Abstract
Mining activities lead to extremely harsh environmental conditions, generally reduce soil biodiversity, and limit natural revegetation and ecological restoration. Biotechnological treatments, based on the use of living microorganisms as arbuscular mycorrhizal fungi (AMF), are essential for the recovery of soils degraded by mining. This study was performed in an open-pit gypsum mining site in northwestern Algeria. We compared the effects of three different strategies of AMF inoculation (a commercial AMF inoculum of Rhizophagus irregularis, a native AMF inoculum cultured with leek as nurse plant and a native AMF inoculum made of root fragments and rhizospheric soil from Lavandula spp.) and of one organic amendment. Inoculum were applied at the time of planting of Olea europaea young trees, and the chemical and biological properties of the soil, the structure of the AMF community and the plant physiology were assessed. Two years after plantation, and whatever the AMF treatment, the concentration of the assimilable phosphorus and of the total glomalin-related soil protein has increased into the soil compared to the bare soil. The composition of the AMF community changed and AMF diversity increased over the years regardless of AMF inoculation. This reflects the low presence of AMF in the soil prior to rehabilitation and therefore the benefits of rehabilitation. The highest AMF diversity was measured when using native inoculum. Mineral analysis of O. europaea leaves has revealed an increase in the concentrations of few nutrients including phosphorus when inoculated with a AMF community. Our study participates to show that AMF can promote ecological restoration of mining-impacted sites, by improving soil structure and quality, plant mineral acquisition, and AMF diversity with time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mining lands are degraded ecosystems facing a reduction in their physical and chemical properties, fertility and biodiversity (Salami et al. 2003; Peña et al. 2015; Ukaogo et al. 2020; Worlanyo and Jiangfeng 2021). Algeria is the largest country in Africa with underground deposits of precious metals such as iron and gold, rare earth minerals such as tungsten and uranium, and industrial minerals such as gypsum and phosphate rocks (Bencharef et al. 2022). Among these deposits, gypsum is a key building component that is excavated from the soil, leading to ecosystem disruption and the disappearance of cover plants (Mota et al. 2004). Restoration of mining sites is generally a difficult process (Wiegleb and Felinks 2001), particularly for gypsum, leading to physical and chemical soil alterations, and a reduction in soil fertility and biodiversity (Salami et al. 2003; Pan and Li 2016; Hao et al. 2016; Peña et al. 2015; da Silva et al. 2017). Fertility of nutrient-deficient gypsum quarry backfills is also one of the main obstacles to the rehabilitation of degraded sites through an in situ ecological restoration process (Schmalenberger et al. 2013). The use of living organisms, as arbuscular mycorrhizal fungi (AMF), is considered as a valuable tool in the rehabilitation of disturbed and degraded soils to mitigate the consequences of mining operations and to improve the quality of degraded soils. In Algeria, a program of progressive ecosystem and site rehabilitation was established in 2014, and one part is based on the use of selected rhizospheric symbionts, as AMF, to exploit their bioremediation properties (Crossay et al. 2018).
Arbuscular mycorrhizal fungi (AMF) are known to establish symbiotic associations with roots of more than 72% of land plants species (Genre et al. 2020). The arbuscular mycorrhizal (AM) symbiosis is essential for the functioning of soil ecosystems (Rillig 2004) and agroecosystems (Solís-Ramos and Andrade-Torres 2020), by improving nutrient cycling (Johnson et al. 2016), tolerance to biotic or abiotic stresses (Gianinazzi et al. 2010), and soil stability (Daynes et al. 2013). Several studies have shown the importance of AMF in the rehabilitation of degraded areas (Maltz and Treseder 2015; Wildman 2015; Cortese and Bunn 2017; Wang 2017; Neuenkamp et al. 2019). However, mine ecosystem restoration is generally a difficult process (Shackelford et al. 2018) as the loss or low-level of AMF propagules after degradation of vegetation cover could further inhibit natural and assisted revegetation processes (Barea et al. 2011; Asmelash et al. 2016). In these soils, AMF inoculation may help plant establishment (Requena et al. 2001; Bi et al. 2014).
Since 1970, many experiments have been carried in greenhouses with mainly positive results on plant growth, using native and introduced plants, and native and commercial inoculum (Wang 2017). Studies have shown that inoculations with multiple AMF have a stronger effect on plants than with a single AMF (Frew 2021). Studies have also shown that inoculations with native AMF remained more efficient than with commercial AMF, suggesting different growth response of crops to commercial AMF, and competition among native and commercial AMF (Frew 2021). The use of native AMF was also used in few field studies for the rehabilitation of degraded sites after coal mining (Frost et al. 2001) and contamination with metals (i.e., Cu, Zn, Pb and Cd) (Wang et al. 2007). The only study on the effects of AMF inoculation after gypsum mining (Khabou et al. 2014) was performed with one AMF. Actually, the effects of AMF inoculum on plant growth and on indigenous AMF communities are still difficult to predict.
