Abstract
Groundwater quality in rural Chandwara block in Jharkhand State in India is crucial to human livelihood in the region. A study undertaken to examine groundwater quality and consumption patterns in the region revealed that average water consumption per family and per capita were 200–517 and 19.7–47 L per day, respectively, while per capita potable water consumption was 1.2‒4.37 L per day. Groundwater samples were collected primarily from tube-wells and a few from dug-wells (n = 143) in the block during post-monsoon season of 2018–2019 (October 2018 to March 2019). Water quality parameters like Fe, TDS, Total hardness, Ca and turbidity were above the permissible limits of Bureau of Indian Standards (BIS) and WHO in 68%, 36%, 39%, 58% and 15.4% of groundwater samples, respectively. Most of the samples (67.9%) belonged to Ca–Mg–HCO3 class while a few (28.6%) were Ca-Mg-SO4-Cl type and the rest (3.6%) were in Ca–Mg–Cl class. As per Gibbs Plot, most samples belonged to the rock weathering region, implying contribution of rock forming minerals to groundwater chemistry. Factor analysis revealed that water quality was controlled by four principal factors to the extents of 43.3, 13.5, 10.9 and 7.3%, respectively, making a cumulative contribution of about 75%. Parameter loading in Factor 1 strongly indicated to various salts dissolving in groundwater from the rocks while Factor 2 was loaded by Fe coming from Fe-bearing rocks and turbidity that is positively influenced by the former. Factor 3 is loaded by pH and alkalinity, pointing to the role of carbonates contributed by the rocks while factor 4, which is loaded by F− and Na, indicates to F−incorporation in water from NaF-bearing rocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is the primary source of potable water in many regions across India where surface water is mostly found unsuitable for drinking or falls short of demand. Groundwater also finds major use in agriculture and industry. Like India, large populations in several countries in Asia and Africa have a strong dependence on groundwater (Singh et al. 2017; Ishwar et al. 2014). Groundwater is constantly under threat of contamination from fertilizers and pesticides used in agriculture (Krishnakumar et al. 2014), human and animal wastes and clandestine dumping of industrial effluents on land (Kumar and Shah 2006). Groundwater quality is also largely influenced by lithological, pedogeochemical compositions, anthropogenic activities and local geochemistry (Adimalla et al. 2018). In mountain regions also, groundwater quality may be substantially influenced by geology and anthropogenic activities that could significantly control chemicals facies and TDS content, depending on extent of interaction with aquifer materials (Thakur et al. 2018).
As per Kumar and Shah (2006), arsenic (As), fluoride (F−), nitrate (NO3−), iron (Fe), salinity and heavy metals are the major pollutants in groundwater in India. Inadequate strategy on groundwater management, rampant industrial development, improper waste disposal, perpetually growing population, urbanization, improper usage of fertilizers etc. are the predominant reasons for deterioration of groundwater quality in India (SubbaRao et al. 2018; Adimalla et al. 2019; Khanoranga Khalid 2019).
As per Central Ground Water Board (CGWB) in India, groundwater in various places in Jharkhand state suffers from contamination of Fe, NO3− and F− but Koderma District is not listed amongst Fe affected districts (http://cgwb.gov.in/gw_profiles/st_Jharkhand.htm). Govt. of Jharkhand reports that groundwater in Koderma is Calcium-Magnesium bicarbonate dominated while Satgawan and Koderma Blocks are affected by unsafe levels of F− in groundwater. In Bokaro District of Jharkhand, groundwater is slightly acidic to slightly alkaline and Ca2+ and Na+ are the dominant cations, while HCO3− and Cl− were dominant anions during pre-monsoon season and post-monsoon seasons. TDS, hardness, Ca2+, Na+, HCO3−, and Cl− are the major parameters of concern while groundwater is mostly suitable for irrigation (Singh et al. 2018). In Ranchi city of Jharkhand, high urban built-up area (39.0%) and population growth (32.6%) were found to have put pressure on groundwater and on the top of this, existence of hard rock formations and ever growing urbanization retarded recharge of groundwater (Krishna Kumar et al. 2015).
Chandwara community development block in Koderma District of Jharkhand state of India represents an area that does not have abundant rainfall and is classified as a semi-arid area. This block is mostly covered by hills and has lateritic soil where villages depend primarily on groundwater for drinking, domestic uses and irrigation. Koderma district has 105 piped water schemes (PWS), 77 open wells, 1401 hand pumps and 445 other types of tube wells as per Govt of Jharkhand estimate in 2013 (http://documents.worldbank.org/curated/en/369471468041971982/pdf/E41820v60EA0P10MF0JHARKHAND0Vol-0I.pdf). Therefore, groundwater quality is extremely important for livelihood and sustainability of the predominantly rural and tribal populace of the region. We report groundwater quality over a large area in 39 villages within Chandwara Community Development Block in Koderma District of Jharkhand state in India, where micro-level baseline information on groundwater quality is lacking. Water quality was assessed comprehensively in terms of its suitability for drinking and irrigation and hydrochemistry is evaluated in terms of chemical facies. Spatial variation in groundwater quality and possible sources of anthropogenic and geological contamination were evaluated. This study also presents new information on regional groundwater consumption patterns by different economic and social classes that would go on to add valuable clues for understanding and assessing regional water management issues, environmental carrying capacity and policy implications with respect to groundwater quality, availability and consumption.
