Abstract
To investigate the suitability of the marine diatoms, Chaetoceros curvisetus and C. simplex for the removal of macronutrients from different wastewater, the growth and nitrate-phosphate removal properties were studied with nitrate, ammonium and urea nitrogen sources. Three separate experiments were conducted using modified F/2 medium with 12.35 mg L−1 total nitrogen and 1.12 mg L−1 total phosphorous (simulating the typical concentration of nitrogen and phosphorus in secondary effluent) as growth medium. The maximum cell densities of C. curvisetus and C. simplex were 7.16 ± 0.34 × 104 cells mL−1 in \( {\text{NO}}_{3}^{ - } \) and 3.88 ± 0.32 × 105 cells mL−1 in urea, respectively. The maximum chlorophyll a per cell was 1.7 and 4.7 pg for C. simplex and C. curvisetus, cultured with urea and nitrate, respectively. The high protein contents of 4.7 pg cell−1 in C. simplex with urea and 19.7 pg cell−1 in C. curvisetus nitrate nitrogen sources were found. The higher cell density and protein content of both species from urea and nitrate nitrogen sources (p < 0.05) have shown that these were utilized by microalgae and were converted to protein. The C. simplex and C. curvisetus showed maximum removal efficiencies of nitrate by 97.86 and 91.62 % and phosphate by 98.5 and 100 %, respectively when urea used as nitrogen source than ammonia. The results indicated the C. simplex was more efficient than C. curvisetus and suitable for the removal of macronutrients when cultured with urea and nitrate nitrogen sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The input of nutrients especially nitrogen and phosphorus by human activities increased into biochemical cycles through agricultural practices, urbanization, industrialization and other activities. The widespread use of nitrogen fertilizers, following discharges of agricultural and industrial effluents resulted in the pollution of aquatic environment and is the main cause of eutrophication (Karthikeyan et al. 2010; Manimaran et al. 2011). Nutrient enrichment or eutrophication of aquatic ecosystems can cause an increase in algae and aquatic plants, loss of component species and loss of ecosystem function. In connection with these problems, numerous studies have aimed on nitrogen and phosphorus removal from wastewater (Thakur and Kumar 1999). Most of these studies are based on biological processes and different combinations of anaerobic, aerobic and anoxic zones (Aslan and Kapdan 2006). It was reported that nitrate and phosphate could be effectively taken up by photosynthetic microorganisms namely, cyanobacteria and green algae. The phytoplankton has many advantages for application to nitrate and phosphate removal than macrophytes. It proliferates throughout the entire year with high growth rates when environmental conditions such as light, nutrients and water temperature are suitable (Alexander and Goldman 1994). Free-living and immobilized cells of microalgae have been extensively studied and used for the removal of the inorganic compounds from water (Garbisu et al. 1992; Urrutia et al. 1995; Gonzalez-Bashan et al. 2000; Tam and Wong 2000).
The advantages of using algae includes the low cost of the operation, the possibility of recycling assimilated nitrogen and phosphorus into algae biomass as a fertilizer, avoiding a sludge handling problem and the discharge of oxygenated effluent into the water body (Hu et al. 2000). In addition, the process has no carbon requirement for nitrogen and phosphorus removal, which is attractive for the treatment of secondary effluents (Aslan and Kapdan 2006). The concentrations of nitrate, phosphate in different sources (urea, nitrate and ammonia) in the secondary effluents vary depending on the wastewater characteristics (Aslan and Kapdan 2006). The total nitrogen concentration was reported as 788 mg L−1 in pretreated piggery wastewater by An et al. (2003) and around 1,450 mg L−1 by Olguin et al. (2003). The average values of \( {\text{NH}}_{ 4}^{ + } \) and \( {\text{PO}}_{ 4}^{ - } \) were 27.4 mg L−1 and 11.8 mg L−1, respectively in the conventional secondary treatment effluent of domestic wastewater (Martinez et al. 2000) whereas they were 48 mg \( {\text{NH}}_{ 4}^{ + } \) L−1and 16 mg \( {\text{PO}}_{ 4}^{ - } \) L−1 in the effluent of up-flow sludge blanket reactor, fed with domestic sewage (Van der Steen et al. 1999). The wastewater treatment with microalgae was reported in the use of high-rate algae ponds (HRAP) (Cromar et al. 1996; Deviller et al. 2004). Recently corrugated raceways (Olguin et al. 2003), a triangular photobioreactor (Dumas et al. 1998) and a tubular photobioreactor (Cattaneo et al. 2003) have been developed for nutrient removal. The microalgae Chlorella sp., Scenedesmus sp., Spirulina sp., Nannochloropsis sp. and Phormidium sp. have been widely investigated and used for nutrient removal from wastewater (Lee and Lee 2001; Gonzales et al. 1997; Martinez et al. 1999; 2000; Olguin et al. 2003; Jimenez-Perez et al. 2004; Laliberte et al. 1997; Dumas et al. 1998).