Our experiment was designed to participate for the first time to the restoration of a gypsum mining. We compared the effects of the introduction of the native plant Olea europaea, either with two native AMF inocula (spores or roots with rhizospheric soil) or an AMF commercial inoculum or a green compost, on chemical and biological soil properties, the structure and composition of AMF communities colonizing plant roots and present in the rhizosphere, and on plant nutrition. The results could be incorporated into new strategies for mine site restoration and ecosystem sustainability and productivity.
2 Material and methods
2.1 Studied site
The experimental site is a gypsum quarry, active from 2014 to 2017, and located in the exploitation area of a Knauf plaster factory in Ben Friha, in Algeria (N 35°43′4.11'', W 0°20′32.63'', altitude: 115 m) (Supp. Fig. S1). The climate is semi-arid Mediterranean, characterized by a dry summer, with a mean temperature of 19.1 °C and an average precipitation level of 347.4 mm/year (national meteorology office Algeria; http://www.meteo.dz). The site has a surface area of 1.7 ha, formed by a limestone fill giving the topography a marked relief at the sloping cliff. The soil is made of a high clay content (from 44 to 56%), of fine and/or rocky fractions from extraction debris or excavations, and sometimes also of blasting debris. These high levels of clay made the soil very firm, compact and difficult to break leading to poor ventilation, waterproofing (low draining) and poor root penetration. The soil was not cultivated and was left open to vegetation spontaneously after exploitation until Olea europaea plantation in May 2019.
2.2 Experimental design
Olea europaea was chosen as an indigenous species, known both for its resistance to water stress (Fernandez 2014) and its dependence on AMF in hot and dry Mediterranean climates (Mekahlia et al. 2013). Two hundred seedlings of young olive trees produced in a nebulization greenhouse were selected according to certain criteria (age, size and origin) to be uniform.
The experimental site had a random block design with five treatments on O. europaea trees (Supp. Fig. S1): (i) no inoculation (Ni), (ii) a commercial AMF inoculum of Rhizophagus irregularis (Schenck & Smith) (AmfRi); (iii) a native AMF mixture inoculum made of isolated spores from site and cultured on leek as nurse plant for four months (from January to April 2019) in autoclaved sand and vermiculite in a greenhouse (AmfComLeek) (iv); a native AMF inoculum made of root fragments and rhizospheric soil (spores and hyphae) of Lavandula spp. plants (a pioneering shrub plant species that is highly mycotrophic and tolerant to the environmental stresses of the site) sampled in an undamaged part of the site (AmfComLav) in April 2019 (Ouahmane et al. 2006); (v) an organic amendment (GC), which is a dried and autoclaved green compost with the following properties: nitrogen, 0.46 g kg−1; carbon, 29.76 g kg−1; phosphorus, 2.68 ppm; organic matter, 50.43 g-1 and pH, 7.44.
To reduce wind damage, a belt of 144 two-month-old seedlings of Casuarina equisetifolia was installed around the site. The experiment was carried out under strictly natural conditions, without any watering or chemical fertilizer. The experiment started in May 2019 and ended in May 2021.
2.3 Soils and plants sampling
Bare soil samples were collected on site, before restoration without vegetation, at a depth of 0–20 cm in May 2019 (year 0; Y0) and analyzed for their chemical, enzymatic, mycorrhizal characteristics and for AMF identification. Two years after planting in May 2021 (year 2; Y2), rhizospheric soil samples were taken from roots of O. europaea plants for each treatment (inoculated or non-inoculated), with five replicas to evaluate changes in soil characteristics. Each soil sample was divided in three sub-samples after homogenization: the first sub-sample was air-dried for soil physico-chemical analysis; the second sub-sample was stored at 4 °C for soil microbial activity and the last sub-sample was stored at -20°c to analyze AMF community. Leaf samples were also harvested; defined along the stem of five olive tree plants for each treatment. Leaves were dried in an oven at 70 °C, weighted and ground, to measure the concentration of essential nutrients.
2.4 DNA extraction, PCR amplification and sequencing of soil AMF communities
Genomic DNA from soil samples was extracted using FastDNA SPIN kit for soil (MPBio®) following the manufacturer's instructions. A nested PCR was performed using two primer pairs targeting the D2 variable region of the rDNA large ribosomal subunit (LSU) gene. The first PCR reaction was performed with 50 ng of DNA template and primers LR1 (5' GCATATCAATAAGCGGAGGA 3') and NDL22 (5' TGGTCCGTGTTTCAAGACG 3') (van Tuinen et al. 1998). The second PCR was performed in a final volume of 50 μL, taking 5 μL of the 1:100 diluted PCR1 template and using primers FLR3 (5' TTGAAAGGGAAACGATTGAAG 3') and FLR4 (5' TACGTCAACATCCTTAACGAA 3') (Gollotte et al. 2004) modified with an adaptor sequence for Illumina® sequencing (2 × 250 bp MiSeq system). Amplicons were sent to Genewiz (GENEWIZ Germany GmbH, Leipzig) for library preparation and sequencing.
