Abstract
Himalayan foothills streams, Pakistan play an important role in living water supply and irrigation of farmlands; thus, the water quality is closely related to public health. Multivariate techniques were applied to check spatial and seasonal trends, and metals contamination sources of the Himalayan foothills streams, Pakistan. Grab surface water samples were collected from different sites (5–15 cm water depth) in pre-washed polyethylene containers. Fast Sequential Atomic Absorption Spectrophotometer (Varian FSAA-240) was used to measure the metals concentration. Concentrations of Ni, Cu, and Mn were high in pre-monsoon season than the post-monsoon season. Cluster analysis identified impaired, moderately impaired and least impaired clusters based on water parameters. Discriminant function analysis indicated spatial variability in water was due to temperature, electrical conductivity, nitrates, iron and lead whereas seasonal variations were correlated with 16 physicochemical parameters. Factor analysis identified municipal and poultry waste, automobile activities, surface runoff, and soil weathering as major sources of contamination. Levels of Mn, Cr, Fe, Pb, Cd, Zn and alkalinity were above the WHO and USEPA standards for surface water. The results of present study will help to higher authorities for the management of the Himalayan foothills streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inorganic, organic and thermal pollutions have led to accelerate the deterioration of the freshwater resources (Khound and Bhattacharyya 2016; Qadir et al. 2008). Contaminants related to urban and agricultural runoff are the primary sources of non-point pollution. Industrial and municipal effluents which comprised organic and inorganic chemicals are directly or indirectly discharged into water bodies and constitute the punctual point sources of contamination (Allan and Castillo 2007). Toxic inorganic chemicals are also released from weathering of rocks, carried by runoff into water bodies and/or percolate into groundwater (Li and Zhang 2010). Among inorganic toxic chemicals, heavy metals are of greater concern. These are highly persistent, ubiquitous, non-biodegradable, accumulate and biomagnify in food chain and have cumulative effects in human (Hashmi et al. 2012). Other inorganic materials, such as, acids, salts and chlorine which are not toxic at low concentrations may become concentrated, deteriorate water quality and adversely affect biological organisms.
There has been long-time history of study on water pollution caused by heavy metals at variable scales from global to regional and in different aquatic systems such as streams, rivers, and lakes (Okiongbo and Douglas 2015; Singh 2002; Vega et al. 1998). Pakistan is amongst countries facing scarcity, shortage and wide-spread heavy metal contamination of water resources. Few studies exist in Pakistan to characterize water quality including nutrients, and hydro-geochemistry using multivariate techniques (Qadir et al. 2008), whilst concentrations and spatial and seasonal distributions of metals, nutrients and other physiochemical parameters and source identification of contaminant of surface water of the Himalayan streams remain unavailable. The Rumli stream consists of three major tributaries. The first tributary originates from Jalwaian, cutting through Rumli, Dhok Kamlar villages, Quaid-i-Azam University Colony and Nur Pur Shahan village. The second tributary originated from Sarra valley Margalla Hills, passes through Mandiala and Bari Imam Villages and joins first tributary at Labour Colony. The third tributary originates from Gagal Pani in Margalla Hills, cutting through Ratta Hotter and Diplomatic Enclave. These tributaries meet near the end of southern edge of Diplomatic Enclave before falling into Lake Reservoir. Quaid-i-Azam university stream originates from Mohra village in close vicinity of the university transverse through university hostels, and Kalapani Kas before descending into the Lake Reservoir. The Rumli and Quaid-i-Azam University streams fall into Rawal lake reservoir on its north east edge. Taxi driving is the major source of income of the local inhabitants. Most of the area is hilly terrain either used for building new colonies or as recreational parks. Though farming is not a major source of earning livelihood but selected terrain is used to cultivate crops. Industrial activity is almost absent in the catchment. However, abandoned crushers were found at a few places indicate the use of land for stone quarries in recent past. Streams are used for all domestic purposes. Raw sewage is drained directly or in directly without any prior treatment into these streams and solid waste is dump near stream banks. The unmanaged urbanization is also a source of major concern. The present study is a first such document to assess the contamination of water quality of the Himalayan foothills streams which feed the Rawal Lake Reservoir with total catchment of 275 km2. Lake Reservoir is the main drinking water source of fourth largest city of Pakistan, Rawalpindi. Thus, its water quality is of a great concern for human well being. The main objectives of this study were: to assess the spatial and seasonal variability of heavy metals and nutrients in surface water of perennial streams feeding the Lake Reservoir.
