Abstract
The potential interference of Frankia asymbiotica NRRL B-16386 (nitrogen-fixing but non-infective) during nodule establishment on Alnus glutinosa seedlings by Frankia torreyi CpI1 (infective and effective) was investigated. Basic plant growth characteristics (stem and root elongation, dry weight production and total chlorophyll) and nodulation were assessed. The analysis showed that NRRL B-16386 did not greatly affect strain CpI1 during root nodule establishment and seedling growth of A. glutinosa despite the increase in its quantity and decrease in the symbiotic strain in the inocula. An increase in plant growth was noted even when the level of the effective Frankia strain decreased. The asymbiotic strain appeared to have an inhibitory effect on seedling growth and to thrive on the surface of root nodules well established by the symbiotic strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alnus glutinosa Gaertn., widely called black alder is an actinorhizal plant that forms symbiosis with members of the genus Frankia (McEwan et al. 2017; Roy et al. 2017). This actinobacterial genus encompasses endophytic actinobacteria that have been isolated primarily from nitrogen-fixing nodules in the roots of dicotyledonous host plant designated as actinorhizal plants (Gtari et al. 2013). Based on Koch's postulates of infectivity (nodulation) and effectivity (nitrogen fixation), Frankia strains were assigned into symbiotic and asymbiotic endophytes (Gtari et al. 2019). Symbiotic strains are phylogenetically affiliated within cluster 1 (microsymbionts of Betulaceae, Casuarina, Allocasuarina, Comptonia, and Myrica species), cluster 2 (microsymbionts of Coriaria, Ceanothus, Datisca, Cercocarpus, Chamaebatia, and Purshia species and of Dryas drummondii), and cluster 3 (microsymbionts of Colletieae, Elaeagnaceae, Gymnostoma and Myricaceae species). Cluster4 contains asymbiotic strains which lack one or two symbiotic characteristics i.e., infectivity and/or effectivity (Gtari et al. 2019). With respect to symbiotic strains, it has been shown that cooperation and competition can occur between various Frankia and non-Frankia microorganisms during the nodulation process and thus drive nodule occupancy in actinorhizal plants (Kurdali et al. 1990; Hahn et al. 1990; Russo et al. 1993; Sempavalan et al. 1995). A role for host plants has also been reported through selectivity for individual Frankia strains or populations (Kurdali et al. 1988; Huss-Danell and Myrold 1994; Mirza et al. 2009; Pokharel et al. 2011; Pozzi et al. 2015). However, the situation remains unclear between asymbiotic and symbiotic Frankia strains. Although Ramírez-Saad et al. (1998) reported the co-occurrence of symbiotic and asymbiotic Frankia strains in the same Ceanothus root nodule samples, the questions of whether asymbiotic strains co-occur as "opportunistic" or "synergistic" and whether they are "parasite" or "beneficial" endophytes remain unanswered.
The ultimate goal of studying nitrogen-fixing symbioses is to maximize their potential to improve plant productivity by accessing an inexhaustible source of nitrogen from the atmosphere (Herridge et al. 2008; Peoples and Craswell 1992). Fundamental, yet challenging, biotechnology practices for nitrogen-fixing symbioses aim to select the most appropriate bacterial inocula (Dommergues 1995; Olivares et al. 2013; Sayed 2011; Zhong et al. 2019). The main selection criteria for field inocula are (i) symbiotic performance (ii) ability to accommodate pedoclimatic conditions and (iii) outperformance in relation to less effective indigenous strains.
The aim of the present study was to evaluate the nodulation capabilities of the host plant species A. glutinosa with symbiotic Frankia torreyi strain CpI1, in the presence of the asymbiotic Frankia asymbiotica NRRL B-16386. Basic plant growth characteristics of A. glutinosa seedlings, including stem length, root elongation, total chlorophyll, and dry weight, were assessed upon dual inoculation with both Frankia strains.
2 Materials and methods
2.1 Plant growth
Alnus glutinosa seeds were collected locally (alder stand in Tamra, Tunisia; 37° 3′ N, 9° 7′ E, 150 m elevation) disinfected by stirring in H2O2 (40%) for 30 min, washed several times with sterile distilled water, and germinated in a sterile mix of sand/vermiculite (v/v). The three-week-old seedlings were then grown under hydroponic conditions in a one litter bottle containing Broughton and Dilworth's solution (Broughton and Dilworth 1971) supplied with a nitrogen source, i.e., 5 mM KNO3. The plant culture in this study was carried out at 20 °C and under a light/dark photoperiod of 16/8 h.