2.5 Diversity analyses
Raw sequences were bioinformatically treated on a Galaxy server using the FROGS metabarcoding pipeline (Escudié et al. 2018). First, the raw data (R1 & R2 reads) were assembled. Next, operational taxonomic units (OTUs) were formed using the SWARM algorithm with an aggregation distance of 5. Once chimeric sequences were removed, a final filter was applied to retain the OTUs present in at least two samples and whose abundance was greater than or equal to 0.005% of the total number of sequences. After the assembly of the OTU table, taxonomic affiliation was performed for each OTU sequence using sequences available on the NCBI and the MaarjAM database (Öpik et al. 2010), reworked with sequences from recent publications. OTUs were named 'sp.' when species identification failed. The Rényi diversity profile gives information about the diversity, richness and evenness of AMF communities among treatments. An alpha variable determines the value of each Rényi diversity profile (Lupatini et al. 2013). The Rényi diversity index is represented by H-alpha (Hα). The scale parameter α has a value ranging from zero to infinity. Tóthmérész (1995) derived diversity profile values (Hα) from the frequencies of each species (proportional abundances = abundance of species/total abundance). The formula is used to calculate the value of a diversity profile: Hα = ln(∑pαi)/1 − α, with Pi defined as proportional abundances (or, pi = abundance of species i/total abundance). H0 = species richness, H1 = Shannon diversity index, H2 = Simpson diversity index, and H∞ = Berger–Parker diversity index are the four frequently studied diversity indices derived from Rényi’s entropy formula (Kindt and Coe 2005).
2.6 Analytical methods and control of soil properties
Chemical soil analyzes were carried out at the FERTIAL agronomic laboratory (Arzew). Total carbon and nitrogen were quantified using the Dumas method (FLASH 2000, Thermo, NF ISO 10390). P Olsen was determined by the extraction of P with sodium bicarbonate by a spectrometric method (NF ISO 11263). The following elements: K, Ca, Mg and Na were extracted with 1 mol/L of ammonium acetate (pH 7.0) and analyzed by ICP-OES 6000 (Thermo Scientific standard, NF ISO 23470).
Glomalin-related soil protein (GRSP), including total GRSP (T-GRSP) and simply extracted GRSP (EE-GRSP), measured in one gram of each soil sample (Wright and Upadhyaya 1998). The EE-GRSP was extracted with sodium citrate (20 mM, pH 7.0) by autoclaving the samples at 121° C for 30 min and then centrifugation at 10,000 rpm for 5 min. The T-GRSP was extracted with 8 ml of sodium citrate (50 mM pH 8.0), autoclaved at 121° C for 60 min and centrifuged at 10,000 rpm. Extraction stopped when the red-brown color of the supernatant was no longer observed. Proteins in the supernatant were assayed by the Bradford test, with bovine serum albumin as standard. Absorbance was measured at 595 nm by spectrophotometer UV/microplate (SPECTRO star Nano, BMG LABTECH, Germany).
Total microbial activity was determined by the hydrolysis of 3,6-diacetylfluorescein (FDA) (Schnürer and Rosswall 1982). FDA hydrolysis was measured as described by Dick et al. (1997), with slight modifications. Briefly, one gram of soil samples was placed into a 50 ml plastic tube and mixed with 10 ml of sodium phosphate buffer 60 mM (pH 7.6), and the reaction was started with the addition of 100 µl of substrate solution FDA 4.8 mM. After 2 h incubation at 25 °C under shaking, the reaction was stopped by adding 10 ml of acetone. Before reading at 490 nm, the tubes were centrifuged for 5 min at 5000 rpm.
2.7 Analytical methods and control of plants properties
The concentration of essential nutrients was measured in the leaves of O. europaea. Total carbon and nitrogen were measured by combustion with Flash 2000. Concentrations of P, K, Ca, Mg, Fe, Mn, Cu and Zn were determined using inductively coupled plasma optical emission spectrometry (ICP-OES; 6000 Thermo Scientific), after digestion of 0.4 g of leaf sample, with 6 ml of nitric acid and 2 ml of hydrogen peroxide, incubated in a microwave at 200 °C for 45 min. Pigments chlorophyll (Chl) and carotenoids (Car) were extracted by grinding 0.1 g of fresh leaves of O. europaea in 1 ml acetone (90%) for 1 day in the dark. The extract was filtered and centrifuged at 15,000 g for 5 min. The supernatant was collected and read at 663 and 647 nm for Chl a and Chl b, respectively, and at 470 nm for Car content (Muneer et al. 2020). The concentrations of pigments were calculated according to formula reported by Lichtenthaler (1987) and Shabala et al. (1998). Soluble sugar concentrations in leaves of O. europaea was determined for each sample using the phenol–sulfuric acid method (Robyt and White 1987).
2.8 Statistical analyses
The statistical analysis was carried out on the R software (R Core Team 2021) and more specifically using the vegan ggplot 2 and tidyverse package (Wickham 2009; Oksanen et al. 2019; Wickham et al. 2019). Using other statistical commands, we determined the effect of the years on the revegetation of the soil, a first Student test was applied to compare (Ni) of second year with the bare soil (Y0). Analysis of variance ANOVA test is applied to study the combinatorial effect of treatment for second year on the various soil and plant parameters. The multiple comparison is applied by post-hoc comparisons, followed by Tukey's HSD test to find significant differences between the treatment. Two-dimensional relationships were analyzed by Spearman's correlation between elements available in soil and in leaves of O. europaea plants.