Many studies have focused on the objective of optimization of nitrogen and phosphorous removal and environmental factors influencing on microalgal growth, such as nutrient concentration (Aslan and Kapdan 2006), nutrient type (Hyenstrand et al. 2000), light availability (Janssen et al. 2000) and pH (Xin et al. 2010). Wastewater may contain different forms of nitrogen (nitrate, ammonium, urea, etc.), so it is important to know the effect of different nitrogen compounds on microalgal growth and nutrient removal (Xin et al. 2010). Among the phytoplankton, diatoms are contributing higher diversity and biomass production in marine environment and not well recognized for bioremediation purposes. The centric diatoms, C. curvisetus and C. simplex were studied extensively for their biochemical composition and used for larval diet in aquaculture practices. But the reports are very limited in the use of diatoms for wastewater treatment. Hence the present investigation focused on the objective of growth, biochemical composition and NP removal properties of these two marine diatoms with three different nitrogen sources viz. nitrate, urea and ammonia.
Materials and methods
Microalgae
The marine centric diatoms, C. curvisetus and C. simplex were isolated by serial dilutions in F/2 media from Vellar estuary, Southeast coast of India. The stock cultures were maintained in Guillard F/2 media following the methods described in Andersen (2005).
Experimental setup
The modified F/2 medium with 12.35 mg L−1 TN and 1.12 mg L−1 TP (simulating the typical concentration of nitrogen and phosphorus in secondary effluent) was used as growth medium. In all experiments, K2HPO4·3H2O (5.6 mg L−1) was used as phosphorus source. Besides nitrogen and phosphorus, the composition of other elements was the same with F/2 medium.
All the experiments were conducted in 5,000-ml Erlenmeyer flasks with 3,000-ml algal cultures in three replicates. The subcultures of C. simplex and C. curvisetus were maintained with three kinds of nitrogen sources, NaNO3 (75 mg L−1 and 24.72 mg L−1 of NaNO3 and TN, respectively), NH4Cl (47 mg L−1 and 24.72 mg L−1 of NH4Cl and TN, respectively) and urea (53 mg L−1 and 22.35 mg L−1 of urea and TN, respectively) for 7 days. The cell density was estimated at every 24 h intervals; the total nitrogen and phosphorous were analysed in culture medium, before inoculation of algal cells and after 7 days of culture. The protein and carbohydrate contents were estimated at the end of the experiment.
Growth rate
The growth rate was calculated using the following formula (OECD 2002),
Where N 0 is the number of cells in time zero, N x is the number of cells in time x, t 0 is the starting time (0) and t x is the time x (in days).
Doubling time
The doubling time was calculated by the following formula (Karthikeyan et al. 2011),
where, N 0 is the number of cells in time zero, N t is the number of cell in time t, t is the time in hours. The results were presented in doubling time (DT) in hours.
Parameters maintained
During isolation, stock culture maintenance and experiments were conducted; the parameters viz. temperature, light intensity, photoperiod and salinity were maintained as 24 ± 2 °C, 4500 ± 500 Lux, 14 ± 1:8 ± 1 h (light:dark) and 30 psu, respectively.