Materials and methods
Study area
Chandwara (24°23′42″ N and 85°29′4″E) community development block is a region in the northern end of Chotanagpur Plateau in Koderma district in Jharkhand state of India. Average altitude is about 397 m (1306 ft.) above mean sea level and has 232.05 km2 coverage. The hilly topography is mostly covered by forests, wasteland, pasture lands and cultivated lands. Upper part of Chandwara block consists of dissected plateau and lowest part by pediplain. About three-fourth the area is covered by biotite granite gneiss and one-fourth by Phyllite mica-schist and Quartzite (Govt. of Jharkhand, https://cdn.s3waas.gov.in/s3087408522c31eeb1f982bc0eaf81d35f/uploads/2018/05/2018050235.pdf). Alluvium is also present in South and South-West part of the District (Govt. of Jharkhand, https://cdn.s3waas.gov.in/s3087408522c31eeb1f982bc0eaf81d35f/uploads/2018/06/2018062339.pdf). The study area is also a part of a laterite soil belt and is dominated by rural areas with 40–50% of families living below poverty line. Chandwara CD Block has a population of 84,914 (all rural) and sex ratio of the block is 932. Majority of cultivated land in India is fully dependent on monsoon rain. The proportion of main workers is 22.82% in Chandwara CD Block. The Chandwara CD Block consists of 15 numbers of Gram Panchayats (i.e. smallest local governing body in India) consisting of 88 villages in which 75 are inhabited (Census of India, 2011). Barakar River with its tributaries is the main river flowing through southern part. This area is dry except in the monsoon season (June to August). Average rainfall is 127 cm per annum while temperature varies from 5 to 45 °C annually.
Socio-economic survey
A socio-economic survey was conducted in 39 villages involving 2507 family members within the study area. Feedback on the number of family members, mean family income, profession, sources of potable water, potable and non-potable water consumption per family, perceptible problems of water quality etc. was noted in a questionnaire through personal interviews conducted by the survey team. It was also attempted to understand popular perception on local water quality issues vis a vis economic conditions and awareness levels and also, their willingness to address drinking water quality problems.
Sampling of groundwater
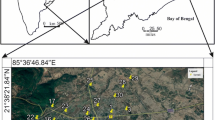
Groundwater samples were collected from 143 numbers of tube-wells and dug-wells in the selected villages of CD block during post-monsoon season (October 2018 to March 2019) (Fig. 1). Information on the depth of tube wells and dug wells were collected from respective owners and neighbours and also, local municipal offices wherever available. Depth of the selected dug wells ranged from about 20‒50 feet while the tube wells had depths ranging from about 60–550 feet. In post-monsoon, depth of groundwater table varies between 3‒4 and 2‒3 m below ground level (mbgl) in northern and southern parts, respectively, as per District Survey Report of Koderma, indicating elevated water table near Tilaiya reservoir and Barakar river (Govt. of Jharkhand, https://cdn.s3waas.gov.in/s3087408522c31eeb1f982bc0eaf81d35f/uploads/2018/05/2018050235.pdf). The well water samples were collected only from those predominantly used by villagers for drinking and domestic purposes. Water samples were collected in clean polypropylene bottles for analysis of physico-chemical parameters while for microbiological analyses, sterilized glass bottles were used. Water pumped out from hand-pumps was allowed to flow for about 1–2 min before sampling to dispose of stagnant groundwater in the pipes, if any, and temperature, pH and electrical conductivity (EC) were measured in situ. The groundwater samples were visibly non-turbid, colourless and odourless immediately after withdrawal and were later analysed for other physico-chemical parameters {n = 143, except for As (29), Na+ (n = 41), K (n = 41)} e.g. TDS, total hardness (TH), Ca2+, Mg2+, Na+, K+, alkalinity, Cl−, SO42− by methods prescribed by American Public Health Association (APHA 2005). The pH and EC were measured by a pH meter (TOSHCON) and portable conductivity meter (HANNA, France). Flame emission photometry method for Na+ and K+ (Flame Photometer model 129, Systronics), turbidimetric method for SO42− (Merck, model Spectroquant Move 100), visible spectrophotometric method for Fe and phosphate (Merck, model Spectroquant NOVA 60A) and nephalometric method for turbidity (model HI98703, HANNA Instruments) were used. Classical physico-chemical methods were followed for the rest of the parameters viz. filtration followed by drying and gravimetric method for TDS, Argentometric Method for Cl−, EDTA titrimetric method for TH, Ca2+, Mg2+ and Titrimetric method for alkalinity. Arsenic was analysed by Arsenic Test Kit (Merck), F− by ion selective electrode (Thermo Fisher Scientific and Orion Ion Selective Electrode, Orion 9609BNWP), total bacterial count (TBC) and E. coli by Bactaslyde (Rakiro Biotech Systems Pvt. Ltd).
Villages selected for groundwater collection in Chandwara block vis a vis Koderma District in Jharkhand State in India (Depth of Water Table Map Courtesy: Govt. of Jharkhand, https://cdn.s3waas.gov.in/s3087408522c31eeb1f982bc0eaf81d35f/uploads/2018/05/2018050235.pdf)
Water quality indices
Water quality indices viz. Water Quality Index (WQI) calculated by using weighted arithmetic index method (Satish Chandra 2017; Gupta et al. 2017), Pollution Index (PI) (Sidabutar et al. 2017; Yadav et al. 2018), Comprehensive Pollution Index (CPI) (Krishna Kumar et al. 2015; Wang et al. 2018) were determined to classify groundwater and understand its quality for drinking and irrigation. Table 1 presents a summary of applied indices for groundwater evaluation with their respective formulae. Details on the classification of water under selected indices are presented in Supplementary Tables 1, 2, 3.