3 Results
3.1 AMF community composition and diversity
In soil samples from Y0 and Y2, 96 OTUs grouped into 28 species corresponding to 12 genera were identified (Ambispora, Archaeospora, Claroideoglomus, Diversispora, Dominikia, Funneliformis, Kamienskia, Nanoglomus, Paraglomus, Rhizophagus, Sclerocystis and Septoglomus). Significant differences were also observed in the distribution and abundance of AMF genera with the abundance increasing over the years (Fig. 1A). This may reflect a lower presence of AMF in the soil before rehabilitation. A strong increase in the genus Rhizophagus was observed after the restoration (Fig. 1A). This result is correlated with a significant difference observed through a permanova among Y0 and Y2 (R2 = 0.06299, p-value = 0.0235).
Diversity analysis among treatments and year of sampling from the studied mine site, Mnatsia Oran (Algeria). (A) Histogram representing the average number of reads of AMF diversity of soil samples, between Y0 (2019) and after restoration Y2 (2021). (B) Rényi diversity computed in Y2 from the OTU table comparing five different treatments: (i) no inoculation (Ni), (ii) a commercial AMF inoculum of Rhizophagus irregularis (AmfRi); (iii) a native AMF inoculum made of isolated spores from site and cultured on leek as nurse plant in a greenhouse (AmfComLeek) (iv); a native AMF inoculum made of root fragments and rhizospheric soil (spores and hyphae) of Lavandula spp. plants sampled in the site (AmfComLav); (v) an organic amendment (GC). (C) Histogram showing the relative abundances of sequences of AMF species in the bared soil (Y0) and two-years (Y2) after planting for the different treatments
The AMF species found in the soil samples were common in the bared soil (Y0) and two-years (Y2) after planting, but with a change in diversity between Y0 and after site restoration (Fig. 1A). Permanova analysis has shown no significant difference among treatments, except for the GC treatment compared to the Ni treatment (R2 = 0.24944, p-value = 0.0155). However, the diversity of AMF communities, described by Hα, differed among the conditions. In addition, the AmfComLav and AmfComLeek treatments had a higher diversity than in the other treatments (Fig. 1B). Although diversity seems to differ among treatments and years, the Shannon and Simpson diversity indices were not significantly different (results not shown). Relative abundance of AMF was different among treatments two-years (Y2) when comparing with the bared soil (Y0) (Fig. 1C).
3.2 Soil chemical properties in response to rehabilitation
The initial soil chemical analysis was made in 2019 before planting. The high limestone (pH [H2O] 7.67; CaCO3 ˃ 30%) has a clay texture: total nitrogen (< 0.05%), total carbon (5.24%), soil organic matter content (7.98%), P Olsen (0.05 ppm) (Supp. Table S1). No heavy metal pollution was measured. The introduction of O. europaea trees significantly decreased Mg concentration and increased assimilable P, T-GRSP and FDA hydrolysis compared to bared soil two-years after planting (Supp. Table S2). No significant differences were measured for the other elements.
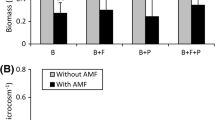
The effects of AMF inoculation and green compost did not differ significantly when comparing with non-inoculated (Ni) soils (Supp. Table S3). P level was significantly higher in the three AMF treatments (AmfRi, AmfComLeek and AmfComLav) compared to the Ni and GC treatments, with the exception of the commercial inoculum R. irregularis which had no significant difference with the GC treatment (Fig. 2A). AmfComLav treatment also had a significant effect on EE-GRSP compared to the GC treatment (Fig. 2B). The combinatorial effect of the four treatments was not statistically significant for many soil elements (N, C, K, Ca, Na, Mg, T-GRSP and FDA) compared to the Ni condition, except for the K and Mg concentration that were significantly enhanced (Supp. Table S3).
ANOVA test box plot representing significant soil parameters (A: Phosphorus (P); B: Easily extractable glomalin-related soil protein (EE-GRSP)) and leaves nutriments (C: Phosphorus (P); D: Total sugar) in response to the different soil treatments in second year. a, b, c, d different letters indicate significant differences between samples with P < 0.05 (n = 5; Two-way ANOVA followed by Tukey's HSD test)
3.3 Variation of leaves nutrients concentration in response to AMF and GC treatments
Sugar, P, Zn and Cu were significantly more concentrated in leaves of O. europaea plants under treatments than under Ni condition (Supp. Table S4, Fig. 2C). In particular, the P which remains the most significant element for mycorrhizal treatments (AmfRi, AmfComLeek and AmfComLav) compared with the Ni conditions (Fig. 2D), and especially for the AmfComLav condition. All four treatments had no effect on other elements.