Materials and methods
Study area
The study area lies in the Islamabad district (latitude 33°41′23.9994–39°51′35.9994N and longitude 73°4′47.9994–73°20′24E) and is characterized by a sub-humid climate. The monthly mean air temperature ranges from 9 to 32.5 °C with a yearly average of 21.96 °C. The lowest ever temperature recorded is −4 °C in January, while the highest is 48 °C in June. The relative humidity in Islamabad ranges from 19 to 54% recorded in the months of May and August, respectively. The annual precipitation ranges from 1000 to 1200 mm. The highest precipitation occurs in the months of July and August followed by dry spell which is broken by slight rain showers from January to March. The study area is strongly influenced by the monsoon rain. The rocks are sedimentary in origin and mainly consisted of red and maroon shale, siltstone, and sandstone which are extensively excavated and used for construction of building and bridges.
The Lake reservoir is fed by five perennial streams (Rumli, Shahdara, Baroha, Malachh, Quaid-i-Azam University and Korang). The Korang is the main tributary which originates from the numerous springs of Chara pani located in the Murree Hills and flows downstream passing through small villages, i.e., Treet, Salgara, Baroha, Arna, Thalan, Torimma, Thrat Kalam, Bharakao, Mal Pur and Bani Gala before drop into the Lake Reservoir. The stream in the start runs as two parallel tributaries, which meet at Thrat Kalam and flow as a single tributary. The upper tributary of the Korang flows through Treet, Salgara and Baroha villages while lower tributary runs through Arna, Thalan and Torimma villages before joining at Thrat Kalam. The upper tributary is more disturbed due to extensive road network, and receives more water from numerous seasonal and perennial streams throughout the year. The Korang stream during its course joins two other streams (Shahdara and Baroha which originates from eastern margins of Margalla Hills). Baroha joins Korang stream in upper tributary while Shahdara joins it at Bharakao, i.e., from where the Korang stream travels as single tributary. At Bani Gala village it drains into the Lake Reservoir.
Shahdara stream originates from the springs located in Ranias, Padu and Shahdara Kalan villages and travels through Shahdara, Mandha and Mill villages before meeting Korang nallah at Bharakao. Baroha stream originates from the Haicht Bagla transverse through Hassah, Tail Ban, Baroha villages and meets Kaurang stream in its upper tributary, i.e., before Thrat Kalam.
Water sampling and laboratory analyses
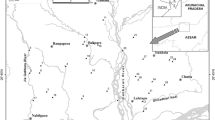
Grab surface water samples were collected from 16 sites (Fig. 1) at approximate 5–15 cm water depth in pre-washed polyethylene containers during pre-monsoon (May 2007 and 2008) and post-monsoon (September 2007 and 2008). Water sampling survey reach length was defined at each site which was 10 times the stream width. One sample was collected each from up, mid, and downstream point of defined survey reach length, and composite sample was filtered through pre-washed 0.45 µm Millipore nitrocellulose filters in polyethylene containers and kept at 4 °C. Water samples for trace metal analysis were acidified to pH <2 with ultra-purified 6 M HNO3 and stored in pre-cleaned polyethylene containers.
Water quality parameters such as Dissolved Oxygen (DO), Dissolved Oxygen saturation (DOS), pH, Electric Conductivity (EC), and temperature were measured from each site using Hydrolab (Hach MS5). Geographic coordinates were determined using Global Positioning System. Water samples were kept at 4 °C till further processing. Dissolved oxygen (DO), Electric Conductivity (EC), and temperature were measured in situ, whereas, Alkalinity (Alk), Chlorides (Cl) Ortho-Phosphates (PO4 3−), Nitrate–nitrogen (NO3–N), NH4–N, and sulfate (SO4 2−) were determined in the laboratory using standard procedures (APHA 1998). PO4 3−, NO3–N, SO4 2− and NH4–N were determined spectrophotometrically (HACH DR/5000 U) using Ammonium molybdenum method, phenol-disulfonic acid method, barium-chloride method and phenate method, respectively. Cl was measured titrimetrically with AgNO3. Total Dissolved Solids (TDS) were measured using conductivity meter (Hi 8033 Hanna Hungry).
Metal analysis and quality assurance and control (QA/QC)
Concentration of metals such as Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mn, Mg, Na, Ni, Pb, and Zn were measured using Fast Sequential Atomic Absorption Spectrophotometer (Varian FSAA-240) in triplicate. Reagent and procedural blanks were measured in parallel to sample treatment using identical procedures. Each calibration curve was evaluated by determination of quality control standards before, during and after set of sample measurement. The recovery rates of studied metals were within 90 ± 10%.