Analytical methods
The total nitrogen and total phosphate were estimated at the beginning and the end of the experiment in cell-free media by centrifugation followed by Strickland and Parsons (1972). After 7 days of culture, the cells were isolated by centrifugation and the pellet used for further analysis of protein and carbohydrate. The protein was estimated by Folin’s phenol reagent method (Lowry et al. 1951) and the carbohydrate was estimated by phenol sulphuric acid method (Dubois et al. 1956).
Data analysis
Three replicates of each sample were used for statistical analysis and the values were reported as mean ± SD. One-way ANOVA and Duncan’s multi-variant analysis were carried out using SPSS, version 16.0 software to study the difference between nitrogen sources and species.
Results
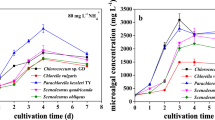
The growth curves of C. curvisetus and C. simplex with different nitrogen sources are shown in Fig 1. The C. curvisetus was grown fastest in \( {\text{NO}}_{3}^{ - } \) followed by urea whereas C. simplex was grown fastest in urea followed by \( {\text{NO}}_{3}^{ - } \). The organisms grew slowest on ammonia. The maximum cell density of 7.16 ± 0.34 × 104 cells mL−1 (growth rate 1.16 μ day−1) and 3.88 ± 0.32 × 105 cells mL−1 (growth rate 0.59 μ day−1) of C. curvisetus and C. simplex were found in \( {\text{NO}}_{3}^{ - } \) and urea, respectively (Fig. 1 and Table 2). The maximum chlorophyll a per cell was 1.7 and 4.7 pg for C. simplex and C. curvisetus which were cultured with urea and nitrate, respectively. The Chl a of 3.9 pg cell−1 was observed in urea used as nitrogen source in the medium for C. curvisetus and 1.6 pg cell−1 for C. simplex in nitrate (Fig 5). The protein content of C. simplex did not showed any significant variations with urea and nitrate nitrogen sources (4.7 pg cell−1 in urea and 4.6 pg cell−1 in \( {\text{NO}}_{3}^{ - } \)) where it was 3.4 pg cell−1 in ammonia nitrogen. The maximum protein of 19.7 and 16.8 pg cell−1 was found in C. curvisetus cultured with nitrate and urea, respectively. The carbohydrate contents of C. simplex were 0.86, 0.78 and 0.62 pg cell−1, whereas it was 3.6, 4.5 and 0.4 pg cell−1 in C. curvisetus with urea, nitrate and ammonia nitrogen, respectively (Fig. 6). The order of specific growth rate, chlorophyll, protein and carbohydrate contents with different nitrogen sources were \( {\text{NH}}_{ 4}^{ + } \) < urea < \( {\text{NO}}_{3}^{ - } \) of C. curvisetus and \( {\text{NH}}_{ 4}^{ + } \) < \( {\text{NO}}_{3}^{ - } \) < urea of C. simplex, respectively (Table 1; Figs. 2, 3, 4).
The capacity of nitrate and phosphate removal differed between the species as well as among the nitrogen sources used (Fig. 4). The observations in the present study showed that both species performed well in the removal of both N and P when urea used as nitrogen source in culture medium. Next to urea, the nitrate was observed as a good source of nitrogen for each species in the removal of N and P. The removal of nitrate was 97.86 and 91.62 %; the phosphate was 98.5 and complete removal by C. simplex and C. curvisetus, respectively cultured with urea source for 7 days (Fig. 3). When compared to urea and nitrate the slow growth of the both species was observed with ammonia (Fig. 1). The order of nitrate and phosphate removal capacities with different nitrogen sources was \( {\text{NH}}_{ 4}^{ + } \) < \( {\text{NO}}_{3}^{ - } \) < urea of C. curvisetus and \( {\text{NH}}_{ 4}^{ + } \) < urea < \( {\text{NO}}_{3}^{ - } \) of C. simplex respectively (Table 1). The one-way ANOVA results that showed the nitrogen sources and the two species were significantly different for their growth, nitrate phosphate removal and biochemical compositions.