4 Discussion
The introduction of plants either with organic amendment releasing nutrients (Gryndler et al. 2006) or with mutualistic microorganisms (e.g. AMF) providing plant benefits (Yang et al. 2017) may be an effective biotechnological tool to assist in the recovery of degraded soils. In this work, we have studied the impact of revegetation assisted by AMF inoculation and organic amendment on chemical soil proprieties, plant nutrition, and structure of AMF communities at the mine site.
4.1 Diversity of native AMF
Analysis of AMF diversity revealed a lower abundance in soils before rehabilitation, reflecting a lower flatness, characteristic of disturbed soils. Our results were consistent with the study of Garcia de Leon et al. (2018) who showed that the structure of the AMF community was modified by anthropogenic activities. After two years of revegetation, the diversity has changed and the abundance of AMF was about doubled. It was already shown that soil rehabilitation promotes AMF abundance (Asmelash et al. 2016; de Oliveira Prado et al. 2019; de Aguiar Santiago et al. 2022) and revegetation modifies the diversity and composition of AMF communities (Faggioli et al. 2019; Xiang et al. 2014; Xu et al. 2017). In our study, we have observed a strong increase in the genus Rhizophagus, suggesting a better adaptation to the stressful soil conditions of the gypsum quarry, as mentioned by Mergulhão et al. (2014). The study of Rényi diversity (Hα) index showed a greater diversity or a more balanced distribution of AMF in the AmfComLav and AmfComLeek treatments compared to the Ni, AmfRi and GC treatments. The AmfComLav and AmfComLeek inocula were prepared from root fragments, rhizospheric soil and isolated spores from the experimental site. A native inoculum therefore seems to be more suitable and appropriate for the rehabilitation of a site. AMF species were already considered as a good indicator of soil changes (Säle et al. 2015).
4.2 Effect of vegetation on remediation of degraded soil
The revegetation process leads to improve the physical and chemical properties of soil degraded by anthropogenic activities (Gunina et al. 2017; Yu et al. 2020). In our experiment, already two-years after planting, soil P content as well as the total microbial activities (FDA activity) and glomalin-related soil protein were significantly enhanced when O. europaea trees were mycorrhizal. Moreover, these results were consistent with the significant higher number of AMF reads measured after O. europaea tree plantation than before. Revegetation accelerates the productivity of degraded and disrupted soils through the development of extensive root systems; they provide nutrients and exudates (i.e. organic acids; Shan et al. 2008), enhancing and facilitating the increase of local soil microbial activity (Sheoran et al. 2010; Levy and Cumming 2014; Singh et al. 2018).
According to Zhang et al. (2017), glomalin content was positively correlated with soil edaphic factors and AMF root colonization, and glomalin can be used to monitor the recovery of degraded soils. In our study, the glomalin level increased under the vegetation. This may be related to the greater abundance of soil AMF, that could potentially increase the production of glomalin through their external mycelium (Driver et al. 2005; Wang et al. 2016; Holátko et al. 2021). In our study, AmfComLeek, AmfComLav and AmfRi treatments have significantly improved soil P availability compared with the Ni control and the GC treatment. In addition, glomalin (GRSP) was positively correlated with soil P as in Balota et al. (2016), suggesting a role of AMF, but also of other soil microbes in soil P mobilization (Smith and Read 2008; Yang et al. 2014; Dezam et al. 2017).
In general, results indicate that O. europaea is a very tolerant plant as able to grow without any treatment. The concentration of Mg in the soil has significantly decreased suggesting its uptake (Berthrong et al. 2009) by olive trees. However, the relation among plant nutrition, AMF and other soil nutrients was difficult to explain as quite inconsistent regarding the short period of the study (Lilleskov 2005; Oehl et al. 2010; Van der Putten et al. 2013; Xiang et al. 2014). In our study, we found that AMF inoculation did not influence nutrient concentration in leaves, as already reported by Berdeni et al. (2018). In addition, no direct relation between available soil nutrients and foliar nutrient concentrations of Olea europaea was shown in our study as already mentioned by Marañón et al. (2020).
5 Conclusion
In our study, the application of AMF inoculum and organic matter did not improve all soil parameters. However, a long period of revegetation is required to achieve significant changes in soil (Mensah 2015; Gu et al. 2019). Anyway, restoration of the gypsum deposit with O. europaea ensured the survival of the AMF community and increased AMF diversity and abundance independently of the treatment. In details, the native inoculum, AmfComLav and AmfComLeek, gave better results for diversity index analysis only, providing the highest Rényi diversity index (Hα). This result was suggesting that preparing inocula using indigenous spores and roots of an endemic plant (here Lavandula spp.) would be of interest to promote indigenous AMF for rehabilitation and also to build common mycorrhizal networks among plants (i.e. O. europaea and Lavandula spp.). Long-term analysis (> 10 years) will be also necessary to evaluate the effects of AMF and GC treatments after plantation and to provide tools to propose several procedures to restore soils degraded by mining. A better understanding of mechanisms is necessary to better use indigenous and locally stress-adapted soil microorganisms to improve the physiological performance and survival of host plants.