All chemicals used were of analytical grade (Merck Darmstadt, Germany). Ultra pure water was used throughout the analysis. Standard solutions of metals were prepared by dilution of 1000 ppm certified standard solutions (Fluka Kamica Busch, Switzerland) of corresponding metal. Polyethylene containers, glass and plastic ware used in field and for laboratory analyses were cleaned with washing detergent (Decon 90), rinsed many times with deionised water, soaked in HNO3 (10%, v/v) for 24 h, and finally rinsed with ultra pure water.
Multivariate statistical analysis
Hierarchical Cluster Analysis
Spatial variability in surface water in terms of water quality parameters was determined using CA. Ward’s method which uses analysis of variance was applied as clustering technique and squared Euclidean methods as distance matrix. Cluster analysis was applied on the total dataset from the two seasons.
Discriminant function analysis
Spatial and seasonal variations in surface water were evaluated using DFA. In spatial DFA, sites groups categorized using CA were treated as spatial independent variables whereas in seasonal DFA, seasons were constituted as independent variables. Measured physicochemical water quality parameters were considered as dependent variables.
Factor analysis based on principal component analysis (FA/PCA)
Factor Analysis based on PCA was employed to interpret underlying dataset and to identify possible sources of contamination through reduce new set of orthogonal variables called Principal components (PCs) which provide information in decreasing order of importance. Factor analysis revealed underlying structure of dataset by means of distance between variables in multi-dimensional space. Varimax rotation of PCs was used to derive and extract the Varimax Factor (VFs). Varimax factors with eigenvalue >1 were retained. FA/PCA was applied on raw data separately for seasonal groups (i.e., pre-monsoon and post-monsoon season) and spatial groups (HIIG, MIG, and LIG defined by HACA). Input data matrices (sites × variables) for FA/PCA in seasonal groups for pre-monsoon and post-monsoon season were (16 × 25). FA and PCA with Varimax rotation of standardized component loadings were conducted for extracting and deriving factors, respectively. Input data matrices (sites × variables) for spatial PCA/FA were (3 × 25) for HIG, (5 × 25) for MIG, and (8 × 25) for LIG, respectively. Statistical analyses were made using Excel and STATISTICA software.
Results and discussion
Classification of sites and source identification of spatial variations
Cluster analysis produced three groups of sites with similar physiochemical water quality characteristics (Fig. 2). Sites K1, K4 and R3 were categorized as highly impaired group (HIG) which receives greater input of metals and PO4 3−. Spatial FA/PCA for HIG, five VFs explained 100% variations in the total dataset (Table 1). VF1 explained 50.35% of the total variance, and strong positive loadings on Cu, Mn, Cr, Co, Fe, Pb, Cd, Zn, K, Mg and Li indicating their common origin related mainly with anthropogenic activities such as car and animal washing, recreational use, construction and domestic waste and grazing. This confirms the results of CA. VF2 explained 26.53% of the total variance, had positive loadings on pH, DOS, DO, SO4 2− and negative on Ni while moderately negative on PO4 3− indicated that high level of nutrients decreases DO and DOS in surface water. VF3 explained 9.93% of the total variance with eigenvalue of 2.48 and had strong negative loadings on NO3–N and Na highlighting their input from anthropogenic activities such as the use of nitrogenous fertilizers and effluent containing municipal waste. VF4 and VF5 explained 8.48 and 4.71% of the total variance with eigen value of 2.12 and 1.18. VF4 showed negative loadings on TDS and ALK and VF5 was correlated with temperature, Cl and Ca content inferring their source from parent rock material.
Moderately impaired group (MIG) comprised of sites such as K3, K5, R2, R4, S2 and M1 which were surrounded by scattered dwellings and agricultural activities. High contents of NO3–N and SO4 2− were measured in this group. Animal waste and surface runoff were the main sources of contamination. For MIG, PCA/FA represented 89.22 (%) of the total variance in physicochemical parameters along five VFs with eigen values >1. VF1 explained 51.09% of total variance with eigenvalue of 12.77 and showed positive loadings on pH, EC, SO4 NO3–N, Co, Fe, Pb, Cd, Zn, K, Ca and Li. VF2 explained 17% of the total variations, had strong positive loadings on TDS, ALK and Cl VF3 explained 13.03% of total variance; strong negative loading on Na. VF4 and VF5 explained 5.78 and 4.11% of the total variance with positive loading on Mn and Ni and Mg, respectively. High loading of parameters of VFs indicated that the sources are mainly related to anthropogenic activities (Tiri et al. 2015).
