Abstract
The quality of river and borehole water fluctuates because of both agricultural and industrial pollutants. Surface runoff during the rainy seasons is high which promote increased turbidity levels in water sources, and this exerts pressure on the quality and usability of the water for domestic use. Unfortunately, most municipalities in developing countries are poor to afford conventional water treatment methods. This study assessed the use of natural coagulants extracted from sunflower seeds for turbidity treatment. Water samples were collected during summer, winter, and autumn from 10 randomly selected groundwater sources and three segments of the Mwerahari River in Buhera District, Zimbabwe. Results captured seasonal turbidity variations across the river segments and the boreholes. Summer season recorded the maximum average levels of turbidity (76 NTU) while autumn and winter recorded 38.7 NTU 36.7 NTU, respectively. Water turbidity levels were above the acceptable 5 NTU Standard Association of Zimbabwe and WHO. The maximum removal efficiency of turbidity was achieved at 80 min at the dose of 4 g/l. These results revealed that the removal efficiency of 95% with 4.6 NTU turbidity is a function of dose; removal efficiency increases as dose of coagulant increases. These results demonstrated that sunflower seed is an effective low-cost natural coagulant for turbidity water treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a global growing concern of environmental pollution which continues to hinder communities from having access to safe drinking water, constituting considerable challenges to water security. According to UNESCO (2019), roughly one-third of the world population have insufficient potable drinking water facilities; a scenario exacerbated by environmental degradation, climate change, population growth and rapid urbanisation, among other factors. In 2015, the WHO and UNICEF Joint Monitoring Programme (JMP) reported that more that 663 million people from low- and middle-income countries live without access to safe drinking water and at the same time 2.1 billion people use water sources that are polluted (WHO 2020). The situation is quite severe in most African countries where access to safe drinking water is compounded by insufficient funding, limited technical expertise as well as lack of appropriate infrastructure coupled with infrastructure deficits and absence (Allen et al. 2017; Herslund and Mguni 2019). As such, most households opt for water treatment methods that in most cases do not eliminate any of the pathogens let alone get rid of undesirable parameters such as turbidity, taste, and smell.
Water turbidity describes the clearness of water and is the major visible indicator of the quality of river systems, and aesthetic destructions in surface waters (Zounemat-Kermani et al. 2021). Turbidity in water systems is influenced by discharge of suspended sediments through erosion of silt, organic matter emanating from construction and agricultural activities as well as natural sources (Yunus et al. 2020; Zounemat-Kermani et al. 2021). The small, suspended solids consist of very small colloidal particles which rarely settles at the bottom of containers. Thus, turbid water presents both safety and aesthetic concerns in consumable water (WHO 2017a). While turbidity does not translate to a direct hazard to public health, it could indicate the prevalence of harmful microbes (WHO 2017b) and suggestive of poor removal of pathogens through the filtration supply system (Awotedu et al. 2020).
In high turbid waters, harmful microorganisms tend to attach themselves to the particles. Besides human health risks, survival of aquatic life is compromised due to limited sunlight and oxygen exhibited by high turbidity (Tahiru et al. 2020). Numerous researchers have reported the relationship linking high turbidity in drinking water and the higher risk of gastro-intestinal diseases among humans (Huang et al. 2021; De Roos et al. 2017; Tinker et al. 2010). A study by Huang et al. (2021) in eastern China reported positive correlation between occurrence of opportunistic pathogens and turbidity; De Roos et al. (2017) found positive associations between turbidity in drinking water and acute gastro-intestinal illness incidences in different metropolitan areas across diverse time periods, and with both unfiltered and filtered supplies. Therefore, it is always vital to treat turbid water before human consumption to avoid subjecting households to infectious microorganisms such as viruses and bacteria that bind themselves to the suspended solids.
Provision of fresh potable water devoid of turbidity is implicitly significant for the acceleration of social-economic advancement in most sub-Saharan African countries (Kitheka et al. 2022). To speed up the coalescence and agglomeration of suspended particles, coagulation and flocculation should be employed since they are most critical processes used to accelerate the elimination of turbidity, remove the colloidal particles such as algae/silt, and ultimately minimise the risk of gastro-intestinal diseases and prevent the blockage of filters (Aboubaraka et al. 2017). However, using aluminium in the treatment of drinking water is ideal when used in moderation since its increased use pose some human health concerns. Kramer (2014) observed that high aluminium intake by humans has been linked with several possible neuropathological diseases including presenile dementia and Alzheimer’s disease. Further, the use of aluminium for water treatment may not be cost effective and ideal for most rural poor communities; in addition to requiring proper training and technical skills (Pooi and Ng 2018).