Data availability
Dataset are either provided in the manuscript in the main document or as supporting information, or could be requested to the corresponding author.
References
Asmelash F, Bekele T, Birhane E (2016) The potential role of arbuscular mycorrhizal fungi in the restoration of degraded lands. Front Microbiol 7:1095
Balota EL, Machineski O, Honda C, Yada IF, Barbosa GM, Nakatani AS, Coyne MS (2016) Response of arbuscular mycorrhizal fungi in different soil tillage systems to long-term swine slurry application. Land Degrad Dev 27:1141–1150
Barea J, Palenzuela J, Cornejo P, Sánchez-Castro I, Navarro-Fernández C, Lopéz-García A, Estrada B, Azcón R, Ferrol N, Azcón-Aguilar C (2011) Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J Arid Environ 75:1292–1301
Bencharef MH, Eldosouky AM, Zamzam S, Boubaya D (2022) Polymetallic mineralization prospectivity modelling using multi-geospatial data in logistic regression: The Diapiric Zone, Northeastern Algeria. Geocarto Int 1–36
Berthrong ST, Jobbágy EG, Jackson RB (2009) A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol Appl 19:2228–2241
Bi Y, Wang J, Feng Y, Yu H, Qin Y, Yu M (2014) Effect of arbuscular mycorrhiza on root self-repairing action of Amorpha fruticose L. in coal mining subsidence land in arid areas. J China Coal Soc 39:1758–1764
Cortese AM, Bunn RA (2017) Availability and function of arbuscular mycorrhizal and ectomycorrhizal fungi during revegetation of dewatered reservoirs left after dam removal. Restor Ecol 25:63–71
Crossay T, Cilia A, Cavaloc Y, Amir H, Redecker D (2018) Four new species of arbuscular mycorrhizal fungi (Glomeromycota) associated with endemic plants from ultramafic soils of New Caledonia. Mycol Prog 17:729–744
da Silva IR, da Silva DKA, de Souza FA, Oehl F, Maia LC (2017) Changes in arbuscular mycorrhizal fungal communities along a river delta island in northeastern Brazil. Acta Oecol 79:8–17
Daynes CN, Field DJ, Saleeba JA, Cole MA, McGee PA (2013) Development and stabilisation of soil structure via interactions between organic matter, arbuscular mycorrhizal fungi and plant roots. Soil Biol Biochem 57:683–694
de Aguiar Santiago FL, Silva AO, Batista ÉR, Kemmelmeier K, Gastauer M, Ramos SJ, Siqueira JO, Carneiro MAC (2022) Rehabilitation promotes rapid recovery of arbuscular mycorrhizal fungi in iron mining areas. Pedobiologia 95:150838
de Oliveira Prado IG, da Silva MdCS, de Oliveira Prado DG, Kemmelmeier K, Pedrosa BG, da Silva CC, Kasuya MCM (2019) Revegetation process increases the diversity of total and arbuscular mycorrhizal fungi in areas affected by the Fundão dam failure in Mariana, Brazil. Appl Soil Ecol 141:84–95
Dezam A, Vasconcellos V, Lacava P, Farinas C (2017) Microbial production of organic acids by endophytic fungi. Biocatal Agric Biotechnol 11:282–287
Dick RP, Breakwell DP, Turco RF (1997) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. Methods Assessing Soil Qual 49:247–271
Driver JD, Holben WE, Rillig MC (2005) Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol Biochem 37:101–106
Escudié F, Auer L, Bernard M, Mariadassou M, Cauquil L, Vidal K et al (2018) FROGS: find, rapidly, OTUs with galaxy solution. Bioinformatics 34:1287–1294
Faggioli VS, Cabello MN, Grilli G, Vasar M, Covacevich F, Öpik M (2019) Root colonizing and soil borne communities of arbuscular mycorrhizal fungi differ among soybean fields with contrasting historical land use. Agr Ecosyst Environ 269:174–182
Fernandez JE (2014) Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Environ Exp Bot 103:158–179
Frew A (2021) Contrasting effects of commercial and native arbuscular mycorrhizal fungal inoculants on plant biomass allocation, nutrients, and phenolics. Plants, People, Planet 3:536–540
Frost SM, Stahl PD, Williams SE (2001) Long-term reestablishment of arbuscular mycorrhizal fungi in a drastically disturbed semiarid surface mine soil. Arid Land Res Manag 15:3–12
Garcia de Leon D, Davison J, Moora M, Öpik M, Feng H, Hiiesalu I, Jairus T, Koorem K, Liu Y, Phosri C (2018) Anthropogenic disturbance equalizes diversity levels in arbuscular mycorrhizal fungal communities. Glob Change Biol 24:2649–2659
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649–660
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Gollotte A, Van Tuinen D, Atkinson D (2004) Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 14:111–117
Gryndler M, Larsen J, Hršelová H, Řezáčová V, Gryndlerová H, Kubát J (2006) Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 16:159–166
Gu LP, Kong JJ, Chen K, Guo YQ (2019) Monitoring soil biological properties during the restoration of a phosphate mine under different tree species and plantation types. Ecotoxicol Environ Saf 180:130–138
Gunina A, Smith AR, Godbold DL, Jones DL, Kuzyakov Y (2017) Response of soil microbial community to afforestation with pure and mixed species. Plant Soil 412:357–368