2.2 Frankia strains
Strain CpI1 was isolated from root nodules of Comptonia peregrina (Callaham et al. 1978) and proposed as a type strain of the species F. torreyi by Nouioui et al. (2019). Strain NRRL B-16386 was isolated from Morella californica (Lechevalier 1986) and proposed as a type strain of the species F. asymbiotica by Nouioui et al. (2017). Both strains were grown and maintained in BAP medium (Murry et al. 1984) supplemented with 2.5 mM propionate as a carbon source at 28 °C without shaking.
2.3 Inoculation experiment
The first set of A. glutinosa seedlings was depleted of nitrogen by omitting 5 mM KNO3 from the DB nutrient solution (BD-N) while the other set was maintained in DB nutrient solution supplemented with 5 mM KNO3 (BD + N). Four-week-old cultures of each strain were washed with sterile distilled water and syringed several times with sterile 21G needles. Equal stocks of inocula of each strain were prepared on the basis INT (2-(p-iodophenyl-3-( p-nitrophenyl)0.5-phenyl tetrazolium chloride) reduction activity (IR) as described by Prin et al. (1990) and total cellular protein estimated by the BCA (bicinchoninic acid) method (Smith et al. 1985). Briefly 100 µl of 0.2% INT was added to 1 ml of homogenized Frankia cells and incubated for one hour at 28 °C in the dark. After methanol extraction for 2 h at 70 °C, the intracellular INTFormazan (INTF) was spectrophotometrically determined at 490 nm. Homogenized Frankia cells were solubilized by heating for 15 min at 90 °C in 1.0 N NaOH and total proteins were measured using BCA Protein Assay Kit (Thermo Scientific). All inocula were prepared as an equivalent of 10 μg of protein. To achieve this quantity of inocula, varying amounts of each strain were combined; 0%/100%, 25%/75%, 50%/50%, 75%/25%, and 100%/0% of CpI1 and NRRL B-16386, respectively. Six-week-old seedlings were inoculated and 10 replicates were considered for each inoculum. As a negative control, 10 seedlings were not inoculated.
2.4 Nodulation assessment
After two months, the nodules were harvested from the plants, previously infected with the different combinations of inoculum, numbered and weighed.
2.5 Plant growth assessment
Plant growth was assessed using basic growth parameters. At the end of the experiment, the lengths and dry weights of the stem part and roots were measured.
2.6 Total chlorophyll
Chlorophyll was measured by the method of Arnon (1949) using 200 mg of fresh material in 80% acetone in test tubes for 72 h in the dark and at + 4 °C. After 72 h, the suspension was filtered and the optical density was measured by a spectrophotometer at three wavelengths: 663, 652 and 645 nm and finally the concentrations of chlorophyll ‘a’ and ‘b’ were determined from the following formulas:
2.7 DNA extraction, 16S-23S rDNA ITS-PCR amplification and capillary electrophoresis
To check for the potential co-occurrence of NRRL strain B-16386 with CpI1 in the induced nodules, the automated ribosomal intergenic spacer analysis (ARISA) method was used (Gtari et al. 2007). Nodule lobes were disinfected by immersion in 30% v/v hydrogen peroxide for 15–20 min, rinsed several times with sterile distilled water containing 2% w/v polyvinylpyrrolidone (PVPP). Peeled and unpeeled lobes from each nodule were crushed separately in 500 µL of extraction buffer (100 mM Tris–HCl pH 8, 20 mM ethylenediaminetetraacetic acid (EDTA) pH 8. 2, 1.4 M NaCl, 2% (w/v) cetyl trimethyl ammonium bromide (CTAB), 3% w/v PVPP and incubated for 1 h at 65 °C. Afterwards chloroform: isoamyl alcohol (24:1) was added, mixed by inversion and centrifuged at 12 000 9 g for 15 min at room temperatue. A 2/3 volume isopropanol was added to aqueous phase and DNA was precipitated by centrifuging at 12 000 g for 15 min at 4 °C. The resulting DNA pellet was then washed twice in ice-cold 75% ethanol and resuspended in 100 lL of 5 mM Tris–HCl buffer (pH = 8.5). The DNA pellet was then dissolved in 10 ml TE (10 mM Tris–HCl pH 8 20 mM EDTA pH 8.2) (Gtari et al. 2007). Amplification of the ITS 16S-23S rDNA was performed using the forward primer S-d-Bact-1494-a-S-20 (5'-GTCGTAACAAGGTAGCCGTA-3'), labeled at the 5' end with the phosphoramidite dye 6-carboxyfluorescein, and the reverse primer L-D-Bact-0035-a-15 (5'-CAAGGCATCCACCGT-3'). Capillary electrophoresis was performed in an ABI Prism 3710 capillary sequencer as described previously (Gtari et al. 2007; Ghodhbane-Gtari et al. 2010). Aliquots (1–5 µl) of the PCR products were mixed with 1 µl of the 1000-bp internal size standard (Applied Biosystems) labeled with the phosphoramidite dye 6-carboxyrhodamine and 20 µl of deionized formamide. The mixture was denatured at 95 °C for 5 min and cooled in an ice bath. PCR products were then run-on the ABI Prism 3710 genetic analyzer (Applied Biosystems) through a 47 cm/50 µm capillary filled with 4% performance-optimized polymer (Applied Biosystems).