According to Talnikar (2017), flocculants and all plant-based bio-flocculants have recently attracted global recognition in water treatment due to their easy to grow, biodegradability and the fact that they generate lower amounts of sludge. Choy et al. (2014) applauds bio-flocculants for their ability to produce environmentally friendly, non-corrosive and cheap coagulants. Aslamiah et al. (2013) and Megersa et al. (2014) observed capability of coagulants from plants like Moringa-Oliefera has received attention from various researchers. Various species of Moringa, M. subcordata and M. stenopetala were tested as coagulants in river water by Megersa et al (2014). Effective removal of turbidity to 5 NTU and 3.5 NTU was achieved with maximum doses of 70 mg/l and 80 mg/l, respectively. Adams and Mulaba-Bafubiandi (2014) also report that turbidity removal efficiency of rice husks material can be up to 96%. Natural coagulants from Musa paradisica (banana peels) powder reported efficient turbidity removal of > 83% at pH values of 3.0–12.0 with highest turbidity elimination of 98.14% at pH 11 (Daverey et al. 2019). Unnisa and Bi (2018) experimented papaya seed protein as a natural coagulant employing jar tests supported by pre-treatment disinfection to remove turbidity. The results exhibited potential to remove turbidity at 100% when C. papaya seeds were combined with alum at coagulant doses of about 0.2–0.6 mg/L at 30 min.
However, available literature reports few studies that have used sunflower as a coagulant for turbid water treatment. Few studies have reported on the efficiency of sunflower in combination with other bio-flocculants as a coagulant for turbidity water treatment. Belbahloul et al. (2014) confirmed with results obtained through comparative studies they carried out in Morocco and Tunisia, respectively, using biomaterials such as Aloe vera, tannin, Coccinia indica, Moringa oleifera, sunflower and Nirmali seed that these biomaterials exhibit flocculation performance, and sunflower extracts exhibited the highest flocculation rate and lowest residual turbidity. To bridge the existing knowledge gap, this paper reports on the ideal doses for different turbidity levels using sunflower extracts as natural coagulants at household level. Additionally, Kamal (2011) and Weisz et al. (2009) acknowledged that there are various naturally occurring compounds produced by different organs of the sunflower plants with great phytochemical properties. Sunflower plants contain organic compounds such as alkaloids, saponin, polyphenols, fatty acids as well as tannins that have antimalarial, antimicrobial, antiasthma, and antioxidant benefits to humans (Ngibad 2019; Mokif et al. 2020; Rauf et al. 2020; Mahamba and Palamuleni 2022). Therefore, the main aim of this study was to assess the coagulation potential of locally grown sunflower plants (Helianthus annuus) for the treatment of water turbidity in Buhera District Zimbabwe. Specifically, the study sought to establish optimal doses and time required to treat turbid water using sunflower extracts at household level. Establishing optimum dosage is imperative to avoid overdose or insufficient dosage which could result in low performance of coagulation process (Rozainy et al. 2014).
Materials and methods
Study area location and description
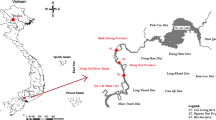
Buhera district is in Manicaland province, Zimbabwe between latitude 19° 20′ 0″S and longitude 31° 26′ 0″E (Fig. 1A). Manicaland province has seven districts namely Buhera, Chimanimani, Chipinge, Makoni, Mutasa, Mutare, and Nyanga and Fig. 1(B) shows the location of Buhera in the province. The delimitation of the study was Buhera district because the climate of the area is suitable for the cultivation of sunflower plants since it lies in ecological region iv; characterised by average rainfall of 650 mm, which is ideal for sunflower cultivation (ZSA 2012). The district ranks second in terms of rural population in Zimbabwe, and it is the most impoverished of the seven districts. As such, most of the households are not connected to clean and potable conventional water treatment facilities (ZNSA 2014). The Mwerahari River, which streams across the district before joining Save River, is the main source of domestic water in the district. For groundwater, access is achieved through various boreholes that are spread out within the study area to augment the surface water supply.