Water quality for irrigation was evaluated by various other indices and ratios as prescribed by various researchers over the last few decades (Table 2). Most of the indices are fundamentally based on Na content of water viz. Sodium index, Sodium Adsorption Ratio (SAR), Kelly’s ratio, Puri’s Salt Index. Irrigation water containing high Na enhances the exchange of alkaline earth metals (e.g. Ca2+ and Mg2+) between water and soil, affecting soil permeability, rendering the soil hard, increasingly impermeable and unsuitable for seedling emergence (Karanth 1987). The Na/alkali hazard is also customarily expressed as SAR, an index that quantifies the proportion of Na+ to Ca2+ and Mg2+ ions in water (Richards 1954).In Kelly’s Ratio (KR) (Kelly 1963). Na+ is quantified against Ca2+ and Mg2+ and water KR > 1 is generally considered unsuitable for irrigation. Puri’s salt index (PSI) (Puri 1949) also uses Na content to determine quality of water for irrigation. On the other hand, Magnesium Hazard (MH) that addresses the equilibrium between Ca2+ and Mg2+ and potential decline in crop yield with increasing concentrations of Mg in water, is also used to determine suitability of groundwater for irrigation. Source of Mg2+ in groundwater is mainly dolomitic rocks (Keesari et al. 2016) and intake of water with high Mg2+ levels causes laxative effect while Mg2+ deficiency may cause structural and functional changes in human body. According to this index, a low MH value (i.e. < 50) of water is suitable for irrigation while high values (i.e. > 50) makes soil more alkaline upon application, badly affecting yield of crops. Another index, Potential Salinity (PS) of water (Doneen 1964) works on the logic that suitability of irrigation water (PS < 10) not only depends on soluble salts but also on the proportion of sparingly soluble salts that precipitate and accumulate in soil with time.
Statistical analyses
Apart from descriptive statistical analysis (mean, SD, range, 90th percentile), Pearson Correlation analysis was undertaken to understand inter-relationships amongst water quality parameters. Further, Factor Analysis preceded by Principal Factor Extraction was undertaken to evaluate number of principal factors influencing groundwater quality. All statistical analyses were undertaken by Statistica (Dell Software, USA). Kriging method, a tool used in geostatistical analysis, that involves interpolation estimates for points not actually sampled from values at adjacent locations and information on spatial relationships in the data set (Datta Chowdhury et al. 2017) was undertaken to generate isotropic variograms. The said interpolation analysis (ordinary Kriging) based on available data points, was used to construct spatial distribution maps of Fe and TDS present in groundwater. Before generation of the said maps, cross-validation analysis of data was conducted to plot and validate estimated versus actual values within in the domain.
Results and discussion
Water quality
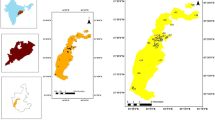
Groundwater in the region is characterized by notable levels of EC, TH, TDS, turbidity and dominant presence of Cl− and Fe. Groundwater pH was always alkaline while electrical conductivity ranged from 125.5 to 2120 µS cm−1 (90th percentile: 1303 µS cm−1). Total hardness ranged from 66 to 1684 mg l−1 while Ca2+ and Mg2+ hardness ranged from 15.2 to 496 and 8 to 444 mg l−1, respectively. Mean TDS was found to be 455 mg l−1, while a maximum TDS of 1272 mg l−1 was observed (Table 3). Water samples were categorized into various groups based on coefficient of spatial variation (CV) in water quality parameters viz. < 20% CV (pH, temperature), 20‒ < 50% CV (TA, Si, F−, Na, k), 50‒80% CV (TH, TDS, EC, Ca2+, Ca-Hardness, SO42−), and > 80% CV (Fe, Cl−, turbidity, Mg2+, Mg-hardness) for understanding the extents of spatial variation in terms of dispersion of data points around respective means. The spatial variation in TDS and Fe was also studied by geospatial data analysis where distribution of Fe and TDS concentration was converted into spatial coverage format (Fig. 2a, b). Groundwater in the southern and central areas of the block is mostly affected by breaching levels of Fe i.e. > 1 mg l−1 while groundwater in the northern part of the block is mostly affected by TDS of > 500 mg l−1.
As per Pearson’s correlation analysis, TH, EC, Ca2+, Mg2+ and total alkalinity were significantly correlated (r = 0.91, 0.91, 0.98, 0.82, 0.55, respectively, p = 0.01) (Table 4), confirming that these parameters positively influenced one another. Further, EC also had strong positive correlations with Cl− (r = 0.93, p = 0.01), SO42− (r = 0.59, p = 0.01) and alkalinity(r = 0.61, p = 0.01). The Ca2+, Mg2+, Na+ and Cl− had concomitantly linear increase with TDS (Fig. 3), indicating that high TDS build-up at some pockets can be attributed to geogenic hydrochemical processes involving long interaction of groundwater with rocks. Factor analysis revealed that water quality was mainly controlled by 4 principal factors to the extents of 43.3 and 13.5, 10.9 and 7.3%, respectively, making a cumulative contribution of about 75% (Table 5). Factor 1 can be explained by strongly correlating TH, Cl−, TDS, EC, Ca2+, Mg2+ and Ca2+ and Mg2+ hardness which is indicative of geochemical influence via salt incorporation in water while Factor 2 was dominated by strongly correlating Fe (geochemical dominance of Fe) and turbidity, the latter known to be influenced by the former. Evidently, Fe is the crucial geochemical factor influencing water quality of this region and it also had a strong influence on turbidity imparted to groundwater of this region. Factor 3 is loaded by pH and alkalinity, pointing to the role of carbonates contributed by the rocks while factor 4, which is loaded by F− and Na+, indicates to F− incorporation in water from NaF-bearing rocks (Biglari et al. 2016). On the other hand, high groundwater Fe observed in some pockets is a manifestation of release of Fe from ferruginous lateritic soil present in Chandwara block, as subsurface weathering and release of Fe into groundwater from Fe-bearing rocks is well known (Raju 2013; CGWB 2013). As per partially available data, depth of tube wells (n = 56) and dug wells (n = 6) ranged from about 60–550 feet and 20–50 feet, respectively, and no statistically significant correlation of depth of tube wells was found with most water quality parameters except for Si (− 0.42, p = 0.01) and K (− 0.27, p = 0.05), indicating that levels of Si and K decreased with increasing depth of tube wells.