2.8 Statistical analysis
Basic growth parameters, including stem and root length and dry weight, total chlorophyll, and nodule number and weight, were analyzed using a simple linear model with fixed effects (Inoculum, Medium and their interaction). For each variable, residual normality and homogeneity of variances were assessed with Shapiro–Wilk and Levene tests, respectively. Adjusted means (LS-means) were calculated and compared with the Tukey–Kramer (for an equal-variance model) or Dunnett T3 test (for an unequal-variance model) of PROC MIXED of SAS, version 9.4. Table S1 summarizes the main statistical analysis performed in this study.
3 Results and discussion
It is well established that inoculation with compatible Frankia strains increases nodulation and growth performance of actinorhizal host plant species even in the presence of indigenous Frankia strains (Weber et al. 1989; Sanginga et al. 1989; Nickel et al. 2001). In this study, it was proposed to investigate nodulation and growth of A. glutinosa seedlings upon co-inoculation with two Frankia strains, one symbiotic F. torreyi CpI1 and the other asymbiotic F. asymbiotica NRRL B-16386. The potential interaction between the two strains was then analyzed.
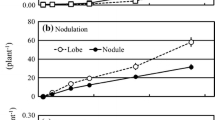
Root nodules were first observed after 3 weeks of inoculation in all tested A. glutinosa seedlings, except those inoculated with NRRL B-16386 strain only and the negative control. This result is concordant with previous studies reporting that root nodules appeared 2–3 weeks after inoculation in members of Alnus species (Huss-Danell 1978; Kohl and Baker 1989; Yamanaka et al. 2016). The assessed time course for nodule appearance was not affected by the presence or absence of the asymbiotic strain NRRL B-16386. The nodulation results obtained in the present study also confirm previous reports on the ability of the CpI1 strain (from phylogenetic cluster 1) to efficiently nodulate A. glutinosa (Lalonde 1979; Nouioui et al. 2019) and the complete inability of strain NRRL B-16386 (phylogenetic cluster 4) to nodulate any of the actinorhizal species tested, including its original host of isolation, Morella californica and Alnus species (Lechevalier 1986; Huguet et al. 2005; Nouioui et al. 2017). ARISA performed on selected root nodules confirmed the omnipresence of the CpI1 strain in all induced root nodules, whereas the strain NRRL B-16386 was detected in only 10% of unpeeled lobes and 3% of peeled lobes (Fig. S1). Thus, strain NRRL B-16386 is more likely to be a contaminant of the surface of root nodules of Morella californica (Lechevalier 1986). Similarly, incompatible R43 strain (cluster 3) does co-infect the root nodules formed by compatible F. casuarinae CcI3 strain (cluster 1) in Casuarina equisetifolia (Vemulapally et al. 2019) and is likely being a surface contaminant (Zhang et al. 1984).
The establishment of optimal symbiosis between F. torreyi strain CpI1 and A. glutinosa seedlings was significantly influenced (p < 0.05) by the availability of mineral nitrogen in the medium (Table S1). The presence of nitrogen in the plant growth medium significantly affected nodulation and the number of nodules induced (Fig. 1A). Thus, the number of nodules in plants grown in BD + N and inoculated only with the symbiotic strain CpI1 was significantly lower compared with the number of nodules found in BD-N. Yamanaka et al. (2016) reported that the number of nodules formed on A. sieboldiana seedlings inoculated with Frankia was reduced when sufficiently supplied with mineral nitrogen. The seedlings appear to have no need to form nodules in a nitrogen-rich environment since nodule formation is energetically costly. This result is similar to those reported previously (Griffiths and McCormick 1984; Knowlton and Dawson 1983; MacConnell and Bond 1957; Smolander and Sundman 1987; Stewart and Bond 1961).