The least impaired group (LIG) consisted of sites viz., S1, H1, B1, K2, R1, R5, and R6. Sites S1, B1, R1 and R5 were located in the upstream region with relatively less impact from anthropogenic activities. Most of the sites were surrounded by thick forest vegetation/scrub. For source identification in LIG, FA/PCA highlighted seven significant VFs. VF1 explained 39.77% of total variance and eigenvalue of 9.94 with strong positive loadings on EC, SO4 2−, Mn, Cu, Co, Pb, K and Li. VF2 explained 15.29% of the total variance and eigen value of 3.82 with having positive loadings on Cr, Zn and Cd. LIG had lowest concentration of metals (such as Cu, Mn, Co, Fe, Pb, K, Na, Ca, Mg, and Li) and nutrients such as NO3–N, SO4 2−, Cl and PO4 3− and highest concentration of DO highlighting least impact from anthropogenic activities. VF3 explained 9.47% of the total variations and eigenvalue of 2.37 had strong positive loadings on DOS and DO and negative on PO4 3−. Mean DO level and DOS were highest in sites located upstream region. Negative association of PO4 3− with VF3 indicated discharge of animal and human waste. VF4, VF5 and VF6 explained 7.82, 6.85 and 5.41% of total variation and showed positive loading on Fe, Cl1−, TDS and ALK indicated input from parent rock material and negative loading on NO3–N highlighting source related to domestic and agricultural activities. VF7 explained 4.64% of the total variance and had strong positive loadings on temperature indicating that LIG is located in upstream segment of the stream at higher elevation and had lower temperature indicating influence of climate.
Seasonal variations of heavy metals and source identification
Seasonal backward stepwise mode DFA identified sixteen important variables viz., pH, TDS, DOS, DO, ALK, PO4 3−, NO3–N, Cl, Ni, Cu, Mn, Co, Fe, Pb, Na and Mg as (Fig. 3; Table 2) with classification accuracies of 100% which bring variations in pre- and post-monsoon seasons. In pre-monsoon, concentration of metals followed the order: Ca > Fe > Mg > Na > K > Zn > Cr > Ni > Li > Mn > Pb > Cu > Co > Cd; whereas, in post-monsoon, slightly different trend was recorded: (Ca > Fe > Mg > Na > K > Zn > Cr > Li > Pb > Co > Ni > Cu > Mn > Cd (Table 3). Fewer seasonal variations were recorded in temperature, EC and TDS. Dissolved oxygen contents were lower in pre-monsoon as compared to post-monsoon. Alkalinity and Cl showed similar seasonal trend. Among nutrients, less concentration of NO3–N and PO4 3− was recorded in post-monsoon season; whereas higher SO4 2− contents were measured in the pre-monsoon. Concentration of TDS, NO3–N, PO4 3−, Ni, Cu and Mn decreased during post-monsoon season highlighting influence from monsoon rains that causes dilution of surface water. This also confirms that sources are linked with municipal activities. Concentration of contaminant from point sources becomes low due to high flow during rainy season and high during dry season when stream exhibits low flow rate (Ding-jiang et al. 2006; Jaji et al. 2007; Shrestha and Kazama 2007). However, greater alkalinity, Co, Fe, Pb, Na, and Mg concentration in post-monsoon can be related to surface runoff and soil weathering of parent rock material (Jaji et al. 2007; Qadir et al. 2008). Erosion is an active phenomenon in catchments area during rainy season indicating a contribution from non-point sources during post-monsoon season (Ding-jiang et al. 2006).