Hydrogeochemistry
Hydrochemistry and hydrochemical facies of water are crucial benchmarks to evaluate possible sources of contamination and chemistry of water. Piper diagram was used to understand the sources of dissolved constituents in water and identification of hydrochemical facies while Gibbs Diagram was used to establish the relationship of water composition with aquifer lithological characteristics under three distinct fields viz. precipitation dominance, evaporation dominance and rock–water interaction dominance. A 6-segment Piper Diagram indicated the following dominant water classes: 1. Ca–Mg–HCO3 type, 2. Na–Cl type, 3. Ca–Mg–Cl type, 4. Ca–Na–HCO3 type, 5. Ca–Cl type, 6. Na–HCO3 type (Fig. 4). The diagram reveals that most of the samples that were analysed for all required parameters belonged to Ca-Mg-HCO3 class (67.9%) while a few were Ca–Mg–SO4–Cl type (28.6%) and the rest 3.6% were in Ca–Mg–Cl class. This implies that in the majority of samples, alkaline earths and weak acidic anions, respectively, exceeded alkali metals and strong acidic anions as indicated by dominance of Ca–Mg–HCO3 type of water. The role of geogenic hydrochemical processes in causing high TDS is further strengthened by the distribution of hydrochemical facies in the Piper Diagram. High bicarbonate content depicted in Piper Diagram indicates to recharging water type and implies sufficiently long interaction of these groundwater samples with rocks, resulting in salt dissolution from rocks contributing to TDS. From the diamond field in the Piper Diagram, two main groundwater groups could be identified viz. Group 1 samples that are primarily the recharging ones having low salt concentration (average TDS 307 mg l−1) and little contact with aquifer material and Group (3) samples that had more interaction with aquifer material leading to higher incorporation of ions (average TDS 712 mg l−1). Therefore, Group (3) groundwater is relatively older than recharging water types in group (1). With respect to TDS, two primary groups of groundwater types were identified: (i) High TDS (> 500 mg l−1), Ca–Mg–HCO3 and Ca–Mg–Cl–HCO3 types that ought to have undergone substantial interaction with subsurface geologic and aquifer materials (ii) Low to moderate TDS (< 500 mg l−1) and Ca–HCO3 and Ca–Na–HCO3 types that are mainly recharging waters with lesser geological interaction.
In general, evaporative dissolution, ion exchange and rock-water interaction are the primary geochemical processes that influence prevalence of salts and groundwater chemistry (Roy et al. 2018). Keesari et al. (2013) reported salt incorporation in root zone, incongruent dissolution of carbonate minerals and attributed high salt levels in shallow groundwater to its mixing with surface water. In a study in Bathinda of Punjab in India, groundwater was found to be affected by dissolution of minerals and ion exchange during pre-monsoon season while leaching of salts from vadose zone during post-monsoon contributed to the dissolved salts in groundwater (Sharma et al. 2017). Iqbal et al. (2021) suggested that high Ca2+ and Mg2+ in higher topographic regions might be a result of inverse ion exchange process, where Ca from aquifer is replaced by Na+ present in groundwater. The hydrochemistry of samples were also evaluated in terms of concentrations of SO4-HCO3− and Na–K-Ca (Fig. 5) that also indicated dominance of Ca2+ bicarbonate class of water. Further, the hydrochemical characteristics of water samples were plotted in Gibbs diagrams to show Gibbs ratio (cation or anion) against TDS (Fig. 6). All the groundwater samples belonged to the rock weathering region, implying incorporation of the geogenic salts in groundwater from rock-forming minerals during chemical weathering, eventually influencing groundwater TDS as also reported by Nagaraju et al. (2016).
Ionic ratios viz., HCO3−/Si and Mg2+/(Mg2+ + Ca2+) are assessed to evaluate the contribution of rock weathering processes on groundwater quality (Thakur et al. 2018). In the collected samples, HCO3−/Si ratio ranged from 1.4 to 31.4. In about 22% of samples the ratio was > 10, implying the role of carbonate weathering, while in 28%, the ratio was < 5, indicating silicate weathering. Further, ratio of Mg2+/(Mg2+ + Ca2+) ranged from 0.04 to 0.5, showing that both limestone and dolomite weathering influenced the hydrochemistry.
Water quality assessment for drinking and irrigation
TDS, Fe, F− and As are the principal drinking water quality parameters that were assessed to evaluate groundwater samples for potability. Iron ranged from 0.02 to 6.64 mg l−1 (90th percentile: 1.7 mg l−1) and 28.7% of samples exceeded the revised acceptable limit of 1.0 mg l−1 Fe prescribed for potable water in India by Bureau of Indian Standards (Amendment No. 1, June 2015 to IS: 10,500–2012). High turbidity in 15.4% groundwater samples (highest 59.4 NTU; 90th percentile 12.1 NTU) (Table 3) breached acceptable limit for potable water owing to the influence of Fe on the former. TDS concentration, the sum of all dissolved organic and inorganic substances (salts of Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, NO3−, and Si), varied from 75.3 to 1272 mg l−1 and 36% exceeded acceptable limit of 500 mg l−1 for TDS (BIS Standard, IS 10500–2012) in potable water (Table 6). On the other hand, total hardness and Ca breached their respective acceptable limits in 39% and 58% of samples. Chloride was widely variable, ranging from 4.5 to 600 mg l−1, breaching the acceptable limit in several samples. A crucial potable water quality parameter like F− ranged from 0.2 to 1.2 mg l−1, never exceeding its acceptable limit of 1.5 mg l−1. Other parameters like PO43−, As, free residual chlorine (FRC), E. Coli and Cu were not detected in any sample, the LDL being < 0.03 mg l−1 (PO43−), < 0.05 mg l−1 (As), 100 CFU ml−1 (E. Coli) and < 0.02 mg l−1 (Cu), respectively, in the deployed methods. Arsenic could be detected down to 50 µg l−1 only and was not found in any sample. The Bureau of Indian Standards (BIS) permissible limit for As in absence of any alternative potable water sources is 50 µg l−1 but the acceptable limit being 10 µg l−1 (both BIS and WHO), none of the samples could be assessed for acceptability in terms of As.