The number of nodules formed following co-inoculation with symbiotic strain CpI1 and the asymbiotic strain NRRL B-16386 was significantly reduced relative to inoculation only with strain CpI1 (Fig. 1A). These results seem to be in agreement with those obtained for A. incana plants (Kurdali et al. 1990) where the double inoculation showed that the spore positive strain reduced the number of nodules induced by the spore negative strain. Martin et al. (2003) reported that co-inoculation with two symbiotic Frankia strains, AvcI1 and ArI5, increases nodulation in A. rubra compared to simple inoculation with each strain.
Although the number of nodules induced in BD-N (Fig. 1A) was significantly different, the total nodule weight for each plant was not significantly affected (Fig. 1B, Fig. S2). Wheeler et al. (1981) and Hooker and Wheeler (1987) reported that, to some extent, increased nodule weight may be a compensatory effect for low specific activity in nitrogen fixation. For the rhizobia-Legume symbiosis, this compensatory mechanism has also been reported when inoculation of legume species was done by effective strains and in the presence of ineffective indigenous strains (Singleton and Stockinger 1983; Singleton and Tavares 1986).
Growth characteristics of seedlings assessed by stem length and dry weight (Fig. 2A, Figs. S3-S4) and roots (Fig. 2B, Figs. S5-S6), showed that nodulated seedlings grown in BD + N or in BD-N had the same growth performance than those grown. In BD-N, the growth of seedlings inoculated with Frankia strain CpI1 and seedlings co-inoculated with both strains at the same time did not differ significantly (Fig. 2, Figs. S3-S6). In fact, seedlings infected only with Frankia NRRL B-16386 showed no significant difference in growth compared with the negative control (non inoculated seedlings). This suggests that seedlings are not able to profit from the nitrogen fixed by asymbiotic strain NRRL B-16386 (Nouioui et al. 2017). Kurdali et al. (1990) show that co-inoculation with spore-positive (Sp +) and spore-negative (Sp-) strains did not influence the growth of the host plant. Growth parameters (length and dry weight) of the aerial part as well as root dry weight showed no significant difference compared to simple inoculation (Kurdali et al. 1990). Upon an analysis of eight Alnus species co-inoculated with an equal mixture of three symbiotic strains of Frankia: ACN1AG, ACoN24d, and AG10AI, Prat (1989) showed that co-inoculation generally provided better seedling growth than the best individual strain. Similarly, co-inoculation with AvcI1 and ArI5, two symbiotic Frankia strains, resulted in increased A. rubra seedling growth and nodulation compared to inoculation with each strain alone (Martin et al. 2003). Using PCR–RFLP analysis of the glnII gene, the authors reported that the ArI1-like pattern was most prevalent in the co-inoculation experiment. Another study showed that double infection of A. glutinosa plants with two strains of Frankia, one symbiotic and the other asymbiotic, caused an increase in plant growth but the number of nodules was not affected (Hahn et al. 1990). This suggests the various asymbiotic strains have diverse effect on the establishment and functioning of actinorhizal symbioses ranging from inhibition to stimulation.
Stem/root weight and length ratios showed that in the presence of nitrogen (BD + N), the seedlings tended to significantly expand the aerial part in terms of weight and length more than the root part (Fig. S7-S8). In BD-N and in the presence of the symbiotic CpI1 strain or both strains at the same time, the root part was significantly more developed than the aerial part compared to the negative control or to the seedlings inoculated only with the strain NRRL B-6386 (Fig. S7-S8). This root extension may be attributed to an expanding nutrients uptake afterward active nodule induction or to vitamins or phytohormones like auxins or cytokinine produced by the microsymbiont Frankia (Hahn et al. 1990).