Water sampling and site selection
Water samples were collected from the main drinking water supplies in the study area (Fig. 1C). For precision purposes, water samples were collected in triplicates at three main locations along Mwerahari River, namely: upper section at Nerutatanga (point near the source), middle section at Nyashanu mission high school, and lower section at Fari, a point before the Mwerahari River joins the Save River. Like many other rivers, Mwerahari River with a catchment area of 172 km2 is heavily impacted by a variety of anthropogenic activities including settlements, agriculture, small-scale industries among others. Therefore, the study used purposive sampling targeting the identified surface and groundwater domestic water sources in the district governed by the dominant land uses prevalent in the study area.
Sampling of the boreholes was governed by the report from ZNSA (2014) which indicated that Buhera had 1242 boreholes. However, 931 were functional boreholes, representing 75% as working boreholes. To ensure coverage of all the boreholes, the district was divided into four quadrants on a Cartesian plane (i.e., North, South, East, and West). From each quadrant, functional boreholes were identified and at the time of water sample collection, Buhera South had 320 working boreholes, Buhera North 245, Buhera Central 221 and Buhera West had 145. Only 1% of the functional boreholes was chosen from each quadrant. Therefore, samples were fetched from boreholes and labelled as 01, 02 and 03; 04 and 05 from Buhera North; 06 and 07 from Buhera Central and 08, 09 and 10 from Buhera South. The dimensions of all the ten boreholes were analysed and recorded in Table 1. It was found necessary to establish the dimensions because the depth and geology of the area where the borehole is located, and the water level would have natural impact on the turbidity of the water (Moulin et al. 2019).
Collection of water samples
For this research, water sampling was conducted using pre-cleaned 1 L high-density polyethylene (HDPE) bottles washed in 1% HNO3 to collect water from the different identified water sources using grab sampling technique. It has been reported that HDPE bottles are able to preserve the sampled water for an extended time without modifying the parameters (APHA 2005). Prior to collection of the water samples, the polyethylene bottles were rinsed several times with the sample water for acclimatisation. Therefore, from Mwerahari River, the study collected a total of 72 water samples and 270 from the ten boreholes for turbidity measurement across the three seasons (summer, winter, and autumn).
After collecting the samples, the turbidity was measured using the Hach turbidimeter 2100 Q Model made in South Africa. This model was chosen because it has the capacity to measure turbidity from as low as 0 NTU and as high as 1000 NTU at a resolution of 0.01 NTU. The model has also a very high datalog of 500 records which enabled the records of the whole research to be captured by one instrument.
Analysis of results
For this research, three samples were collected at each sampling point. This was done to enhance the validity of the results. Means and standard deviations were calculated and expressed using ANOVA single factor analysis. To ascertain the quality of the water, results were compared with the Standards Association of Zimbabwe (SAZ) (Environmental Management Agency 2008) and the World Health Organisation (WHO 2017a) drinking water quality standards.
Experiment using sunflower organs as coagulant
The purpose of the study was to test the efficacy of Sunflower (Helianthus annuus) extracts as coagulant for turbid water treatment. For this study, the sunflower plants were cultivated at Nyashanu high school in Buhera district of Manicaland province in Zimbabwe for a period of 10 weeks. The mature plants at height approximately 2.2 m with a flowering head were picked and transported to the laboratory for processing.
Sample preparation
Different organs of the sunflower plants were used for the experiment. The study used four leaves measuring about 22 cm long with a diameter of 9 cm. For the stem, one piece measuring 30 cm cut from the mature 120 cm stem was used in this experiment as it was able to produce sufficient powder required for the coagulation process. Mature sunflower seeds weighing 2 kg were used for the experiment. All the roots from one plant were used for the experiment. The sunflower plant organs (i.e., leaves, stem, seeds, and roots) used for the experiment were washed twice, first with tap water and then with distilled water. The sunflower organs were oven dried at 65 °C for 24 h in a Jouan EU28. According to Foster (2020), 24-h drying period for the various plant organs is sufficient to evaporate all the moisture from the sunflower organs. The dried sunflower organs were milled separately into powder using a standard bench-top blender.