In Jharkhand, groundwater in some areas of Sahibganj and Ranchi district have substantial As (Nayek et al. 2008; Tirkey et al. 2017) while some areas in Deoghar, Dhanbad and Bokaro districts are affected with high Fe in groundwater (Banerjee and Mukherjee, 2013; Singh et al. 2017). Singh et al. (2012) has reported high salinity and Mg hazard at a few districts of Jharkhand state. On the other hand, F− is reported to have been found beyond the safe limit in some parts of Palamu, Garhwa, Koderma and Pakur Districts in the state (Central Ground Water Board, 2016). As per Singh et al. (2012), F− levels were < 1.0 mg l−1 in most of the samples collected from Dumka and Jamtara Districts in Jharkhand state. Some other districts also in the state have areas where groundwater F− is above the permissible level of BIS (2012) (Kumari and Pathak, 2014; Avishek et al. 2010; Patolia and Sinha, 2017).
The groundwater samples could be classified into low, medium and high salinity (classes 1, 2 and 3) (Fig. 7) and those in ‘high’ salinity hazard category are considered detrimental to soils and crops in the long run and are suitable for applying to medium and highly salt-tolerant crops only. Further, Class 4 (very highly saline) water is suitable for only highly salt-tolerant crops but none of the tested water samples belonged to this category. The potential salinity (PS) in the water samples ranged from 0.24 to 17.08 me l−1 that is indicative of their suitability for irrigation. About 46% of samples had low PS (< 3 me l−1) that are considered suitable for irrigating fine, medium and coarse textured soils. On the other hand, about 52% of samples had PS of 3‒15 me l−1, implying their suitability for medium and coarse textured soils. Rest can be used only in coarse textured soils. Sodium percent is another important indicator of irrigation water. Dissolution of alkali feldspar minerals, ion exchange processes, sewage contamination and infiltration from agricultural run-off may impart Na in groundwater. Repeated use of Na contaminated water for irrigation areas also contributes to Na in groundwater (Keesari et al. 2016, 2019). As per Na% assessment, about 29% samples could be categorized under the class ‘good’ (20–40) while 71% samples could be categorized under the ‘permissible’ range (40–60). Further, as per Sodium Absorption Ratio (SAR) which is a standard indicator for suitability of irrigation water, about 12% samples were categorized under excellent class (i.e. 0–10) while 88% were in good class (i.e. 10–18).
The mean water quality index (WQI) of water samples was calculated to be 208.7, that renders water unfit for drinking in general. But, when the index is calculated without Fe, mean WQI sharply declined to 8.57, putting the same under ‘excellent’ class, suitable for drinking, irrigation and industrial purposes. On the other hand, mean Pollution Index (PI) was calculated to be 3.92 making the water ‘lightly polluted’ in general while without Fe, mean PI came down to 2.74, keeping water quality in the same category. Mean Comprehensive Pollution Index (CPI) was calculated to be 1.004, categorizing general water quality into ‘polluted’ class that is of very poor quality and requires restriction for irrigation. But, without taking Fe into consideration, CPI was 0.81 that came under ‘Basically Qualified’ class meant for irrigation only. On the other hand, Puri’s Salt Index (PSI)values ranged from − 2236.1 to 95.78 and about 90% samples belonged to ‘good class’ while the rest were categorized under poor quality. Index of Magnesium Hazard (MH) also determines suitability of groundwater for irrigation. Generally in water, equilibrium is established between Ca and Mg but with increasing concentration of Mg, salinity of water increases and soil is affected by such water, affecting crop yield. Water with MH < 50 is suitable for irrigation while > 50 makes soil alkaline on continuous application. In our study area, MH ranged from 4.2 to 49.5, making all samples suitable for irrigation. Also, Kelly’s Ratio (KR) in all the samples were < 1, making them suitable for irrigation.
Water consumption patterns vis a vis socio-economic conditions
Groundwater is the principal source of potable water in this region and it is mostly drawn from tube- and dug-wells. The dug-wells are mostly private properties that are mostly dug during house construction and accessed by only family members while tube-wells are mostly put up by Gram Panchayats for public access. About 49% of interviewees perceived that there was at least one problem with potable water while another 49% agreed that water quality was either satisfactory or good. High Fe content is the principal water quality problem in this block and visible reddening of water and red scale formation in storage vessels on standing are common in some areas. The study area being a part of laterite soil belt, high Fe content in water can be expected as reported from many other parts of India (Girish and Seralathan, 2006; SubbaRao, 2008; Achary, 2014). Central Groundwater Board (CGWB) of India lists Fe as one of the prominent groundwater pollutant in India and mentioned Jharkhand as one of the affected states (http://cgwb.gov.in/WQ/GROUND%20WATER%20QUALITY%20SCENARIO%20IN%20INDIA.pdf). As household water purification systems were not found in the surveyed households, Fe and taste imparted by it in potable water remains largely unaddressed.