The assay showed that the amount of total chlorophyll was significantly higher in the BD-N than in the BD + N medium (Fig. S9). In BD-N, seedlings inoculated with either strain CpI1 or both strains showed higher amounts of total chlorophyll than seedlings inoculated only with strain NRRL B-16386, which showed the lowest amount of total chlorophyll of all treated seedlings. However, this difference was only significant for seedlings inoculated with a 75%/25% mix (CpI1/NRLL B-16386) (Fig. S9). Although increased photosynthetic activity is expected to improve vital plant activities, it also appears essential to support the additional metabolic expense due to the intracellular growth of microsymbionts. Alder plants inoculated with Frankia strains have higher productivity as assessed by shoot and root length, dry matter production, and chlorophyll content (Vendan et al. 1999). These results illustrate that the symbiotic strain CpI1 promotes plant growth in a nitrogen-poor medium, whereas the nitrogen-fixing and asymbiotic NRRL B-16386 strain alone inhibits seedling growth and/or induces defensive plant immune response. It appears that this effect is delayed in the presence of the symbiotic strain CpI1. This may be due to evasion or suppression of host immune responses by symbiotic CpI1 effectors as has been suggested in the legume-rhizobia symbiosis (Yasuda et al. 2016). The symbiosis between F. torreyi CpI1 strain and A. glutinosa promotes plant growth even in a nitrogen-replete environment, while the presence of the CpI1 strain and the formation of nodules cover the nitrogen needs of the plant. Black alder co-infection did not influence plant development, but it did influence nodule number.
In conclusion, our current analysis suggests that the asymbiotic strain NRRL B-16386 did not greatly affect the symbiotic strain CpI1 during root nodule establishment and growth of A. glutinosa seedlings despite the increase in its amount along with the decrease in the amount of the symbiotic strain in the inocula. A compensatory mechanism for the nodule number may be achieved by increasing the nodule weight in order to sustain nitrogen-fixation rate and satisfy plant demand. The NRRL B-16386 strain did not penetrate inside root nodules, regardless of its amount in the inocula, but may occur as a contaminant of the nodule surface. The ability these asymbiotic strains to fix nitrogen in the rhizosphere should be assessed. Assessments of basic growth characteristics and nodulation of A. glutinosa seedlings upon dual inocula suggest that the competition result depends on the symbiotic capacity of strains used for inoculation.
References
Arnon D (1949) Copper enzymes in isolated chaloroplasts. Poly-phenoloxidase in beta vulgaris. Plant Physiol 24:1–15
Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125:1075–1080
Callaham C, Deltredici P, Torrey JG (1978) Isolation and cultivation in vitro of the actinomycete causing root nodulation in comptonia. Science 199:899–902
Dommergues YR (1995) Nitrogen fixation by trees in relation to soil nitrogen economy. Fertilizer Res 42(1):215–230
Ghodhbane-Gtari F, Nouioui I, Boudabous A, Gtari M (2010) 16S–23S rRNA intergenic spacer region variability in the genus Frankia. Microb Ecol 60(3):487–495
Griffiths AP, McCormick LH (1984) Effects of soil acidity on nodulation of Alnus glutinosa and viability of Frankia. Plant Soil 79(3):429–434
Gtari M, Brusetti L, Cherif A, Boudabous A, Daffonchio D (2007) Heteroduplex structures in 16S–23S rRNA intergenic transcribed spacer PCR products reveal ribosomal interoperonic polymorphisms within single Frankia strains. J Appl Microbiol 103(4):1031–1040
Gtari M, Nouioui I, Sarkar I, Ghodhbane-Gtari F, Tisa LS, Sen A, Klenk HP (2019) An update on the taxonomy of the genus Frankia Brunchorst, 1886, 174 AL. Anton Leeuw 112(1):5–21
Gtari M, Tisa LS, Normand P (2013) Diversity of Frankia strains, actinobacterial symbionts of actinorhizal plants. In Symbiotic endophytes. Springer, Berlin, Heidelberg, pp. 123–148
Hahn D, Starrenburg MJC, Akkermans ADL (1990) Growth increment of Alnus glutinosa upon dual inoculation with effective and ineffective Frankia strains. Plant Soil 122(1):121–127
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311(1):1–18
Hooker JE, Wheeler CT (1987) The effectivity of Frankia for nodulation and nitrogen fixation in Alnus rubra and A. glutinosa. Physiol Plantarum 70(2):333–341
