Abstract
The sorption of Cr(VI) and As(V) from the aqueous solutions with the polyacrylate anion exchangers of the strong base functional groups Amberlite IRA 458 and Amberlite IRA 958 was studied. The studies were carried out by the static-batch method. The concentration of Cr(VI) and As(V) ions in the aqueous solution was determined by the UV–VIS spectrophotometer. The influence of several parameters was studied with respect to sorption equilibrium. The phase contact time and the concentration affect the sorption process. The equilibrium state was established already after 15 min of phase contact time. Maximum uptake of Cr(VI) and As(V) occurred at pH 5 and 10, respectively. The determined kinetic parameters imply that the sorption process proceeds according to the equation type of pseudo second-order. Sorption equilibrium data were correlated with the Langmuir and Freundlich isotherms. Removal of As(V) ions on macroporous Amberlite IRA 900 decreased about 12 % in presence of other anions (Cl−, NO3−, SO42−) in the solution. The sorption was temperature dependent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is a naturally occurring element found in soil and groundwater in several different forms. It occurs in the (VI) and (III) oxidation states (Banfalvi 2011). The use of chromium chemicals in several industrial processes (leather tanning, mining of chrome ore, production of steel and alloys, dyes and pigment manufacturing, glass industry, wood preservation, textile industry, film and photography, metal cleaning, plating and electroplating, etc.) leads to contamination of natural waters mainly due to improper disposal methods (Sarin et al. 2006). While Cr(III) is generally non-toxic and is believed to be essential in glucose metabolism in mammals (Rengaraj et al. 2001), Cr(VI) is toxic to animal and plant cells. Furthermore, it is dangerous for human beings due to its mutagenic and carcinogenic properties (Arslan and Pehlivan 2007). Since the compounds of chromium are known to be harmful to human health, the maximum level permitted in wastewater is 5 ppm for trivalent chromium and 0.05 ppm for hexavalent chromium (Namasivayam and Senthilkumar 1999). Arsenic compounds are rather widely distributed in nature and they can be often found in groundwater and drinking water, mainly due to erosion and weathering of As-containing soils and minerals, as well as from industrial effluents and via atmospheric deposition (burning of fossil fuels) (Bortun et al. 2010). Water contaminated by arsenic may cause numerous diseases of the skin and internal organs (Shevade and Ford 2004; Mohan and Pittman 2007). An inorganic form of arsenic is highly toxic compared to organic arsenic. Inorganic arsenate (AsO43−) and arsenite (AsO33−), referred to As(V) and As(III) are most common in natural waters. Although As(V) tends to be less toxic compared to that of As(III), it is thermodynamically more stable due to which it predominates under normal conditions and becomes the cause of significant contamination in ground water (Elizalde-Gonzalez et al. 2001; DeMarco et al. 2003). According to WHO the acceptable arsenic content in drinking water does not exceed 0.01 g/m3 (Dziubek 2006).
There are many different ways of purifying wastewater. Among the various treatment techniques available, the most commonly used ones are: ion exchange, adsorption, reduction and precipitation. A combination of two or more of these processes in many cases is the environmentally most compatible but cost effective solution. Ion exchange is a well-established environmentally friendly technology with metals recovery opportunities. The main advantages of ion exchange are recovery of metal value, selectivity and less sludge volume produced. Ion exchange uses synthetic resins, which are made of a polymeric structure and design for selectivity of various metals with various basic or chelating functional groups (He et al. 2000; Tenorio and Espinosa 2001; Janin et al. 2009).
The purpose of this work is to investigate some aspects of the use of strong basic, polyacrylate anion exchangers with the gel structure Amberlite IRA 458 and with the macroporous structure Amberlite IRA 958. The parameters that influence the sorption process: initial Cr(VI), As(V) concentrations (0.025–0.01 mol/L), pH, contact time (1–180 min) and interfering ions (Cl−, NO3−, SO42−) were investigated. In addition, the obtained results were compared with those for the strongly basic polystyrene anion exchangers Amberlite IRA 402 and Amberlite IRA 900.