Most of the families in the region are involved in crop cultivation while some work as agricultural labourers or workers in shops or businesses. Some have their own businesses while very few are in government jobs. Mean family size ranged from 7 to 17, indicating presence of a joint family system where kin stay together in a house. Mean water usage per family and per capita ranged from about 200 to 517 L per day and 19.7 to 47 L per day, respectively, that included water for drinking and other uses. Per capita potable water consumption was 1.2‒4.37 L per day. A few Indian cities have shown similar water consumption viz. 20–40 L per capita per day (lpcd) in Jaipur (Jethoo and Poonia, 2011) and about 55 lpcd in Chennai (Dhanapal, 2019) while Bangalore reportedly consumed higher e.g. 100–125 lpcd (Ramachandra et al. 2016). Ministry of Housing and Urban Affairs (MoHUA), Government of India has set a benchmark of 135 lpcd for Indian cities (WaterAid India, 2018) and therefore, water consumption in this region is lower than this benchmark set for cities. Mean monthly family income ranged from 6800 to 18,727 Indian rupees (95.7 to 263.6 US $ as per exchange rates on 18.1.2020), indicating that the villages represented lower to lower-middle class Indian population (Table 7). Expectedly, mean family water consumption increased with increasing number of mean family members while the former increased at a lower rate with increasing family income (Fig. 8) which sometimes is aligned with higher water usage in improved toilets. Family income also heavily influences affordability to own household water purifiers that were not found to be popular in this region due to lack of affordability to own and maintain such purifiers in the long-run for large families consisting of 7‒18 members. Family income that can be linked to profession and formal education, has significant influence on development of required perception on importance of water quality on human health while family income would go on to influence the affordability to own household water purifiers or participation in contributing to the procurement and management of community level water treatment plants. As per WHO, increased national income and proportion of population with access to improved water supply are correlated. In Africa, people spend about a third of income on treatment of water-related diseases (WHO, https://www.who.int/water_sanitation_health/watandmacr2.pdf), signifying the importance of affordability and willingness to combat water-borne diseases. Installation of community RO plants may be one of the possible solutions to ensure quality potable water for all in this region. The past experience on the operation and economics of community RO plants from Indian villages (Shah et al. 2004; Sharma and Joshi, 2014; APMAS, http://apmas.org/gagi/A%20Case%20Study%20on%20RO%20Water%20Plant-Mission%20Gagillapur.pdf) could be of great assistance in planning for such endeavour in this region.
Conclusion
This study assessed groundwater quality over a large area in 39 villages in Koderma District in India along with micro-level baseline information on water consumption vis a vis economic classes of consumers. Spatial variation in groundwater quality (TDS, Fe) was analysed by kriging method and mapped along with assessment of anthropogenic and geological sources of contamination by statistical methods. The study provides valuable clues in understanding water management issues and policy implications with respect to groundwater quality, availability and consumption. In terms of water consumption for domestic purposes, the families in Chandwara Block are placed on the same pedestal with a few prominent Indian cities but have lower consumption than a consumption benchmark of 135 lpcd set for Indian cities by Housing and Urban Affairs (MoHUA), Government of India. Most of the groundwater samples in Chandwara CD Block can be deemed unsafe for drinking primarily due to high Fe and TDS, both of which are considered critical potable water parameters. In the southern and central areas of the block, groundwater is affected by Fe (> 1 mg l−1) while in the northern part, it is mostly affected by TDS of > 500 mg l−1. High TDS at some places is due to long interaction of groundwater with rocks and eventual dissolution of minerals in water. On the other hand, Fe is incorporated in groundwater and held in solution in varying amounts through the release of Fe from ferruginous lateritic soil present in study region. As Fe-rich groundwater is exposed to air upon withdrawal, Fe would get oxidized to less water-soluble forms, becomes visible in water and forms white, yellow and finally red-brown particles. Further, high Fe in water would promote growth of non-pathogenic bacteria that feed on iron, forming red-brown iron slime that can foul and clog water systems, often producing a foul odour. Therefore, Fe in groundwater may be a potential problem for drinking and domestic water supply networks in affected regions that needs to be redressed through conscious decision making and using either Point of Use (PoI) or Point of Entry (PoE) water purification before consumption. Groundwater in study region may need to be treated by household reverse osmosis units (PoI) for TDS and Fe removal before consumption, but economic condition of families having mean monthly income of INR 6800 to 18,727 (95.72 to 263.6 US $ as per exchange rates on 18.1.2020), is not conductive for spending towards individual household water purification units that could cost anything around INR (Indian rupees) 15,000–25,000 each (US $ 202.8–338.0, as per exchange rates on 12.1.22), apart from costly maintenance of recommended iron removal pre-units and RO membranes that would need about 20‒25% of capital cost of equipment per year. In terms of irrigation, groundwater withdrawn in some villages are suitable for only salt tolerant crops and hence, needs judicious decision making in terms of their continuous use in regional agriculture. This study would serve as a useful guide in regional groundwater management, livelihood, water carrying capacity analysis and policy-making.