The extraction of phytochemicals involved the use of five solvents namely: ethanol, n-hexane, chloroform, acetone, and cold water. Exactly 2 g of each of the sunflower organ’s powder were agitated in 40 ml of each of the five solvents for a rapid speed of 150 rpm, and then subjected to shaking using an orbital shaker (CS 200 model) for 24 h at 20 °C. When the extraction was completed, the liquid extract was allowed to settle thereafter filtered using Whatman paper no.1. The sunflower liquid extract was dispensed in round-bottomed flasks and concentrated using vacuum rotary evaporator at 50 °C for 4 h. The supernatant solution was then used for the preparation of different coagulant doses to test for turbidity treatment.
Results and discussion
Turbidity levels of borehole and river water
Figure 2 shows the turbidity levels in borehole water during the summer season to be in the range of 6.5 NTU to 10 NTU. Water collected during winter (dry season) was clear with an average turbidity of 6.7 NTU. Turbidity levels from the borehole water were higher than the stipulated WHO and SAZ acceptable turbidity of 5 NTU suggesting that the water is not suitable for human consumption. The elevated turbidity could be attributed to the subsistence agricultural activities practised throughout the study area, where stream bank cultivation is widespread coupled with overgrazing. Subsistence agriculture and overgrazing induce soil erosion fostering higher contents of organic material and sediment discharge in runoff waters, which increases turbidity (Ruiz and Sanz-Sánchez 2020). Any turbidity above 5 NTU gives the water undesirable colour making the water unappealing for consumption.
The results of the analysis (Tables 2 and 3) also showed that there was no significant impact between seasonal turbidity with respect to the calculated F \(<\) F crit values. The study results yielded a standard deviation of < 5, revealing that turbidity of borehole water was not affected by seasonal variations. However, the geological formations of the study area as well as the influence of land uses could be attributed to the chemistry of the borehole water (Kumar et al. 2020).
Generally, the temporal and spatial water turbidity from all the sections of the river averaging 75.3 NTU were all above the recommended SAZ (5 NTU) and WHO (5 NTU) standards (Fig. 3). The maximum turbidity was recorded during summer season across all the river sections. This could be attributed to hydrological response to high rainfall events with increased runoff waters containing contaminants and suspended solids from residential and agricultural areas deposited into the river (Wang et al. 2021). Generally, the deposition of suspended solids is a function of hydraulic size, hydraulic conditions as well as suspended sediment advection (Wilkes et al. 2019). The lower section of the river registered the highest turbidity, and this was attributed to deposition of river’s sediment yield and organic matter load in the lower section because of lower energy associated with reduced river flow (Nel et al. 2018). The lower section of the river is characterised by no gravitational potential energy with less turbulent flow and deposition of all suspended river load (Balasubramanian 2010).
In the study area, rainy season rainfall increases soil erosion and sediment yield, contributing to the turbidity of water (Uwacu et al. 2021). Unsustainable agricultural practices prevalent in the Mwerahari River catchment contribute to high soil erosion which leads to siltation. Additionally, the study area experiences occasional winds during autumn, accelerating deposition of fine sand and dust particles into the river, in that way negatively affecting the turbidity of water. Winter yielded the minimal amounts of turbidity in all the river segments compared to other seasons (32 NTU); however, these turbidities were still high, exceeding the SAZ and WHO acceptable limits of 5 NTU.
The highest turbidity variation was recorded in during the rainy season had ± 36.33 NTU, which was greater than seven times the mean tolerable probable error of ± 5 (Table 3). During the rainy season, the river experiences high levels of runoff from the immediate catchment area and an influx of water from the tributaries; contributing to high turbid waters during summer and autumn. In autumn and winter, the turbidity was ± 1.44 NTU and ± 1.78 NTU, respectively, which was considerably less than the threshold. These results suggest that seasons had an impact on the turbidity variations; with high turbid waters recorded during summer followed by autumn, and winter having the lowest turbidity, F ˃ F crit.
Treatment of water turbidity using sunflower extracts
The study used chloroform solvent to test the potential of sunflower extracts in removing turbidity from borehole and river water. The data in Table 4 shows that using sunflower seed extract has a 60% potential to remove suspended solids from all water samples from the boreholes. The turbidity reduction is attributed to the presence of tannins and fatty acids in sunflower seed extracts (Özacar and Şengil 2003), which are natural products with high anticoagulant activity. The phenolic structure with an anionic character of tannins allows their application as natural coagulants for water treatment (Grenda et al. 2020). The order of turbidity removal efficiency is leaf extracts (50%) > root extracts (27%) and stem extracts (22%).