Materials and methods
Resins
The anion exchangers Amberlite IRA 458, and Amberlite IRA 958 produced by Rohm and Haas were used in the investigation. Amberlite IRA 458 is an acrylic gel type strongly basic anion exchange resin. It combines high operating capacity with low silica leakage values. It is recommended as the working anion exchange resin, for demineralisation of water, when low caustic regenerant consumption and good resistance to organic fouling are primarily required. Its total ion exchange capacity is 1.3 eq/L, the particle size 0.600–0.900 mm and the thermal stability to 333 K.
The resin Amberlite IRA 958 is a polyacrylate, strongly basic, macroporous anion exchanger of type 1. It is used for the removal of organic compounds. Its total ion exchange capacity is 0.8 eq/L, the particle size 0.675–0.875 mm and the thermal stability to 353 K.
For comparison the polystyrene anion exchangers Amberlite IRA 402, and Amberlite IRA 900 (Rohm and Haas) were also applied.
Amberlite IRA 402 resin is a type 1 strongly basic, clear gel, anion exchange resin. It has a crosslinked polystyrene structure that is designed to give an optimum balance of capacity and regeneration efficiency in water treatment applications. Its total ion exchange capacity is 1.3 eq/L, the particle size 0.600–0.750 mm, the thermal stability to 333 K.
Amberlite IRA 900 is a macroporous polystyrene type 1 strong base anion exchange resin containing quaternary ammonium groups. This allows complete removal of all anions, including weakly dissociated ions. Its total ion exchange capacity is 1.0 eq/L, the particle size 0.650–0.820 mm, the thermal stability to 353 K (The Rohm and Haas 2003).
The specific BET surface area and average pore diameter of the studied ion exchangers were measured using ASAP 2405 (Micromeritics Instrument Co., Norcross, USA). These values were as follows: for Amberlite IRA 402 2.45 m2/g and 1.89 nm, for Amberlite IRA 458 2.03 m2/g and 3.60 nm, and for Amberlite IRA 958 2.23 m2/g and 6.46 nm, respectively.
The detailed characteristics of analyzed anion exchangers are presented in Table 1 (The Rohm and Haas 2003). The anion exchangers were washed with 1 M NaOH and 1 M HCl to remove organic and inorganic impurities and then washed several times with deionised water prior to the use.
Chemicals
Cr(VI) and As(V) stock solutions were obtained by dissolving appropriate amounts of potassium salt of dichromate (K2Cr2O7) and disodium salt of hydrogenarsenate (Na2HAsO4) salts in distilled water. They were diluted to get the solutions of various concentrations.
The initial pH values of the solutions were in the range 4.0–5.5 for Cr(VI) and 6.5–7 for As(V). All chemicals used were of analytical reagent grade (POCh S.A. Gliwice, Poland).
Theory and calculation
A series of standard Cr(VI) solution was prepared by appropriate dilution of the stock solution. For Cr(VI) and As(V) ion sorption studies, 0.2 g of anion exchanger was added into 100 mL flasks and mixed in 20 mL solution of Cr(VI) and As(V) at a constant speed 170 rpm using a mechanical shaker Elpin Plus 357 at 25 °C. After filtration, the concentration of Cr(VI) in the filtrate was analyzed spectrophotometrically. The total absorbed Cr(VI) was calculated by taking the difference of the initial concentration and the total Cr(VI) concentration in the filtrate. These concentrations were determined colorimetric at 546 nm by the UV–VIS spectrophotometer (Specord M42 produced by Carl Zeiss-Jena) using 1,5-diphenylcarbazide as a complexing agent. Diphenylcarbazide reacts with Cr(VI) in the acid medium as shown below (Pflaum and Howick 1956):
Where H4L is 1,5-diphenylcarbazide.
Besides the above mentioned method of Cr(VI) determination, the United States Environmental Protection Agency (USEPA) also admits the three methods: co-precipitation, chelation/extraction and differential pulse polarography (Pehlivan and Cetin 2009). For the determination of the total chromium content after complete conversion of trivalent chromium to hexavalent chromium, the comparative study of the three analytical techniques, namely ICP-AES, FAAS and UV–VIS spectrophotometer, is proposed (Balasubramanian and Pugalenthi 1999).
