatesham M, Ayodhya D, Madhusudhan A et al (2014) A novel green one-step synthesis of silver nanoparticles using chitosan: catalytic activity and antimicrobial studies. Appl Nanosci. https://doi.org/10.1007/s13204-012-0180-y
Verma VN (2014) The chemical study of calotropis. Int Lett Chem Phys Astron 1:74–90. https://doi.org/10.18052/www.scipress.com/ILCPA.20.74
Vimala K, Mohan YM, Varaprasad K et al (2011) Fabrication of curcumin encapsulated chitosan-PVA silver nanocomposite films for improved antimicrobial activity. J Biomater Nanobiotechnol 2:55–64. https://doi.org/10.4236/jbnb.2011.21008
Wang X, Liu J, Wang P et al (2018) Synthesis of biomaterials utilizing microfluidic technology. Genes (Basel) 9:E283. https://doi.org/10.3390/genes9060283
Wei W, Mao X, Ortiz LA, Sadoway DR (2011) Oriented silver oxide nanostructures synthesized through a template-free electrochemical route. J Mater Chem 21:432–438. https://doi.org/10.1039/c0jm02214d
Yang CH, Wang LS, Chen SY et al (2016) Microfluidic assisted synthesis of silver nanoparticle–chitosan composite microparticles for antibacterial applications. Int J Pharm 510:493–500. https://doi.org/10.1016/j.ijpharm.2016.01.010
Yousefzadeh S, Rajabi H, Ghajari N et al (2019) Numerical investigation of mixed convection heat transfer behavior of nanofluid in a cavity with different heat transfer areas. J Therm Anal Calorim. https://doi.org/10.1007/s10973-019-09018-6
Zhou N, Li D, Yang D (2014) Morphology and composition controlled synthesis of flower-like silver nanostructures. Nanoscale Res Lett 9:1–6. https://doi.org/10.1186/1556-276X-9-302
Zugic A, Tadic V, Savic S (2020) Nano-and microcarriers as drug delivery systems for usnic acid: review of literature. Pharmaceutics. https://doi.org/10.3390/pharmaceutics12020156
Acknowledgements
This research is supported by Pakistan Institute of Engineering and Applied Sciences (PIEAS), Islamabad, Pakistan. Authors are grateful to Mr. Asghar Ali and Mr. Islam Khattak for providing technical support in various stages of research. We extend our heartful thanks to Engr. Usman Aftab for his significant assistance in proofreading of this research. We are also thankful to Malik Ihsanullah Khan from Division of Molecular Medicine (USTC) for helping in preparation of plant extract and its phytochemical analysis. The authors would like to pay a special thanks to University of Science and Technology of China for providing chitosan (CAS: 9012-76-4, Sigma).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aftab, A., Bashir, S., Rafique, S. et al. Microfluidic platform for encapsulation of plant extract in chitosan microcarriers embedding silver nanoparticles for breast cancer cells. Appl Nanosci 10, 2281–2293 (2020). https://doi.org/10.1007/s13204-020-01433-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01433-8