Seasonal FA/PCA highlighted five VFs for pre-monsoon and seven VFs for post-monsoon season with eigenvalue >1 and represented 87.22 and 83.04% of total variance in water quality data (Table 4). For pre-monsoon season, out of five VFs, VF1, and VF2 represented 47.51 and 12.97% of total variance and eigenvalue of 11.88 and 3.24. Metals such as (Ni, Cu, Mn, Cr, Co, Fe, Pb, Cd, Zn, K, Mg and Li) and nutrients (SO4 2−, NO3–N) showed strong loadings indicated input from untreated discharge of municipal waste, surface runoff and solid waste into streams in pre-monsoon season. VF3 represented 12.38% of total variance and had a strong but positive loadings on DOS and DO and negative on Cl. Chloride is mobilized mainly through weathering of rocks (Fernandez-Turiel et al. 2003; Girija et al. 2007) however, use of salts in dehydrating and antiseptic agent for hides in tanneries (Qadir et al. 2008) is another possible source which adds chlorides in surface waters. Presence of Cl can be attributed from natural and anthropogenic sources. These results are in agreement with the study of Vega et al. (1998). Chloride penetrates into natural waters from soil, natural layers of salts, municipal and industrial sewage and waste of animal origin (Girija et al. 2007). VF4 and VF5 explained 9.85 and 4.52% of total variance and had strong negative loadings on EC, TDS and ALK and positive loading on Ca. TDS which indicates dissolved constitutes in water (Girija et al. 2007) ranged from 0.3 to 0.6 (g L−1) in pre-monsoon and from 0.3 to 0.53 (g L−1) in post-monsoon season. EC of surface water is affected by the presence of inorganic dissolved solids. Stream geology, industrial and urban effluents and surface runoff can be correlated with EC (Kannel et al. 2007). Extended dry periods and low flow conditions contribute to higher EC values. Temperature showed positive correlation with EC. Main source of greater EC values in current study can be linked to the geology of catchment area of the Kurang, soil weathering, surface runoff and dumping of untreated sewage.
For post-monsoon season, out of seven VFs, VF1 explained 25.2% of total variance with eigenvalue of 7.06 and strong loading on TDS, EC, ALK and Pb highlighting sources related with surface runoff (Castañé et al. 2006; Rath et al. 2000; Sundaray et al. 2006). VF2 and VF3 identified influence of climate on water quality and explained 14.92 and 12.54% of total variance. These VFs showed significant loadings on DOS, DO and temperature. Low level of DO was found during low flow period, however, DO level remarkably improved in post-monsoon season, as catchment area is highly dependent on rainfall. VF4 explained 10.22% of total variance with eigenvalue of 2.86 and positive loadings on Zn and Li and negative lading on Ca. Sources of Zn and Li can be associated with anthropogenic activities whereas Ca had an origin from parent rock material. VF5 explained 7.7% of total variance having a strong positive loading on Mn and negative on Fe; Mn being originated from anthropogenic sources and Fe from parent rock material. VF6 and VF7 explained 6.93 and 6.97% of total variance with a strong loading on NO3–N and Cd highlighting sources from surface runoff.
Characterization and comparison of physiochemical parameters with literature and quality guidelines
Spatial backward stepwise mode Discriminant function analysis (DFA) discriminated five parameters viz., temperature, EC, NO3–N, Fe and Pb that cause variations among three groups with classification accuracies of 71.88% (Fig. 4). Impairment of sampling sites and deterioration of surface water quality can be linked to the lack of proper sanitation, unprotected stream sites and anthropogenic activities in their catchment. Sites grouped in impaired and moderately impaired categories had greater impact from anthropogenic activities and were characterized by relatively open vegetation, turbulent flow, input from solid waste, washing activities along the stream banks and urban runoff and municipal effluents. According to Qadir et al. (2008) spatial variations in surface water is related to anthropogenic and natural processes and caused by various factors viz., temperature, solar angle, meteorological events, stream origin, velocity, vegetation types and coverage and land-use types in the basin (Qadir and Malik 2009). Results showed lower temperature in upstream as compared towards downstream. LIG was characterized by less temperature, high DO contents, steep slopes, rocky landscape, forest vegetation and high flow rate. These conditions generally enhanced aeration of surface water resulting greater DO (Kannel et al. 2007). Sites located at higher slopes showed higher DO values. Dissolved oxygen has been used as an indicator for ecological health of aquatic ecosystem (Qadir and Malik 2009). Rath et al. (2000), Sundaray et al. (2006) and Jaji et al. (2007) showed similar trends in DO as recorded in current study (2–8 mg L−1 in pre- monsoon and 4.9–11.6 mg L−1 in post-monsoon). Measured DO contents were higher at most of the sampling sites to those of minimum level of 2.43 (mg L−1 DO at 25.1 °C) required for protection of aquatic life (DeZuane 1997).
Surface water was alkaline in nature and measured pH values were within the EU and USEPA criterion of surface water (6.5–9.5 and 6.5–9). In post-monsoon, pH values reached the upper limit of 9 which is attributed to inputs of metals viz., Na, Ca and Mg from natural sources (Spellman and Drinan 2001). Measured EC was also within the safe limits of EU standard of 2.5 mS-cm and were lower to those reported by other authors (Debels et al. 2005; Singh et al. 2004). TDS values from all sites except at site M1, fall within WHO and EU standard of 0.5 g L−1. TDS values measured in the current study were similar to those reported in study of Vega et al. (1998), but greater to those reported in study of Fernandez-Turiel et al. (2003), Castañé et al. (2006) and (Zhou et al. 2007).