As(V) is determined from the reaction with ammonium molybdate in the presence of ascorbic acid. The concentrations of As(V) were determined spectrophotometrically at 870 nm by the UV–VIS spectrophotometer (Marczenko and Balcerzak 1998).
The sorption of Cr(VI) and As(V) ions on the above mentioned ion exchangers was investigated by batch operation as a function of initial concentration of metal ions, pH of the solution, contact time, temperature and interfering ions. The experiments were performed to determine the concentration of analyzed metals at the equilibrium (qe), at the specific time (qt). The resin phase concentrations of Cr(VI) and As(V) at the equilibrium, qe (mg/g), at the specific time, qt (mg/g) were obtained according to (McKay et al. 1999):
where ce is the concentration of Cr(VI) and As(V) ions in the aqueous phase at equilibrium (mg/L), ct is the concentration of Cr(VI) and As(V) ions in the aqueous phase at time t (mg/L), V is the volume of the solution (mL or L), m is the mass of the anion exchanger (g).
Contact time sorption was carried out at the initial concentration 0.001 mol/L of each solution. The solutions of Cr(VI) and As(V) were agitated in the mechanical shaker in the time range 1–180 min. From the obtained results there were determined kinetic parameters based on the pseudo first-order and pseudo second-order models developed by Lagergren and Ho and McKay, connected with the reactions presented below. The pseudo first-order equation is expressed as (Blanchard et al. 1984):
where qt is the mass of the adsorbed Cr(VI) and As(V) ions at time t (mg/g), q1 the mass of the adsorbed Cr(VI) and As(V) ions at equilibrium for the first-order reactions (mg/g) and k1 is the rate constants of the first-order (1/min) reactions.
The pseudo second-order equation is expressed as (Ho 2006):
where q2 is the mass of the adsorbed Cr(VI) and As(V) ions at equilibrium for the second-order reactions (mg/g) and k2 is the rate constants of the second-order (g/mg min) reactions.
The effect of pH on the Cr(VI) ion sorption was evaluated by adjusting the initial pH of the initial solution within the range 2–7. In the case of pH effect on As(V) ion sorption, the initial pH of the initial solution was adjusted in the range 4–10. The pH of each solution was fitted to the desired value by means of 0.01 mol/L NaOH and H2SO4. In addition, the effects of concentration, temperature and interfering ions were also investigated to determine the optimum conditions for these parameters. All experiments were repeated three times to observe the reproducibility of the measurements (5 %).
To describe variations of sorption with relation to the concentration of sorbate in the solution at constant temperature, adsorption isotherms were studied. Initial concentrations of the studied solutions were prepared in the range 0.00025–0.001 mol/L. The adsorption equilibrium data were fitted into the Langmuir and Freundlich models, which are presented as (Langmuir 1916; Rivero et al. 2004):
where qe is the equilibrium Cr(VI) and As(V) ions concentration on the ion exchanger (mg/g), q0 is the monolayer capacity of the ion exchanger (mg/g), KL is the Langmuir adsorption constant (L/mg) related to the free energy of adsorption (L/mg), KF is the Freundlich adsorption capacity (mg/g [L/mg]1/n) and 1/n is the Freundlich constant related to the surface heterogeneity.
The FT-IR (Fourier transform infrared spectroscopy) spectrum of the anion exchangers Amberlite IRA 458 and Amberlite IRA 402 before and after the sorption of Cr(VI) and As(V) ions was recorded over the interval 400–4,000 cm−1 by means of a Perkin-Elmer 1725 X. The samples were tabulated with KBr. Surface morphology of the anion exchangers was studied by means of the atomic force microscope NanoScope V (Veeco).