Discussion
The microalgae could be used for the removal of phosphate and nitrate from wastewater. In this present study, the order of specific growth rate, chlorophyll, protein and carbohydrate contents with different nitrogen sources was \( {\text{NH}}_{ 4}^{ + } \) < urea < \( {\text{NO}}_{3}^{ - } \) of C. curvisetus and \( {\text{NH}}_{ 4}^{ + } \) < \( {\text{NO}}_{3}^{ - } \) < urea of C. simplex, respectively (Table 1). The order of growth rate and nutrient removal of C. curvisetus was similar to Scenedesmus sp. LX1 after 13 days of cultivation with nitrate, ammonium and urea as nitrogen sources (Xin et al. 2010). But the C. simplex was grown fastest with urea and the higher growth rate was observed in the present study from urea nitrogen (Fig. 2). When urea used as a nitrogen source, the initial pH of the medium is slightly high compared to other nitrogen sources. This might be the reason for highest growth rate of C. simplex in urea (Garcia et al. 2006; Xin et al. 2010). Other than this reason, the C. simplex is very smaller in cell size and bio-volume than C. curvisetus. So the growth rate and doubling time of C. simplex were higher than C. curvisetus in this present study. The algal growth in the present study with ammonium nitrogen was significantly low. However, the ammonium is good nitrogen source for the phytoplankton growth, the unfavorable concentration causes growth inhibition due to toxicity and/or acidifying the culture media during algal growth (Garcia et al. 2006; Xin et al. 2010).
After 7 days of cultivation with nitrate, ammonium and urea as nitrogen sources, TN removal efficiencies of C. simplex was significantly high with urea and nitrate nitrogen. But, C. curvisetus efficiently removed the TN from the media in the presence of nitrate nitrogen. The TP removal capacities were also observed in the same trend as TN removal. The TN and TP removal capacities of the diatoms in this study were slightly lower than Scenedesmus sp. LX1 after 13 days cultivation (Xin et al. 2010). However, the nutrient removal efficiencies of these two diatoms were higher than Chlorella spp. and Phormidium bohneri (Dumas et al. 1998; Lee and Lee 2001; Valderrama et al. 2002). The growth and nutrient uptake properties of these two species significantly varied because each species require unique suitable environmental conditions such as light intensity, temperature and salinity, etc. (Aslan and Kapdan 2006). From the available literatures, the different kinds of biomolecules have been produced in a single species of microalgae with changing environmental parameters.
Aslan and Kapdan (2006) found that the \( {\text{NH}}_{ 4}^{ + } \) was completely removed from the media by Chlorella vulgaris when the initial concentration was between 13.2 and 21.2 mg L−1. Further, the removal efficiency of \( {\text{NH}}_{ 4}^{ + } \) was decreased to less than 24 % when the \( {\text{NH}}_{ 4}^{ + } \) concentration was higher than 129 mg L−1. In the present study, the initial \( {\text{NH}}_{ 4}^{ + } \) concentration was 47 mg L−1 and the nitrate removal was 26.9 and 3.1 % by C. simplex and C. curvisetus, respectively.
The chlorophyll content of C. simplex and C. curvisetus estimated in this study was slightly varied with cell density and nitrogen sources. The nutrient concentrations also influenced the chlorophyll a concentration and light intensity with major significance (Sciandra et al. 2000; Young and Beardall 2003; Lafarga-De la Cruz et al. 2006; Eker-Develi et al. 2006).
The protein and carbohydrate contents of C. simplex and C. curvisetus in this present study are under the range of reported amounts in other diatoms like Chaetoceros calcitrans,C. gracilis, Nitzchia closterium, Phaeodactylum tricornutum, Skeletonema costatum, Thalassiosira pseudonana and Tetraselmis chui by Brown (1991). So, the biomass produced after wastewater treatment could be used for many purposes after required precautions such as sterilization, etc.
Conclusions
Further optimization of other environmental parameters can improve the nutrient removal efficiency of C. curvisetus. If ammonium was the main nitrogen source in wastewater, a buffer solution should be added to keep pH above 7.5 for algal growth and nutrient removal.