Hao X, Wang D, Wang P, Wang Y, Zhou D (2016) Evaluation of water quality in surface water and shallow groundwater: a case study of a rare earth mining area in southern Jiangxi Province, China. Environ Monit Assess 188:1–11
Holátko J, Brtnický M, Kučerík J, Kotianová M, Elbl J, Kintl A et al (2021) Glomalin-Truths, myths, and the future of this elusive soil glycoprotein. Soil Biol Biochem 153:108116
Johnson NC, Gehring C, Jansa J (2016) Mycorrhizal mediation of soil: fertility, structure, and carbon storage. Elsevier
Khabou W, Hajji B, Zouari M, Rigane H, Abdallah FB (2014) Arbuscular mycorrhizal fungi improve growth and mineral uptake of olive tree under gypsum substrate. Ecol Eng 73:290–296
Kindt R, Coe R (2005) Tree diversity analysis: a manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre
Levy MA, Cumming JR (2014) Development of soils and communities of plants and arbuscular mycorrhizal fungi on West Virginia surface mines. Environ Manage 54:1153–1162
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in enzymology. Elsevier. p. 350–382
Lilleskov EA (2005) How do composition, structure, and function of mycorrhizal fungal communities respond to nitrogen deposition and ozone exposure? In The Fungal Community (pp. 789–822). CRC Press
Lupatini M, Suleiman AKA, Jacques RJS, Antoniolli ZI, Kuramae EE, de Oliveira Camargo FA, Roesch LFW (2013) Soil-borne bacterial structure and diversity does not reflect community activity in Pampa biome. PLoS One 8:e76465
Maltz MR, Treseder KK (2015) Sources of inocula influence mycorrhizal colonization of plants in restoration projects: a meta-analysis. Restor Ecol 23:625–634
Marañón T, Navarro-Fernández CM, Gil-Martínez M, Domínguez MT, Madejón P, Villar R (2020) Variation in morphological and chemical traits of Mediterranean tree roots: linkage with leaf traits and soil conditions. Plant Soil 449:389–403
Mekahlia MN, Beddiar A, Chenchouni H (2013) Mycorrhizal dependency in the olive tree (Olea europaea) across a xeric climatic gradient. Adv Environ Biol 7:2166–2174
Mensah AK (2015) Role of revegetation in restoring fertility of degraded mined soils in Ghana: A review. Int J Biodivers Conserv 7:57–80
Mergulhão ACDES, Silva MVD, Lyra MDCCPD, Figueiredo MDVB, Silva MLRBD, Maia LC (2014) Morphological and molecular characterization of arbuscular mycorrhizal fungi isolated from gypsum mining areas, Araripina, Pernambuco State, Brazil. Hoehnea 41:393–400
Mota J, Sola A, Jiménez-Sánchez M, Pérez-García F, Merlo M (2004) Gypsicolous flora, conservation and restoration of quarries in the southeast of the Iberian Peninsula. Biodivers Conserv 13:1797–1808
Neuenkamp L, Prober SM, Price JN, Zobel M, Standish RJ (2019) Benefits of mycorrhizal inoculation to ecological restoration depend on plant functional type, restoration context and time. Fungal Ecol 40:140–149
Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E (2010) Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem 42:724–738
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, ... Imports MASS (2019) Package ‘vegan’. Community ecology package, version, 2(9)
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij, J. M., ... et al Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241
Ouahmane L, Duponnois R, Hafidi M, Kisa M, Boumezouch A, Thioulouse J, Plenchette C (2006) Some Mediterranean plant species (Lavandula spp. and Thymus satureioides) act as potential ‘plant nurses’ for the early growth of Cupressus atlantica. Plant Ecol 185:123–134
Pan Y, Li H (2016) Investigating heavy metal pollution in mining brownfield and its policy implications: a case study of the Bayan Obo rare earth mine, Inner Mongolia, China. Environ Manage 57:879–893
Peña A, Mingorance MD, Rossini-Oliva S (2015) Soil quality improvement by the establishment of a vegetative cover in a mine soil added with composted municipal sewage sludge. J Geochem Explor 157:178–183
Requena N, Perez-Solis E, Azcón-Aguilar C, Jeffries P, Barea JM (2001) Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl Environ Microbiol 67:495–498
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363
Robyt JF, White BJ (1987) Biochemical techniques: theory and practice. Books/Cole Publishing Company, Monterey, pp 267–275
Salami A, Jimoh M, Muoghalu J (2003) Impact of gold mining on vegetation and soil in southwestern Nigeria. Int J Environ Stud 60:343–352
Säle V, Aguilera P, E Laczko E, Mäder P, Berner A, Zihlmann U, van der Heijden MG, Oehl F (2015) Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol Biochem 84:38-52
Schmalenberger A, O’Sullivan O, Gahan J, Cotter PD, Courtney R (2013) Bacterial communities established in bauxite residues with different restoration histories. Environ Sci Technol 47:7110–7119
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA (1998) Salinity effect on bioelectric activity, growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Funct Plant Biol 25:609–616