Comparatively, the results in Table 4 reveal lower turbidity from borehole water than from Mwerahari River (Fig. 3). Surface water like rivers have higher turbidity attributed to their susceptibility because of runoff from anthropogenic activities such as industrial, agricultural, and other human land uses (Singh et al. 2005) than groundwater (boreholes) which are protected against direct runoffs. The Mwerahari River has a catchment area of over 172 km2 and it means the river receives high inflows of water with high suspended solids from runoff and water from tributaries during summer. As such, elevated levels of suspended solids and total dissolved solids are present in surface water than in groundwater sources.
The data in Table 5 shows performance of sunflower extracts as a coagulant for turbidity water treatment during summer season. The experimental jar tests predicted a proper dosage of sunflower extract in reducing turbidity to the required quality. The amounts of sunflower extracts were varied between 0.5 g and 4 g to examine the efficiency of the coagulant. For the different sunflower organs, the treatment dosage of 2 g revealed an efficiency rate of 60% from the seed extract, 50% from the leaf extract, 27% from the stem extract while root extract had the lowest rate of 22%. The obtained values showed reduction of turbidity but not the allowable levels of 5 NTU prescribed by WHO and SAZ standards (Table 5). Although there was a reduction in turbidity, the levels were still above acceptable limits as shown in Table 5.
To determine more favourable coagulant quantity, the amounts of sunflower extracts were increased from 2 to 4 g. The administration of higher dosage concurs with Moulin et al. (2019) who proposed the need for repeated and increased dosages if initial results do not bring about desirable outcomes. Thus, the sunflower seed extracts removed over 95.6% of the turbidity from the water at a dosage of 4 g. Further examination of the data suggests a decreased potential of turbidity removal from leaf extracts by efficiency rate of 84.8%, stem extract (69.5%) while the use of root extracts in water coagulation resulted in turbidity removal efficiency of 65.5%. The results further show that 4 g of the extracts is undeniably optimal dosage for turbidity removal to recommended limits by WHO and SAZ in all segments of the river (Table 5). This indicate that the increment in dosage of sunflower seed extract show a significant relationship with turbidity removal. Additionally, the dosages of 2–4 g of the seed extract would produce satisfactory results in reducing water turbidity to allowable limits for domestic use. However, it should be noted that the extent of water turbidity governs the concentration of the extract to be used for optimal water treatment.
To establish the effectiveness of sunflower seed extracts’ ability to treat turbidity, different time lengths and different dosages were tested. The doses varied from 1 to 4 g at time intervals of ten minutes using the summer water samples collected from Mwerahari River. Summer season recorded the highest turbidity, and this provided a basis for evaluation of the efficiency of the seed extracts in removing the turbidness. Figure 4 elaborates the performance of sunflower seed extracts and shows the impact of contact time required to remove turbidity effectively.
The experiment investigated the effect of contact time and the quantity of the extracts carried out for 2 g, 3 g and 4 g of the natural coagulants. Figure 4 shows the variation in turbidness corresponding to time and dose. It can be observed that at contact time of 20 min with a lower dosage of 1 g achieved turbidity removal effectiveness from 88 to 35 NTU. However, increasing the quantity of the natural coagulant and the contact time exhibited a turbidity reduction trend. Previous studies revealed that turbidity would be reduced with increasing coagulant dosages used (Ahmad et al. 2022; Moran 2018). Ahmad et al (2022) experimented S. palustris (Climbing fern) as a coagulant and results showed that the highest amount of turbidity removal (24.9%) was achieved at 10 g/L where it clearly showed a significant difference compared to the rest of the coagulant dosages in which the obtained turbidity removal was within 13.3% to 17.2%. The use of 2 g of the seed extract at a timeline of 40 min yielded the turbidness removal efficiency of 60%. The minimum recording of water turbidity was 4.6 NTU with a quantity of 4 g at 80 min.
Additionally, the effect of time to test settling efficacy of coagulant was measured for 1 h at 10 min interval. Previous experiments have reported that increasing time, the extracts get enriched with polymeric substances primarily responsible for the turbidity removal. Daverey et al (2019) noted that the turbidity removal increased from 73.21% to 87.71% when settling time increased from 15 to 60 min for Dolichos lablab (Indian beans) seed’s coagulant. In this study, further experimentation suggests increased potential for turbidity removal from 88 to 23 NTU at contact time of 40 min. Based on Fig. 4, the effectiveness of coagulant to be used is influenced by varying the contact time; thus, increasing the dosage of sunflower seed extract and contact time.