References
Alexander JH, Goldman CR (1994) Limnology, 2nd edn. Mcgraw-Hill, New York, pp 193–263
An JY, Sim SJ, Lee JS, Kim BK (2003) Hydrocarbon production from secondary treated piggery wastewater by the green algae. Botrycoccus braunii. J Appl Phycol 15:185–191
Andersen RA (2005) Algal culturing techniques. Elsevier Academic Press, Burlington. ISBN 0-12-088426-7
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae ‘Dokuz eylu’ University, Department of Environmental Sebnem Engineering, Kaynaklar Campus, 35160 Buca-IZMIR. Turk ecol eng 28:64–70
Brown MR (1991) The amino acid and sugar composition of 16 species of microalgae used in mariculture. Aquacult 145:79–99
Cattaneo C, Zolezzi M, Rovatti P, Fabiano M, Povero P, Rovatti M (2003) Growth and nutrient uptake of microalgae (Nannochloropsis sp.) in a tubular photo bioreactor. In: Pierucci S (ed) IcheaP-6 the six italian conference on chemical and process engineering florance, vol 1, pp 1677–1682
Cromar NJH, Fallowfield J, Martin NJ (1996) Influence of environmental parameters on biomass production and nutrient removal in a high rate algal pond operated by continuous culture. Water Sci Technol 34:133–140
Deviller G, Aliaume C, Nava MAF, Casellas C, Blancheton JP (2004) High-rate algal pond treatment for water reuse in an integrated marine fish recirculation system: effect on water quality and sea bass growth. Aquaculture 235:331–344
Dubois M, Gillies KA, Hamilton JK, Rebers F, Smith PA (1956) Calorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dumas A, Laliberte G, Lessard P, Noue J (1998) Biotretament of fish farm effluents using the cyanobacterium Phormidium bohneri. Aquacult Eng 17:57–68
Eker-Develi E, Kideys AE, Tugrull S (2006) Effect of nutrients on culture dynamics of marine phytoplankton. Aquat Sci 68:28–39
Garbisu C, Hall DO, Serra JL (1992) Nitrate and nitrite uptake by free-living and immobilized N-starved cells of Phormidium laminosum. J Appl Phycol 4:139–148
Garcia J, Green B, Oswald W (2006) Long term diurnal variations in contaminant removal in high rate ponds treating urban wastewater. Bioresour Technol 97:1709–1715
Gonzales LE, Canizares RO, Baena S (1997) Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microlagae Chlorealla vulgaris and Scenedesmus dimorphus. Bioresour Technol 60:259–262
Gonzalez-Bashan LE, Lebsky VK, Hernandez JP, Bustillos JJ, Bashan Y (2000) Changes in the metabolism of the microalga Chlorella vulgaris when coimmobilized in alginate with the nitrogen-fixing Phyllobacterium myrsinacearum. Can J Microbiol 46:653–659
Hu Q, Westerhoff P, Vermaas W (2000) Removal of nitrate from groundwater by cyanobacteria: quantitative assessment of factors influencing nitrate uptake. Appl Environ Microbiol 66(1):133–139
Hyenstrand P, Burkert U, Pettersson A, Blomqvist P (2000) Competition between the green alga Scenedesmus and the cyanobacterium Synechococcus under different modes of inorganic nitrogen supply. Hydrobiologia 435:91–98
Janssen M, Bresser LD, Baijens T, Tramper J, Mur LR, Snel JFH, Wijffels RH (2000) Scale-up aspects of photobioreacters: effects of mixing-induced light/dark cycles. J Appl Phycol 12(3–5):225–237
Jimenez-Perez MV, Sanches-Castillo P, Romera O, Fernandez-Moreno D, Perez-Martinez C (2004) Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microbial Technol 34:392–398
Karthikeyan P, Jayasudha S, Sampathkumar P, Manimaran K, Santhoshkumar C, Ashokkumar S, Ashokprabu V (2010) Effect of industrial effluent on marine diatom. Chaetoceros simplex (Ostenfeld, 1901). J. Appl. Sci. Environ. Manage. 14(4):35–37