Greater PO4 3− concentrations measured in pre-monsoon indicated input of nutrients in streams from surface runoff (Majagi et al. 2008). Laundry, car washing and human/cow bathing are common activities along stream banks and high concentration of PO4 3− can also be related to use of detergents (Allan and Castillo 2007). PO4 3− concentration >0.02 mg L−1 causes eutrophication in watercourses and has influence on oxygen budget of fresh water. Studies conducted by Rath et al. (2000), Debels et al. (2005), Crosa et al. (2006) showed relatively lower level of PO4 3− in comparison to those recorded in the current study. However, measured concentrations were within range reported by other authors (Singh 2002; Singh et al. 2004).
Nitrate–nitrogen originates from various sources in surface water viz., human and animal waste, decomposition of organic nitrogenous matter the use of agricultural fertilizers, atmospheric deposition, and surface runoff (Qadir et al. 2008). The presence of nitrate can also be related to land-use in the catchment (Allan and Castillo 2007). Lack of sewerage/drainage system in the catchment area, municipal sewage and solid waste, animals waste and the use of fertilizers can be the main sources of NO3–N in surface water. Measured concentrations (pre-monsoon 15.1–49.2 mg L−1; post-monsoon 0.0–31.0 mg L−1) were within safe limits of WHO i.e. 50 mg L−1 of drinking water. Similar concentrations were found by Vega et al. (1998) and Jaji et al. (2007), However, measured concentrations were higher to those reported in the studies of Crosa et al. (2006), Shrestha and Kazama (2007), and Sojka et al. (2008) and lower to those reported of Rath et al. (2000).
Sulfate concentration in both seasons was within WHO, EU, and USEPA criterion. Measured concentrations were greater to those reported in by Kannel et al. (2007) and Zhou et al. (2007). Weathering of sedimentary rocks and contamination from fertilizers, poultry wastes and fossil fuel burning are the main sources (Qadir et al. 2008).
Alkalinity values recoded in the current study were well above USEPA criterion (20 mg L−1) of surface water whereas Cl contents were within the limits of EU, WHO and USEPA standards. Measured values of alkalinity were greater to those recorded in other studies (Crosa et al. 2006; Kannel et al. 2007; Majagi et al. 2008) and, however, lower to those reported in Reconquista River, Argentina (Castañé et al. 2006). Chloride concentration measured in the current study was lower to those recorded in Llobregat river basin (Fernandez-Turiel et al. 2003), Cao River China (Ding-jiang et al. 2006), Reconquista River, Argentina (Castañé et al. 2006), and Mala Welma River, Poland (Sojka et al. 2008). Nonetheless, the studies accomplished by Singh et al. (2004) showed lower values of chlorides in comparison with the current study.
High concentration of Ca, Mg and Fe in pre- and post-monsoon can be related to their origin from parent rock material. These are the most abundant elements of earth crust and soil (Majagi et al. 2008). Weathering and erosion can mobilize these metals (Qadir et al. 2008). Concentration of Na ranged from 9.80 to 71.38 (mg L−1) and falls within the EU and WHO guidelines. However, measured concentrations were higher to those reported by other authors (Sojka et al. 2008; Vega et al. 1998). Higher concentrations of Fe, Pb Mg, Na, and Co in post-monsoon can be related to surface runoff. Greater Fe concentration is mainly related to parent rock material; however, acid mine discharges, smelting and refining of metals, waste disposals such as landfills and urban runoff, i.e., corrosion of iron, steel structures in buildings, dumpsites and vehicles are possible anthropogenic sources (Jaji et al. 2007). Since industrial activity is absent in the catchment area, th soil leaching and urban runoff are the most likely process contributing in Fe in surface water (Simeonov et al. 2003). Measured Fe concentrations were high to those reported by Fernandez-Turiel et al. (2003) and Zhou et al. (2007). Fe concentration in both seasons was above the standard of EU, WHO and USEPA guidelines.
High concentration of Pb in soil and water is mainly related to human activities. Lead salts are commonly used as pigments in commercial paints and anti-corrosion coatings. Lead is used as anti knocking agent in gasoline in Pakistan. Jaji et al. (2007) pointed out that sites where industrial activity is absent, traffic density is the main source of Pb contamination. Pb contents high in impaired sites group can be related to urban runoff, traffic density, and automobile washing which is common along stream banks. Effluents from Quaid-i-Azam University, Bari Imam, Barakaho, Bani Gala and Diplomatic enclave are other possible sources. Pb concentrations (0.03–2.38 mg L−1) were higher to the EU and WHO standard for drinking water and USEPA standards of surface water. Measured concentrations were also higher to those reported in Adyar River India (Venugopal et al. 2009), and Ogun River Nigeria (Jaji et al. 2007).