References
Achary GS (2014) Studies on ground water pollution due to iron content in Bhubaneswar, Odisha, India. Int J Curr Eng Technol 4(1):88–93
Adimalla N, Vasa SK, Li P (2018) Evaluation of groundwater quality. Peddavagu in Central Telangana (PCT), South India: an insight of controlling factors of fluoride enrichment. Mode Earth Syst Environ 4:841–852. https://doi.org/10.1007/s40808.018.0443.z
Adimalla N, Marsetty SK, Xu P (2019) Assessing groundwater quality and health risks of fluoride pollution in the Shasler Vagu (SV) watershed of Nalgonda, India. Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2019.1594154
APHA, American Public Health Association (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington
Avishek K, Pathak G, Nathawat MS, Jha U, Kumari N (2010) Water quality assessment of Majhiaon block of Garwa district in Jharkhand with special focus on fluoride analysis. Environ Monit Assess 167(1–4):617–623
Banerjee M, Mukherjee A (2013) Status of water quality in the proximity of Deogharh Town in the Jharkhand State of India. Indian J Sci Res 4(2):87–92
Biglari H, Chavoshani A, Javan N, Mahvi AH (2016) Geochemical study of groundwater conditions with special emphasis on fluoride concentration. Iran Deslin Water Treat 57(47):1–8
BIS (2012) Indian standard, drinking water–specification (Second Revision): IS 10500, BIS, Delhi
Brown RM, Mcclelland NI, Deininder RA, Tozer RG (1970) A water quality index: do we dare? Water Sewage Works 117(10):339–343
CGWB (2013) Ground water information booklet Koderma District, Jharkhand State. Ministry of Water Resources (Govt. of India) State Unit Office, Ranchi Mid-Eastern Region Patna, pp 1–25
CGWB (2016) Ground water year book: Jharkhand (2015–2016). Mid-Eastern Region, Patna State Unit Office, Ranchi (http://cgwb.gov.in/Regions/GW-year-Books/GWYB-2015-16/GWYB%20MER%20(Jharkhand)%202015-16.pdf)
Datta Chowdhury T, Abu Zafor M, Chakroborty A (2017) Spatial variation of Iron (Fe) Concentration in groundwater of greater Sylhet district using geostatistical mapping. Nat Environ Pollut Technol 16:789–794
Dhanapal G (2019) Chennai’s water crisis: a lesson for Indian cities. Down to Earth (https://www.downtoearth.org.in/blog/water/chennai-s-water-crisis-a-lesson-for-indian-cities-65606).
Doneen LD (1964) Notes on water quality in agriculture. Published as a water science and engineering paper. 4001, Department of Water Science and Engineering, University of Califonia, US.
Effendi H, Romanto Wardiatno Y (2015) Water quality status of Ciambulawung River, Banten Province, based on pollution index and NSF-WQI. Procedia Environ Sci 24:228–237
Girish G, Seralathan P (2006) Chemistry of groundwater in the laterite formations of the Muvattupuzha river basin. Kerala J Geolog Soc India 68(4):705–714
Gupta N, Pandey P, Hussain J (2017) Effect of physicochemical and biological parameters on the quality of river water of Narmada, Madhya Pradesh, India. Water Sci 31:11–23
Horton RK (1965) An index number system for rating water quality. J Water Poll Cont Fed 37(3):300–306
Ishwar CY, Devi NL, Mohan D, Shihua Q, Singh S (2014) Assessment of 559 groundwater quality with special reference to arsenic in Nawalparasi district, Nepal 560 using multivariate statistical techniques. Environ Earth Sci 72:259–273
Iqbal J, Su C, Rashid A, Yang N, Baloch MYJ, Talpur SA, Ullah Z, Rahman G, Rahman NU, Earjh Sajjad MM (2021) Hydrogeochemical assessment of groundwater and suitability analysis for domestic and agricultural utility in Southern Punjab, Pakistan. Water 13:3589
Jethoo AS, Poonia MP (2011) Water consumption pattern of Jaipur City (India). Int J Environ Sci Dev 2(2):152–155
Karanth KR (1987) Groundwater assessment, development and management. Tata-McGraw-Hill, New Delhi
Keesari T, Kulkarni UP, Deodhar A, Ramanjaneyulu PS, Sanjukta AK, Saravana Kumar U (2013) Geochemical characterization of groundwater from an arid region in India. Environ Earth Sci 71:4869–4888
Keesari T, Ramakumar KL, Chidambaram S, Pethperumal S, Thilagavathi R (2016) Understanding the hydrochemical behaviour of groundwater and its suitability for drinking and agricultural purposes in Pondicherry area, South India: a step towards sustainable development. Groundwater Sust Dev 2–3:143–153
Keesari T, Sinha UK, Kamaraj P, Sharma DA (2019) Groundwater quality in a semi-arid region of India: Suitability for drinking, agriculture and fluoride exposure risk. J Earth Syst Sci 128:24
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95(4):355–391
Khanoranga Khalid S (2019) An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. J Geochem Explor 197:14–26
Krishnakumar P, Lakshumanan C, Pradeep Kishore V, Sundararajan M, Santhiya G, Chidambaram S (2014) Assessment of groundwater quality in and around Vedaraniyam, South India. Environ Earth Sci 71:2211–2225
Krishna Kumar S, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343
Kumar D, Shah T (2006) Groundwater pollution and contamination in India. The emerging challenge. IWMI-Tata Partners Meet. IWMI- Tata water Policy Program.