Karthikeyan P, Jayasudha S, Manimaran K, Sampathkumar P, Suriya S (2011) Effect of cadmium on marine diatom. Chaetoceros curvisetus with different ambient iron concentrations. Asian J of Biochem Pharm Res 2(1):240–248
Lafarga-De la Cruz F, Valenzuela-Espinoza E, Millan-Nunez R, Trees CC, Santamaria-del-Angel E, Nunez-Cebrero F (2006) Nutrient uptake, chlorophyll a and carbon fixation by Rhodomonas sp. (Cryptophyceae) cultured at different irradiance and nutrient concentrations. Aquacult Eng 35:51–60
Laliberte G, Lessard P, Noue J, Sylvestre S (1997) Effect of phosphorus addition on nutrient removal from wastewater with the cyanobacterium Phormidium bohneri. Bioresour Technol 59:227–233
Lee K, Lee CG (2001) Effect of light/dark cycles on wastewater treatments by microalgae. Biotechnol Bioprocess Eng 6:194–199
Lowry OH, Rosebrough NH, Farr AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Manimaran K, Karthikeyan P, Ashokkumar S, Ashok Prabu V, Sampathkumar P (2011) Effect of copper on growth and enzyme activity of marine diatom Odontella mobiliensis. Bull Environ Contam Toxicol 88(1):30–37
Martinez ME, Castillo JM, Yousfi EF (1999) Photoautotrophic consumption of phosphorus by Scenedesmus obliquus in a continuous culture. Influence of light intensity. Process Biochem 34:811–818
Martinez ME, Sanchez S, Jimenez JM, Yousfi FE, Munoz L (2000) Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. 73(3):263–272
OECD (2002) Guidelines for the testing of chemicals, Lemna sp. Growth inhibition test draft guideline 221. Paris, France
Olguin EJ, Galicia S, Mercado G, Perez T (2003) Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycle process under tropical conditions. J Appl Phycol 15:249–255
Sciandra A, Lazzara L, Claustre H, Babin M (2000) Response of growth rate, pigment composition and optical properties of Cryptomonassp. to light and nitrogen stress. Mar Ecol Prog Ser 201:107–120
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res Board Can 167:311
Tam NFY, Wong YS (2000) Effect of immobilized bead concentrations on wastewater nutrient removal. Environ Pollut 107:145–151
Thakur A, Kumar HD (1999) Nitrate, ammonium, and phosphate uptake by the immobilized cells of Dunaliella salina. Bull Environ Contam Toxicol 62:70–78
Urrutia I, Serra JL, Llama MJ (1995) Nitrate removal from water by Scenedesmus obliquus immobilized in polymeric foams. Enzyme Microbial Technol 17:200–205
Valderrama LT, DelCampo CM, Rodriguez CM, De-Bashan LE, Bashan Y (2002) Treatment of recalcitrant wastewater from ethanol and citric acid production using the micro alga Chlorella vulgaris and macrophyte Lemna minuscula. Water Res 36:4185–4192
Van der Steen P, Brenner A, Van Buuren J, Oron G (1999) Post-treatment of UASB reactor effluent in an integrated duckweed and stabilization pond system. Water Res 33:615–620
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater micro alga Scenedesmus sp. Bioresour Technol 101:5494–5500
Young E, Beardall J (2003) Rapid ammonium and nitrate-induced perturbations to chl ‘a’ fluorescence in nitrogen-stressed Dunaliella tertiolecta (Chlorophyta). J Phycol 39:332–342
Acknowledgments
We are thankful to Dr. T. Balasubramanian, Dean, CAS in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai and MoES-ICMAM to carry out the present work for providing facilities and fellowship to Mr. P. Karthikeyan, Mr. K. Manimaran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Karthikeyan, P., Manimaran, K., Sampathkumar, P. et al. Growth and nutrient removal properties of the diatoms, Chaetoceros curvisetus and C. simplex under different nitrogen sources. Appl Water Sci 3, 49–55 (2013). https://doi.org/10.1007/s13201-012-0056-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0056-z