Conclusion
Plant-based coagulants for this research included sunflower seeds, roots, stems and leaves. As observed and reflected by the research findings discussed, sunflower has great promising coagulant activity and turbidity reduction, and is highly recommended for domestic water treatment. The treatment efficiency of turbidity removal increased by increasing the dose of coagulants and settling time. As for the seeds, a dosage of 4 g at 80 min was found to reduce turbidity by 95%, and water meeting WHO and SAZ standards of drinking water for turbidity (< 5 NTU). The experimental study showed that sunflower seed extracts performed better than stem, leaf, and roots extracts. Sunflower seed extracts showed better water turbidity removal efficiency and should be the first choice from sunflower organs as a plant-based environmentally friendly, non-corrosive cheap coagulant agent. The research findings show that plant-based technologies using indigenous available resources could be utilised as alum substitutes for turbidity water treatment among poor households in developing countries. In addition, these local technologies do not require expert training to administer.
Data availability
The datasets generated for this study are included in the manuscript and any additional datasets can be available on request to the corresponding author.
References
Aboubaraka AE, Aboelfetoh EF, Ebeid EZM (2017) Coagulation effectiveness of graphene oxide for the removal of turbidity from raw surface water. Chemosphere. https://doi.org/10.1016/j.chemosphere.2017.04.137
Adams FV, Mulaba-Bafubiandi AF (2014) Application of rice hull ash for turbidity removal from water. Phys Chem Earth 72–75:73–76. https://doi.org/10.1016/j.pce.2014.09.010
Ahmad A, Abdullah SRS, Hasan HA et al (2022) Potential of local plant leaves as natural coagulant for turbidity removal. Environ Sci Pollut Res 29:2579–2587. https://doi.org/10.1007/s11356-021-15541-7
Allen A, Hofmann P, Mukherjee J, Walnycki A (2017) Water trajectories through non-networked infrastructure: insights from peri-urban Dar es Salaam. Cochabamba Kolkata Urban Res Pract 10(1):22–42. https://doi.org/10.1080/17535069.2016.1197306
APHA (2005) Standard methods for examination of water and wastewater. 21st edn. American Public Health Association, American Water Works Association and the Water and Environment Federation, Washington
Aslamiah SS, Yulianti E, Jannah A (2013) Coagulation activity of Kelor seed extract (Moringa oleifera L.) in NaCl solution to liquid waste PT. SIER PIER Pasuruan Alchemy 2(3):178–183
Awotedu OL, Okeke U, Ogunbamowo PO, Ariwoola O, Omolola TO (2020) Extraction of phytochemical compounds of Leea guineensis (G. Don) leaves using non-polar and polar solvents. European J Med Plants. 31(2):24–31. https://doi.org/10.9734/ejmp/2020/v31i230213
Balasubramanian A (2010) Fluvial processes and landforms. Technical Report
Belbahloul M, Anouar A, Zouhri A (2014) Low technology water treatment: investigation of the performance of cactus extracts as a natural flocculant for flocculation of local clay suspensions. Int J Eng Res Technol 3(3):2440–2445
Choy SY, Prasad KMN, Wu TY, Raghunandan ME, Ramanan RN (2014) Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification. J Environ Sci 26(11):2178–2189. https://doi.org/10.1016/j.jes.2014.09.024
Daverey A, Tiwari N, Dutta K (2019) Utilization of extracts of Musa paradisica (banana) peels and Dolichos lablab (Indian bean) seeds as low-cost natural coagulants for turbidity removal from water. Environ Sci Pollut Res 26:34177–34183. https://doi.org/10.1007/s11356-018-3850-9
De Roos AJ, Gurian PL, Robinson LF, Rai A, Zakeri I, Kondo MC (2017) Review of epidemiological studies of drinking-water turbidity in relation to acute gastrointestinal illness. Environ Health Perspect 125(8):086003. https://doi.org/10.1289/EHP1090
Environmental Management Agency (2008) Water quality in Zimbabwe: environmental management authority; environmental management. Harare