Shackelford N, Miller BP, Erickson TE (2018) Restoration of open-cut mining in semi-arid systems: A synthesis of long-term monitoring data and implications for management. Land Degrad Dev 29:994–1004
Shan Y, Cai Z, Han Y, Johnson SE, Buresh RJ (2008) Organic acid accumulation under flooded soil conditions in relation to the incorporation of wheat and rice straws with different C: N ratios. Soil Sci Plant Nutr 54:46–56
Sheoran V, Sheoran A, Poonia P (2010) Soil reclamation of abandoned mine land by revegetation: a review. Int J Soil Sediment Water 3:13
Singh AK, Rai A, Banyal R, Chauhan PS, Singh N (2018) Plant community regulates soil multifunctionality in a tropical dry forest. Ecol Ind 95:953–963
Smith S, Read D (2008) Mycorrhizal Symbiosis Third Edition Introduction. Mycorrhizal Symb 1–9
Solís-Ramos LY, Andrade-Torres A (2020) Arbuscular mycorrhizal fungi in tropical ecosystems: towards its management? Agric Res Tech: Open Access J 24:556279
Tóthmérész B (1995) Comparison of different methods for diversity ordering. J Veg Sci 6:283–290
Ukaogo PO, Ewuzie U, Onwuka CV (2020) Environmental pollution: causes, effects, and the remedies. Microorganisms for sustainable environment and health. Elsevier. p. 419-429
Van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitze JA (2013) Plant–soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276
van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzi-Pearson V (1998) Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol 7:879–887
Wang FY, Lin XG, Yin R (2007) Effect of arbuscular mycorrhizal fungal inoculation on heavy metal accumulation of maize grown in a naturally contaminated soil. Int J Phytorem 9:345–353
Wang F (2017) Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit Rev Environ Sci Technol 47:1901–1957
Wang ZG, Bi YL, Jiang B, Zhakypbek Y, Peng SP, Liu WW, Liu H (2016) Arbuscular mycorrhizal fungi enhance soil carbon sequestration in the coalfields, northwest China. Sci Rep 6:1–11
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A et al (2019) Welcome to the tidyverse. J Open Source Softw 4:1686
Wiegleb G, Felinks B (2001) Primary succession in post-mining landscapes of Lower Lusatia—chance or necessity. Ecol Eng 17:199–217
Wildman H (2015) Improving mine rehabilitation success through microbial management. J Environ Solut Oil Gas Min 1:32–46
Worlanyo AS, Jiangfeng L (2021) Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J Environ Manage 279:111623
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107
Xiang D, Verbruggen E, Hu Y, Veresoglou SD, Rillig MC, Zhou W, Xu T, Li H, Hao Z, Chen Y (2014) Land use influences arbuscular mycorrhizal fungal communities in the farming–pastoral ecotone of northern China. New Phytol 204:968–978
Xu M, Li X, Cai X, Li X, Christie P, Zhang J (2017) Land use alters arbuscular mycorrhizal fungal communities and their potential role in carbon sequestration on the Tibetan Plateau. Sci Rep 7:1–11
Yang G, Liu N, Lu W, Wang S, Kan H, Zhang Y, Xu L, Chen Y (2014) The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J Ecol 102:1072–1082
Yang Y, He C, Huang L, Ban Y, Tang M (2017) The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS One 12:e0182264
Yu J, Liu F, Tripathi BM, Steinberger Y (2020) Changes in the composition of soil bacterial and fungal communities after revegetation with Caragana microphylla in a desertified semiarid grassland. J Arid Environ 182:104262
Zhang Y, He X, Zhao L, Zhang J, Xu W (2017) Dynamics of arbuscular mycorrhizal fungi and glomalin under Psammochloa villosa along a typical dune in desert, North China. Symbiosis 73:145–153
Acknowledgements
This work was carried out as part of a project to rehabilitate a gypsum quarry in western Algeria. The authors would like to thank the Knauf team for their field collaborations; Mr. Fatah Redouane and his team from Fertial Arzew Agricultural Laboratory; The forests Gdyel commune conservation; Mr. Belantar Ibrahim University of Tlemcen; members of the LBRAP Oran laboratory and especially the technician Miss. Bouchiba Chahinez, PhD candidate Miss. Benkritly Sara, PhD candidate Miss. Mesbah Nadjet and Dr. Abed Nour Elhouda and finally the team of the Agroecological Institute of Dijon.
Funding
PHC Magrehb.
Author information
Authors and Affiliations
Contributions
N.M. and C.D made the experiment and analysis. All authors contribute to the writing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pierre-Emmanuel Courty and Abdelkader Bekki are Co-last authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madjoub, N., Durney, C., Sportes, A. et al. Arbuscular mycorrhizal fungi participate to the restoration of a gypsum mining site in western Algeria. Symbiosis 90, 183–192 (2023). https://doi.org/10.1007/s13199-023-00936-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00936-6