Concentrations of Ni, Cu, and Mn were high in pre-monsoon season. Greater Ni concentration may be attributed to the disposal of sewage wastes and waste from household activities. Mean concentration of Ni, Mn in pre- and post-monsoon seasons were higher than the EU and WHO guidelines of drinking water and USEPA standards of surface water quality. In contrast, mean Cu concentration in pre-monsoon (0.21 mg L−1) and post-monsoon (0.08 mg L−1) falls within the USEPA, EU and WHO criterion for water quality. However, measured concentrations are higher to those reported in other studies (Venugopal et al. 2009). Mn concentration (0–7.8 mg L−1) was also higher to those reported in Pisuerga and Llobregat River, Spain; 0.01–0.08 mg L−1 (Vega et al. 1998), 0.008–0.07 mg L−1 (Fernandez-Turiel et al. 2003), Ogun river Nigeria; 0.04–1.89 mg L−1 (Jaji et al. 2007), and Mala Welma river Poland; 0.01–0.22 mg L−1 (Sojka et al. 2008). Mean measured Cd concentration in pre- (0.053 mg L−1) and post-monsoon (0.061 mg L−1) seasons were higher to those of EU and WHO guidelines of drinking water and USEPA of surface water quality. Measured concentrations (0.03–0.25 mg L−1) were within the range (0–0.25 mg L−1) reported in Hudiara drain Pakistan (Khan et al. 2003) and, however, lower than those in Ogun river Nigeria (Jaji et al. 2007). Greater Cr concentrations in pre- (mean 1.58 mg L−1) and post-monsoon (1.08 mg L−1) were recorded to those from Hong Kong (0.001–0.059 mg g−1) (Zhou et al. 2007). Comparatively concentrations measured in current study exceeded the permissible limits of EU, WHO and USEPA water quality. Similarly, Zn concentration was also above USEPA criteria but lower to those of WHO guideline of drinking water quality. Zn concentration recorded in the current study was higher to those reported in other studies (Jaji et al. 2007; Venugopal et al. 2009; Zhou et al. 2007).
Conclusion
In the present study, we concluded that sites located upstream segments of Rumli, Baroha and Shahdara streams were relatively less polluted as compared to the Malachh stream and Kurang. Multivariate analyses (HACA, DFA, FA/PCA) recognized spatio-seasonal patterns of surface water quality and aided in pattern recognition and source identification of pollutants. HACA produced three sites groups based on the impairment of sites. Spatial DFA gave best results for data reduction method. Based on FA/PCA, parent rock material, soil weathering, municipal and poultry waste, surface runoff from urban and agricultural land, automobile and laundry washing are the main source of inputs of contaminants into the feeding streams of Rawal Lake Reservoir. Identified sources of contamination could be useful to local authorities for the control of surface water pollution and the obtained results will help to design a future spatial sampling strategy in an optimal manner for mountainous streams of the region. Results also provide a baseline to compare the physical and chemical changes in surface water that occur at spatial and seasonal scale and to establish a scientific understanding to environmental managers, policy makers, conservationist and researchers working for future planning, restoration and formulation of management strategies.