Foster S (2020) Field guide to medicinal plants and herbs of eastern and central North America. Houghton Mufflin, p 432
Grenda K, Arnold J, Gamelas JAF et al (2020) Up-scaling of tannin-based coagulants for wastewater treatment: performance in a water treatment plant. Environ Sci Pollut Res 27:1202–1213. https://doi.org/10.1007/s11356-018-2570-5
Herslund L, Mguni P (2019) Examining urban water management practices–Challenges and possibilities for transitions to sustainable urban water management in Sub-Saharan cities. Sustain Cities Soc 48:101573. https://doi.org/10.1016/j.scs.2019.101573
Huang J, Chen S, Ma X, Yu P, Zuo B, Shi H, Wang PJJ (2021) Alvarez Opportunistic pathogens and their health risk in four full-scale drinking water treatment and distribution systems. Ecoll Eng. https://doi.org/10.1016/j.ecoleng.2020.106134
Kamal J (2011) Quantification of alkaloids, phenols, and flavonoids in sunflower (Helianthus annuus L.). Afr J Biotechnol 10(16):3149–3151
Kitheka JU, Njogu IN, Muluvi GM, Hunja CW, Mutiso F, Kioko D, Kimatu J (2022) The effectiveness of Moringa oleifera seed coagulant in reducing the turbidity and modifying the physico-chemical characteristics of water. Afr J Environ Sci Technol 16(4):126–145
Kramer MF (2014) Heath Aluminium in allergen-specific subcutaneous immunotherapy–a German perspective. Vaccine 32:4140–4148
Kumar PJS, Mohanan AA, Ekanthalu VS (2020) Hydrogeochemical analysis of Groundwater in Thanjavur district, Tamil Nadu; influences of geological settings and land use pattern. Geol Ecol Landsc 4(4):306–317. https://doi.org/10.1080/24749508.2019.1695713
Mahamba C, Palamuleni LG (2022) Antimicrobial activity of sunflower (Helianthus annuus) seed for household domestic water treatment in Buhera District, Zimbabwe. Int J Environ Res Public Health 19:5462. https://doi.org/10.3390/ijerph19095462
Megersa M, Beyene A, Ambelu A, Woldeab B (2014) The use of indigenous plant species for drinking water treatment in developing countries: a review. J Bio Env Sci. 5(3):269–281. https://doi.org/10.12692/ijb/5.3.269-281
Mokif LA, Al-Sareji OJO, Obaid ZH, Abdulhusain NA, Mohammed-Ali SS (2020) Removal of COD and TOC from domestic wastewater by using alum and peels of sunflowers seeds as natural coagulant. Eurasia J Biosci 14:2011–2014
Moran S (2018) Engineering science of water treatment unit operations. In: Moran S (ed) An applied guide to water and effluent treatment plant design, Butterworth-Heinemann, pp 39–51. https://doi.org/10.1016/b978-0-12-811309-7.00004-7
Moulin M, Mossou E, Signor L, Kieffer-Jaquinod S, Kwaambwa HM, Nermark F, Rennie AR (2019) Towards a molecular understanding of the water purification properties of Moringa seed proteins. J Colloid Interface Sci 554:296–304. https://doi.org/10.1016/j.jcis.2019.06.071
Nel HA, Dalu T, Wasserman RJ (2018) Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci Total Environ 612:950–956. https://doi.org/10.1016/j.scitotenv.2017.08.298
Ngibad K (2019) Phytochemical screening of sunflower leaf (Helianthus annuus) and anting-anting (Acalypha indica Linn.) plant ethanol extract. Borneo J Pharm 2(1):24–30. https://doi.org/10.33084/bjop.v2i1.689
Özacar M, Şengil IA (2003) Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles. Colloids Surf A Physicochem Eng Asp 229:85–96. https://doi.org/10.1016/j.colsurfa.2003.07.006
Pooi CK, Ng HY (2018) Review of low-cost point-of-use water treatment systems for developing communities. NPJ Clean Water 1:11. https://doi.org/10.1038/s41545-018-0011-0
Rauf S, Ortiz R, Shehzad M, Haider W, Ahmed I (2020) The exploitation of sunflower (Helianthus annuus L.) seed and other parts for human nutrition, medicine, and the industry. Helia 43(73):167–184. https://doi.org/10.1515/helia-2020-0019
Rozainy MR, Hasif M, Syafalny PP, Afifi A (2014) Combination of chitosan and bentonite as coagulant agents in dissolved air flotation. APCBEE Proc 10:229–234. https://doi.org/10.1016/j.apcbee.2014.10.044