Results and discussion
Effect of pH
The sorption process of Cr(VI) ions is dependent on pH of the equilibrium solution. The hexavalent chromium exists primarily as chromic acid (H2CrO4) and its salts, hydrogen chromate (HCrO4−) and chromate (CrO42−) ions depending on the sample pH (Kota and Stasicka 2000). The dichromate ion (Cr2O72−) is formed when the concentration of chromium exceeds approximately 1 g/L. In the solution in the whole range of concentrations and when pH > 6.5, only CrO42− ions exist. In the pH range from 0 to 6.5, HCrO4− and Cr2O72− ions are predominant (Fig. 1a). A sorption process between a strong base anion exchange resin with quaternary ammonium –N+(CH3)3 and chromates from the aqueous solution can be described according to the following reactions (Shi et al. 2009):
Based on the speciation As(V) diagram, it can be stated that at pH > 4 mostly H3AsO4 molecules are present in the solution. In the pH range 4–10, the ionic H2AsO4− and HAsO42− forms are dominant. Above pH = 10 As(V) occurs in the AsO3− form (Fig. 1b).
Figure 2 shows the effect of initial pH (2–7) on the removal of chromium (VI) on Amberlite IRA 458, Amberlite IRA 958, Amberlite IRA 402 and Amberlite IRA 900. As follows from the data the capacity of the anion exchangers in question does not change significantly in the pH range 3–6. The maximum sorption capacities are equal 7.7947 mg/g for Amberlite IRA 458, 7.7921 mg/g for Amberlite IRA 958, 7.7960 mg/g for Amberlite IRA 402 and 7.7972 mg/g for Amberlite IRA 900.
Generally for the sorption of As(V) ions it can be seen that increase in the pH values from 4 to 10 results in the increase in metal sorption on the above mentioned anion exchangers. The maximum uptake of As(V) occurred at pH 10. The maximum value 10.9478 mg/g was found for Amberlite IRA 900.
Effect of contact time and kinetic studies
The amount of adsorbed metal ions (qt) from the solutions of the concentration from 0.00025 to 0.001 mol/L depends on time. The results in Fig. 3a–d indicate that for Cr(VI) and As(V) the sorption capacity of Amberlite IRA 458 and Amberlite IRA 402 increases with an increase in the phase contact time before equilibrium is reached. The equilibrium state is established already after 15 min of phase contact time. Metal ions removal by sorption on the above mentioned anion exchangers was also concentration dependent. For the initial concentration 0.001 mol/L the amount of the sorbed metal ions follows the order: As(V) > Cr(VI) and they are equal to 7.3774, 7.4665, 5.1969 and 5.2000 mg/g for Amberlite IRA 458 and Amberlite IRA 402, respectively.
The determined kinetic parameters imply that the sorption process proceeds according to the equation type of the pseudo second-order. Moreover, the experimental data are well correlated by the pseudo second-order equation. The pseudo second-order rate constant (k2) decreases from 2.179 to 0.444 g/mg min for Cr(VI), 1.016 to 0.100 g/mg min for As(V) in the case of sorption on Amberlite IRA 458, 1.416 to 0.385 g/mg min for Cr(VI), 0.704 to 0.147 g/mg min for As(V) in the case of sorption on Amberlite IRA 402 with the increasing metal concentration (Table 2). The equilibrium sorption capacity (q2) and the initial sorption rate (h) increase with the increasing concentration. This is obvious that for higher initial concentration more efficient usage of the sorption capacity of the anion exchangers is expected due to larger driving forces connected with a greater gradient of concentration.
The sorption isotherm studies
The influence of metal ion concentration on effectiveness of sorption process is indicative for the sorption capacity of the microporous anion exchangers Amberlite IRA 458 and Amberlite IRA 402 as well as macroporous Amberlite IRA 958 and Amberlite IRA 900. As follows the increase in the initial metal ion concentration results in the increasing metal sorption (Fig. 4a–b). It was found that the sorption of Cr(VI) and As(V) on the above mentioned anion exchangers is well described by the Langmuir isotherm model. The correlation coefficients of the linear plot of ce/qe vs. ce obtained from them were high, ranging from 0.897 to 0.999. The calculated equilibrium capacities q0 according to the Langmuir isotherm model are in good agreement with the values of experimental capacities qe,exp. These values for the sorption of Cr(VI) and As(V) on Amberlite IRA 458, Amberlite IRA 958, Amberlite IRA 402 and Amberlite IRA 900 are presented in Table 3.