References
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters. Springer, Berlin
Castañé PM, Rovedatti MG, Topalián ML, Salibián A (2006) Spatial and temporal trends of physicochemical parameters in the water of the Reconquista River (Buenos Aires, Argentina). Environ Monit Assess 117:135–144
Crosa G, Froebrich J, Nikolayenko V, Stefani F, Galli P, Calamari D (2006) Spatial and seasonal variations in the water quality of the Amu Darya River (Central Asia). Water Res 40:2237–2245
Debels P, Figueroa R, Urrutia R, Barra R, Niell X (2005) Evaluation of water quality in the Chillán River (Central Chile) using physicochemical parameters and a modified water quality index. Environ Monit Assess 110:301–322
DeZuane J (1997) Handbook of drinking water quality. Wiley, New York
Ding-jiang C, Jun L, Shao-feng Y, Shu-quan J, Ye-na S (2006) Spatial and temporal variations of water quality in Cao-E River of eastern China 环境科学学报 18
Fernandez-Turiel J, Gimeno D, Rodriguez J, Carnicero M, Valero F (2003) Spatial and seasonal variations of water quality in a Mediterranean catchment: the Llobregat River (NE Spain). Environ Geochem Health 25:453–474
Girija T, Mahanta C, Chandramouli V (2007) Water quality assessment of an untreated effluent impacted urban stream: the Bharalu tributary of the Brahmaputra River, India. Environ Monit Assess 130:221–236
Hashmi MZ, Malik RN, Shahbaz M (2012) Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan. Ecotoxicol Environ Saf. doi:10.1016/j.ecoenv.2012.11.029
Jaji M, Bamgbose O, Odukoya O, Arowolo T (2007) Water quality assessment of Ogun River, south west Nigeria. Environ Monit Assess 133:473–482
Kannel PR, Lee S, Kanel SR, Khan SP, Lee Y-S (2007) Spatial–temporal variation and comparative assessment of water qualities of urban river system: a case study of the river Bagmati (Nepal). Environ Monit Assess 129:433–459
Khan M, Khan HN, Aslam H (2003) Hudiara drain—a case of trans-boundary water pollution between India and Pakistan. Pak J Biol Sci 6:167–175
Khound NJ, Bhattacharyya KG (2016) Multivariate statistical evaluation of heavy metals in the surface water sources of Jia Bharali river basin, North Brahmaputra plain, India. Appl Water Sci 1–10. doi:10.1007/s13201-016-0453-9
Li S, Zhang Q (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176:579–588
Majagi SH, Vijaykumar K, Vasanthkaumar B (2008) Concentration of heavy metals in Karanja reservoir, Bidar district, Karnataka, India. Environ Monit Assess 138:273–279
Okiongbo K, Douglas R (2015) Evaluation of major factors influencing the geochemistry of groundwater using graphical and multivariate statistical methods in Yenagoa city, Southern Nigeria. Appl Water Sci 5:27–37
Qadir A, Malik RN (2009) Assessment of an index of biological integrity (IBI) to quantify the quality of two tributaries of river Chenab, Sialkot, Pakistan. Hydrobiologia 621:127–153
Qadir A, Malik RN, Husain SZ (2008) Spatio-temporal variations in water quality of Nullah Aik-tributary of the river Chenab, Pakistan. Environ Monit Assess 140:43–59
Rath P, Bhatta D, Sahoo B, Panda U (2000) Multivariate statistical approach to study physico-chemical characteristics in Nandira-Brahmani River, Angul-Talcher Belt, Orissa, India. Pollut Res 19:701–710
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22:464–475
Simeonov V et al (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Singh AK (2002) Quality assessment of surface and subsurface water of Damodar river basin. Indian J Environ Health 44:41–49
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): a case study. Water Res 38:3980–3992
Sojka M, Siepak M, Zioła A, Frankowski M, Murat-Błażejewska S, Siepak J (2008) Application of multivariate statistical techniques to evaluation of water quality in the Mała Wełna River (Western Poland). Environ Monit Assess 147:159–170
Spellman FR, Drinan J (2001) Stream ecology and self purification: an introduction. CRC Press, Boca Raton
Sundaray SK, Panda UC, Nayak BB, Bhatta D (2006) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the Mahanadi river-estuarine system (India)—a case study. Environ Geochem Health 28:317–330
Tiri A, Lahbari N, Boudoukha A (2015) Assessment of the quality of water by hierarchical cluster and variance analyses of the Koudiat Medouar Watershed, East Algeria. Appl Water Sci 1–10. doi:10.1007/s13201-014-0261-z
Vega M, Pardo R, Barrado E, Debán L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592
Venugopal T, Giridharan L, Jayaprakash M, Velmurugan P (2009) A comprehensive geochemical evaluation of the water quality of river Adyar, India. Bull Environ Contam Toxicol 82:211–217
Zhou F, Liu Y, Guo H (2007) Application of multivariate statistical methods to water quality assessment of the watercourses in Northwestern New Territories, Hong Kong. Environ Monit Assess 132:1–13
Acknowledgements
This research was funded by Higher Education Commission (HEC), Pakistan under Research Project No. 828. Pakistan Wetlands Program (PWP) support towards provision of transport and Hydrolab facility during field surveys is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Malik, R.N., Hashmi, M.Z. Multivariate statistical techniques for the evaluation of surface water quality of the Himalayan foothills streams, Pakistan. Appl Water Sci 7, 2817–2830 (2017). https://doi.org/10.1007/s13201-017-0532-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0532-6