Ruiz I, Sanz-Sánchez MJ (2020) Effects of historical land-use change in the Mediterranean environment. Sci Total Environ 732:139315. https://doi.org/10.1016/j.scitotenv.2020.139315
Singh KP, Malik A, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques—a case study. Anal Chim Acta 538(1–2):355–374
Tahiru AA, Doke DA, Baatuuwie BN (2020) Effect of land use and land cover changes on water quality in the Nawuni Catchment of the White Volta Basin, Northern Region. Ghana Appl Water Sci 10:198. https://doi.org/10.1007/s13201-020-01272-6
Talnikar T (2017) Natural coagulant for wastewater treatment: review. Pravara J Sci Technol 1(1):38–42
Tinker SC, Moe CL, Klein M, Flanders WD, Uber J, Amirtharajah A et al (2010) Drinking water turbidity and emergency department visits for gastrointestinal illness in Atlanta, 1993–2004. J Expo Sci Environ Epidemiol 20(1):19–28. https://doi.org/10.1038/jes.2008.68
UNESCO (2019) The United Nations world water development report 2019: leaving no one behind. UNESCO World Water Assessment Programme, Paris, UNESCO. OPENASFA INPUT, p 201. https://aquadocs.org/handle/1834/42357
Unnisa SA, Bi SZ (2018) Carica papaya seeds effectiveness as coagulant and solar disinfection in removal of turbidity and coliforms. Appl Water Sci 8:149. https://doi.org/10.1007/s13201-018-0791-x
Uwacu RA, Habanabakize E, Adamowski J, Schwinghamer TD (2021) Using radical terraces for erosion control and water quality improvement in Rwanda: a case study in Sebeya catchment. Environ Dev 39:100649. https://doi.org/10.1016/j.envdev.2021.100649
Wang J, Wang W, Xiong J, Li L, Zhao B, Sohail I, He Z (2021) A constructed wetland system with aquatic macrophytes for cleaning contaminated runoff/storm water from urban area in Florida. J Environ Manage 280:111794. https://doi.org/10.1016/j.jenvman.2020.111794
Weisz GM, Kammerer DR, Carle R (2009) Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem 115(2):758–765
WHO (World Health Organisation) (2017a) Guidelines for drinking water quality, World Health Organisation, Geneva
WHO (World Health Organisation) (2017b) Water quality and health—review of turbidity: information for regulators and water suppliers. https://Apps.Who.Int/Iris/Bitstream/Handle/10665/254631/Who-Fwc-Wsh-17.01-Eng.Pdf
WHO (World Health Organisation) (2020) Nitrate and nitrite in drinking-water. Background document for development of WHO Guidelines for drinking-water quality. World Health Organization (WHO/SDE/FWC/16.52), Geneva
Wilkes MA, Gittins JR, Mathers KL et al (2019) Physical and biological controls on fine sediment transport and storage in rivers. Wires Water 6:e1331. https://doi.org/10.1002/wat2.1331
Yunus AP, Masago Y, Hijioka Y (2020) COVID-19 and surface water quality: improved lake water quality during the lockdown. Sci Total Environ 731:139012. https://doi.org/10.1016/j.scitotenv.2020.139012
ZNSA (Zimbabwe National Statistical Agency) (2014) Inventory of facilities and social amenities (IFSA) in rural district councils. Zimbabwe, Harare
Zounemat-Kermani M, Alizamir M, Fadaee M, Sankaran Namboothiri A, Shiri J (2021) Online sequential extreme learning machine in river water quality (turbidity) prediction: a comparative study on different data mining approaches. Water Environ J 35:335–348. https://doi.org/10.1111/wej.12630
ZSA (Zimbabwe Statistical Agency) (2012) Preliminary report census 2012. Harare Zimbabwe, Government of Zimbabwe
Acknowledgements
The authors convey their appreciation to the anonymous reviewers for their constructive comments on this paper.
Funding
Open access funding provided by North-West University. The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All authors agreed to the submission of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahamba, C., Palamuleni, L.G. Sunflower (Helianthus annuus) seeds as a natural coagulant for water turbidity treatment: assessment of efficacy and dosage. Appl Water Sci 14, 168 (2024). https://doi.org/10.1007/s13201-024-02228-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02228-w