The adsorption coefficients (KL) are related to the free energy of sorption and indicate the affinity of the anion exchangers for the studied metal ions. These values were greater for Cr(VI) than for As(V) for all tested anion exchangers.
The degree of suitability of anion exchangers for the sorption of Cr(VI) and As(V) ions under investigations was also estimated from the values of the separation factor constant (RL) according to the following relation (Langmuir 1916):
The value of RL > 1 indicates unfavorable, RL = 1 linear, 0 < RL < 1 favorable or RL = 0 irreversible sorption. The RL values for the studied metal ions on Amberlite IRA 402, Amberlite IRA 900, Amberlite IRA 458 and Amberlite IRA 958 are presented in Table 3.
As it can be seen in Table 3 the Freundlich constant KF values for Cr(VI) are higher than for As(V). The parameter KF related to the sorption density is higher in the case of sorption on the acrylic anion exchangers. The Freundlich constant n between 1 and 10 exhibits a favorable adsorption tendency. Larger value of n (smaller value of 1/n) implies stronger interaction between the sorbent and the heavy metal ions while 1/n equal to 1 indicates the linear adsorption leading to identical adsorption energies for all sites (Febrianto et al. 2009). The R2 values in the case of Freundlich isotherm ranged from 0.8681 to 0.9950.
Effect of interfering ions
The effect of other anions present in the solution on sorption of Cr(VI) and As(V) ions on Amberlite IRA 402, Amberlite IRA 900, Amberlite IRA 458 and Amberlite IRA 958 was also studied. 0.1 mol/L solutions of NaCl, Na2SO4 and NaNO3 were used. As follows from Fig. 5 the addition of salt particularly NaCl decreases effectiveness of Cr(VI) ions sorption by 1 % on the polyacrylate anion exchangers Amberlite IRA 458 and Amberlite IRA 958. In the case of As(V) ions sorption, the presence of NaCl, Na2SO4 and NaNO3 decreases the sorption capacity of the macroporous polystyrene anion exchanger Amberlite IRA 900 by about 12 %.
Effect of temperature
The thermodynamics for the sorption of Cr(VI) and As(V) on the strongly basic anion exchangers Amberlite IRA 402, Amberlite IRA 900, Amberlite IRA 458 and Amberlite IRA 958 was investigated in the range 293–323 K, at initial concentration 0.002 mol/L.
Thermodynamic parameters such as change in the Gibbs free energy (ΔG), enthalpy (ΔH) and entropy (ΔS) were determined using the following equations (Guo et al. 2009):
where KD is the distribution coefficient (L/g), Ce is the concentration Cr(VI) and As(V) at the equilibrium (mmol/L), Qe is the sorption capacity (mmol/g), T is the temperature (K) and R is the gas constant.
ΔH and ΔS were obtained from the slope and intercept of the plots of logKD vs. 1/T (Fig. 6). Table 4 shows the calculated values of the thermodynamic parameters. In the case of Cr(VI) ions sorption on the above mentioned ion exchangers, the negative values of ΔH indicate that the sorption had exothermic nature. On the other hand, sorption of As(V) ions can be characterized by more negative values of ΔG with the increasing temperature (the sorption process is more favorable at high temperature). The positive values of ΔS indicate that there is an increase in the randomness in the solid/solution interface during the sorption process. The ΔH values are in the range 12.212–31.974 kJ/mol which indicates the endothermic nature of the sorption process.