Kumar A, Pandey AC (2016) Geoinformatics based groundwater potential assessment in hard rock terrain of Ranchi urban environment, Jharkhand state (India) using MCDM–AHP techniques. Groundw Sustain Dev 2–3:27–41
Kumari N, Pathak G (2014) A review of groundwater quality issue in Jharkhand due to fluoride. J Eng Res Appl 43(2):65–77
Liu X, Li G, Liu Z, Guo W, Gao N (2010) Water pollution characteristics and assessment of lower reaches in Haihe River Basin. Procedia Environ Sci 2:199–206
Mishra S, Sharma MP, Kumar A (2016) Assessment of surface water quality in Surha Lake using pollution index, India. J Mater Environ Sci 7:713–719
Nagaraju A, Thejaswi A, Sreedhar Y (2016) Assessment of groundwater quality of Udayagiri area, Nellore District, Andhra Pradesh, South India using multivariate statistical techniques. Earth Sci Res J 20(4):E1–E7. https://doi.org/10.15446/esrj.v20n4.54555
Nayek B, Das B, Mukherjee SC, Pal A, Ahamed S, Hossain MA, Maity P, Dutta RN, Dutta S, Chakarborti D (2008) Groundwater arsenic contamination in the Sahibganj district of Jharkhand state, India in the middle Ganga plain and adverse health effects. Toxicol Environ Chem 90(4):673–694
Patolia P, Sinha A (2017) Fluoride contamination in Gharbar Village of Dhanbad District, Jharkhand, India: source identification and management. Arab J Geosci 10:381. https://doi.org/10.1007/s12517.017.3164.0
Puri AN (1949) Soils—their physical chemistry. Reinhold Publishing and Co., p. 550.
Raghunath IIM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Raju NJ (2013) Iron Contamination in groundwater: a case from Tirumala–Tirupati environs. India Research 01(01):32–35
Ramachandra TV, Vinay S, Mahapatra DM, Varghese S, Aithal BH (2016) Water situation in Bengaluru. Energy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore, India
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. US Department of Agriculture, Agriculture Hand book 60, Washington.
Roy A, Keesari A, Mohokar H, Sinha UK, Bitra S (2018) Assessment of groundwater quality in hard rock aquifer of central Telangana state for drinking and agriculture purposes. Appl Water Sci 8:124
Satish Chandra D (2017) Estimation of water quality index by weighted arithmetic water quality index method: a model study. Int J Civ Eng Technol 8(4):1215–1222
Shah VJ, Devmurari CV, Joshi SV, Trivedi JJ, Prakash Rao A, Ghosh PK (2004) A case study of long-term RO plant operation without chemical pretreatment. Desalination 161(2):137–144
Sharma DA, Rishi MS, Keesari T (2017) Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl Water Sci 7:3137–3150
Sharma LM, Joshi RK (2014) Reverse osmosis is not a viable option for water purification in water stressed regions of India. OSR J Environ Sci: Toxicol Food Technol 8(8):23–27
Sidabutar NV, Hartono DM, Soesilo TEB, Hutapea RC (2017) The quality of raw water for drinking water unit in Jakarta-Indonesia. In: AIP conference proceedings. 1823, 020067
Singh AK, Mondal GC, Singh TB, Singh S, Tewary BK, Sinha A (2012) Hydrogeochemical processes and quality assessment of groundwater in Dumka and Jamtara districts, Jharkhand, India. Environ Earth Sci 67:2175–2191
Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J (2017) Multivariate 626 statistical analysis and geochemical modeling for geochemical assessment of 627 groundwater of Delhi, India. J Geochem Explor 175:59–71
Singh PK, Verma P, Tiwari AK (2018) Hydrogeochemical investigation and qualitative assessment of groundwater resources in Bokaro district, Jharkhand, India. Arab J Geosci 11:483. https://doi.org/10.1007/s12517.018.3831.9
SubbaRao N (2008) Iron content in groundwaters of Visakhapatnam environs, Andhra Pradesh, India. Environ Monit Assess 136:437–447
SubbaRao N, Sunitha B, Rambabu R, Rao PVN, Rao PS, Spandana BD, Sravanthi M, Marghade D (2018) Quality and degree of pollution of groundwater, using PIG from a rural part of Telangana State, India. Appl Water Sci 8:227. https://doi.org/10.1007/s13201.018.0864.x
Thakur N, Rishi M, Sharma DA, Keesari T (2018) Quality of water resources in Kullu Valley in Himachal Himalayas, India: perspective and prognosis. Appl Water Sci 8:20
Tirkey P, Bhattacharya T, Chakarborty S, Barik S (2017) Assessment of groundwater quality and associated health risks: a case study of Ranchi city, Jharkhand, India. Groundw Sustain Dev 5:85–100. https://doi.org/10.1016/j.gsd.2017.05.002
Wang XQ, Wen J, Chen P, Liu N (2018) Monitoring and assessment of Youshui river water quality in Youyang. IOP Conf Ser Earth Environ Sci 113:012069. https://doi.org/10.1088/1755-1315/113/1/012069
WaterAid India (2018) State of urban water supply in India. New Delhi, India, p. 36. https://www.wateraidindia.in/sites/g/files/jkxoof336/files/state-of-urban-water-supply.pdf).
WHO (2017) WHO library cataloguing-in-publication data, guidelines for drinking-water quality: fourth edition incorporating the first Addendum. ISBN 978–92–4–154995–0.
Wilcox LV (1955) Classification and use of irrigation water. USDA Circ. 696, Washington DC.
Yadav NS, Kumar A, Mishra S, Singha S (2018) Assessment of water quality using pollution-Index in the study stretch of river Chambal, India. Integrat Res Adv 5(1):20–25
Acknowledgements
The senior author would like to acknowledge Chemistry Department, Raiganj University, West Bengal, India for providing technical and laboratory support during conducting this study and preparing the manuscript. The manuscript has been cross-checked for similarity index by Ithenticate software at CSIR-NEERI Knowledge Resource Centre and the designated manuscript number is CSIR-NEERI/KRC/2020/JUNE/KZC/1.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Human and animal rights
All the authors certify that no animal or human studies were carried out by the authors. Also, the highest level of integrity has been maintained in the content of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pramanik, A.K., Majumdar, D. & Chatterjee, A. Groundwater hydrochemistry and consumption patterns in Chandwara community development block of Jharkhand state in India. Appl Water Sci 12, 60 (2022). https://doi.org/10.1007/s13201-022-01587-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01587-6