FT-IR and AFM analysis
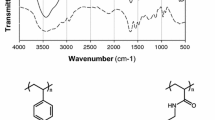
As can be seen in Fig. 7 a broad, intensive peak at 3,360 cm−1 is characteristic of hydroxyl and amine groups. The bands at 3,020 and 2,928 cm−1 are described to the asymmetric and the corresponding symmetric stretch vibrations of the –CH2 groups. The bond related to the presence of water in the ion exchanger phase was found at 1,620 cm−1. The ring carbon–carbon stretching and the scissoring vibrations of the methylene groups occur at 1,499, 1,480 and 1,417 cm−1 as well as at 1,379 cm−1. For the frequencies about 979 and 887 cm−1 there occur deformation vibrations of 1.4 substituted benzene ring (ST with DVB).
Figure 8 presents the surfaces morphology of Amberlite IRA 402 and Amberlite IRA 958 before and after the sorption process. Pictures were reordered by means of atomic force microscopy (AFM). It is noticed that after the sorption process their surface was dense in comparison with the ones not being in contact with Cr(VI) and As(V) ions.
Conclusions
In the presented paper the studies on the sorption of Cr(VI) and As(V) on the strong basic polystyrene Amberlite IRA 402, Amberlite IRA 900 and polyacrylate Amberlite IRA 458 and Amberlite IRA 958 ion exchangers were carried out. Optimal sorption parameters were determined by batch experiments. The obtained results can be summarized as follows:
-
(a)
For the initial concentration 0.001 mol/L the amount of the sorbed metal ions follows the order: As(V) > Cr(VI) and they are equal to 7.3774, 7.4665, 5.1969 and 5.2000 mg/g for Amberlite IRA 458 and Amberlite IRA 402, respectively.
-
(b)
The equilibrium state was established already after 15 min of phase contact time.
-
(c)
The sorption process of Cr(VI) and As(V) ions is dependent on pH of the equilibrium solution. Maximum uptake of Cr(VI) and As(V) occurred at pH 5 and 10, respectively. The maximum values 7.7972 mg/g for Cr(VI) and 10.9478 mg/g for As(V) were found for Amberlite IRA 900.
-
(d)
The determined kinetic parameters imply that the sorption process proceeds according to the equation type of pseudo second-order.
-
(e)
Sorption equilibrium data were correlated with the Langmuir and Freundlich isotherms.
-
(f)
The effect of other anions (NaCl, Na2SO4 and NaNO3) present in the solution indicates that especially removal of As(V) ions on macroporous Amberlite IRA 900 decreased about 12 %.
-
(g)
The sorption was temperature dependent. The equilibrium sorption capacity slightly increased when the temperature of the complex solution increased from 293 to 323 K during the phase contact time. The enhancement in adsorption with temperature may be attributed to the increase in the porosity and in the total pore volume of the anion exchangers.
-
(h)
These results were confirmed by the FT-IR studies and AFM analysis.
References
Arslan G, Pehlivan E (2007) Batch removal of chromium(VI) from aqueous solution by Turkish brown coals. Bioresource Technol 98:2836–2845. doi:10.1016/j.biortech.2006.09.041
Balasubramanian S, Pugalenthi V (1999) Determination of total chromium in tannery waste water by inductively coupled plasma-atomic emission spectrometry, flame atomic absorption spectrometry and UV–visible spectrophotometric methods. Talanta 50:457–467. doi:10.1016/S0039-9140(99)00135-6
Banfalvi G (2011) Cellular effects of heavy metals. Springer, New York
Blanchard G, Maunayue M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507. doi:10.1016/0043-1354(84)90124-6
Bortun A, Bortun M, Pardini J, Khainakov SA, Garcia JR (2010) Effect of competitive ions on the arsenic removal by mesoporous hydrous zirconium oxide from drinking water. Mater Res Bull 45:1628–1634. doi:10.1016/j.materresbull.2010.07.011
DeMarco MI, SenGupta AK, Greenleaf JE (2003) Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res 37:164–176. doi:S0043-1354(02)00238-5
Dziubek J (2006) Badania technologiczne nad usuwaniem związków arsenu ze ścieków przemysłowych. Ochrona Środowiska 4:41–44
Elizalde-Gonzalez MP, Mattusch J, Einicke WD, Wennrich R (2001) Sorption on natural solids for arsenic removal. Chem Eng J 81:187–195. doi:10.1016/S1385-8947(00)00201-1
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645. doi:10.1016/j.jhazmat.2008.06.042
Guo L, Sun CH, Li G, Liu Ch, Ji Ch (2009) Thermodynamics and kinetics of Zn(II) adsorption on crosslinked starch phosphates. J Hazard Mater 161:510–515. doi:10.1016/j.jhazmat.2008.04.003
He Y, Cervera L, Garrido-Eciya MI, Guardia M (2000) On-line bidirectional electrostacking of chromium(III) and chromium(VI) for flame atomic absorption spectrometry determination. Anal Chim Acta 421:57–65. doi:S0003-2670(00)01027-8
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136(2006):681–689. doi:10.1016/j.jhazmat.2005.12.043
Janin A, Blais JF, Mercier G, Drogui P (2009) Selective recovery of Cr and Cu in leachate from chromated copper arsenate treated wood using chelating and acidic ion exchange resins. J Hazard Mater 169:1099–1105. doi:10.1016/j.hydromet.2008.12.002
Kota J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107:263–283. doi:10.1016/S0269-7491(99)00168-2
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Marczenko Z, Balcerzak M (1998) Spektrofotometryczne metody analizy nieorganicznej. PWN, Warszawa
McKay G, Ho YS, Ng JCY (1999) Biosorption of copper from waste waters: a review. Sep Purif Method 28:87–125. doi:10.1080/03602549909351645
Mohan D, Pittman CU Jr (2007) Arsenic removal from water/wastewater using adsorbents- a critical review. J Hazard Mater 142:1–53. doi:10.1016/j.jhazmat.2007.01.006
Namasivayam C, Senthilkumar S (1999) Adsorption of copper(II) by “waste” Fe(III)/Cr(III) hydroxide from aqueous solution and radiator manufacturing industry wastewater. Sep Sci Technol 34:201–217. doi:10.1081/SS-100100645
Pehlivan E, Cetin S (2009) Sorption of Cr(VI) ions on two Lewatit-anion exchange resins and their quantitative determination using UV–visible spectrophotometer. J Hazard Mater 163:448–453. doi:10.1016/j.jhazmat.2008.06.115
Pflaum RT, Howick LC (1956) The chromium diphenylcarbazide reaction. J Am Chem Soc 78:4862–4866
Rengaraj S, Yeon KH, Moon SH (2001) Removal of chromium from water and wastewater by ion exchange resins. J Hazard Mater 87:634–638. doi:10.1016/S0304-3894(01)00291-6
Rivero MJ, Primo O, Ortiz MI (2004) Modelling of Cr(VI) removal from polluted ground waters by ion exchange. J Chem Technol Biotechnol 79:822–829. doi:10.1002/jctb.1049
Sarin V, Sarvinder Singh T, Pant KK (2006) Thermodynamic and breakthrough column studies for the selective sorption of chromium from industrial effluent on activated eucalyptus bark. Bioresource Technol 97:1986–1993. doi:10.1016/j.biortech.2005.10.001
Shevade S, Ford RG (2004) Use of synthetic zeolites for arsenate removal from pollutant water. Water Res 38:3197–3204. doi:10.1016/j.watres.2004.04.026
Shi T, Wang Z, Liu Y, Jia S, Changming D (2009) Removal of hexavalent chromium from aqueous solutions by D301, D314 and D354 anion-exchange resins. J Hazard Mater 161:900–906. doi:10.1016/j.jhazmat.2008.04.041
Tenorio JAS, Espinosa DCR (2001) Treatment of chromium plating process effluents with ion exchange resins. Waste Manag 21:637–642. doi:10.1016/S0956-053X(00)00118
The Rohm and Haas (2003) Company information brochure
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Jachuła, J., Hubicki, Z. Removal of Cr(VI) and As(V) ions from aqueous solutions by polyacrylate and polystyrene anion exchange resins. Appl Water Sci 3, 653–664 (2013). https://doi.org/10.1007/s13201-013-0110-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0110-5