Huguet V, Gouy M, Normand P, Zimpfer JF, Fernandez MP (2005) Molecular phylogeny of Myricaceae: a reexamination of host–symbiont specificity. Mol Phylogenet Evol 34(3):557–568
Huss-Danell K (1978) Nitrogenase activity measurements in intact plants of Alnus incana. Physiol Plantarum 43:372–376
Huss-Danell K, Myrold DD (1994) Intragenic variation in nodulation of Alnus: Consequences for quantifying Frankia nodulation units in soil. Soil Biol Biochem 26:525–531
Knowlton S, Dawson JO (1983) Effects of Pseudomonas cepacia and cultural factors on the nodulation of Alnus rubra roots by Frankia. Can J Bot 61(11):2877–2882
Kohl SJ, Baker DD (1989) Effects of substrate nitrate concentration on symbiotic nodule formation in actinorhizal plants. Plant Soil 118:171–179
Kurdali F, Domenach AM, Bardin R (1990) Alder-poplar associations: determination of plant nitrogen sources by isotope techniques. Biol Fertil Soils 9(4):321–329
Kurdali F, Domenach AM, Fernandez M, Capellano A, Moiroud A, De la Paz-Fernandez M (1988) Compatibility of Frankia spore positive and spore negative inocula with Alnus glutinosa and Alnus incana. Soil Sci Plant Nutr 34:451–459
Lalonde M (1979) Immunological and ultrastructural demonstration of nodulation of the European Alnus glutinosa (L.) Gaertn. host plant by an actinomycetal isolate from the North American Comptonia peregrina (L.) Coult. root nodule. Bot Gaz 140:S35–S43
Lechevalier MP (1986) Nitrogen-fixing actinomycetes of the genus Frankia. In: Megusar F, Gantar M (eds) Perspectives in Microbial Ecology. Slovene Society for Microibiology, Ljubljana, Yugoslavia, pp 253–256
MacConnell JT, Bond G (1957) A comparison of the effect of combined nitrogen on nodulation in non-legumes and legumes. Plant Soil 8(4):378–388
Martin KJ, Tanaka Y, Myrold DD (2003) Dual inoculation increases plant growth with Frankia on red alder (Alnus rubra Bong.) in fumigated nursery beds. Symbiosis 34:253–260
McEwan NR, Wilkinson T, Girdwood SE, Snelling TJ, Collins T, Dougal K et al (2017) Evaluation of the microbiome of decaying alder nodules by next generation sequencing. Endocyt Cell Res 28:14–19
Mirza BS, Welsh A, Rasul G, Rieder JP, Paschke MW, Hahn D (2009) Variation in Frankia populations of the Elaeagnus host infection group in nodules of six host plant species after inoculation with soil. Microb Ecol 58:384–393
Murry M, Fontaine M, Torrey J (1984) Growth kinetics and nitrogenase induction in Frankia sp. HFPArI3 grown in batch culture. Plant Soil 78:61–78
Nickel A, Pelz O, Hahn D, Saurer M, Siegwolf R, Zeyer J (2001) Effect of inoculation and leaf litter amendment on establishment of nodule-forming Frankia populations in soil. Appl Environ Microbiol 67:2603–2609
Nouioui I, Ghodhbane-Gtari F, Jando M, Tisa LS, Klenk HP, Gtari M (2019) Frankia torreyi sp. nov., the first actinobacterium of the genus Frankia Brunchorst 1886, 174 AL isolated in axenic culture. Anton Leeuw 112(1):57–65
Nouioui I, Gueddou A, Ghodhbane-Gtari F, Rhode M, Gtari M, Klenk HP (2017) Frankia asymbiotica sp. nov., a non-infective actinobacterium isolated from Morella californica root nodule. Int J Syst EvolMicrobiol 67(12):l4897-4901
Olivares J, Bedmar EJ, Sanjuán J (2013) Biological nitrogen fixation in the context of global change. Mol Plant Microbe Interact 26(5):486–494
Peoples MB, Craswell ET (1992) Biological nitrogen fixation: investments, expectations and actual contributions to agriculture. Plant Soil 141(1):13–39
Pokharel A, Mirza BS, Dawson JO, Hahn D (2011) Frankia populations in soil and root nodules of sympatrically grown Alnus taxa. Microb Ecol 61:92–100
Pozzi AC, Bautista-Guerrero HH, Nouioui I, Cotin-Galvan L, Pepin R, Fournier P, Menu F, Fernandez MP, Herrera-Belaroussi A (2015) In-planta sporulation phenotype: a major life history trait to understand the evolution of Alnus-infective Frankia strains. Environ Microbiol 17:3125–3138
Prat D (1989) Effects of some pure and mixed Frankia strains on seedling growth in different Alnus species. Plant Soil 113(1):31–38
Prin Y, Neyra M, Diem HG (1990) Estimation of Frankia growth using Bradford protein and INT reduction activity estimations: application to inoculum standardization. FEMS microbiology letters 69(1–2):91–95
Ramírez-Saad H, Janse JD, Akkermans AD (1998) Root nodules of Ceanothus caeruleus contain both the N2-fixing Frankia endophyte and a phylogetically related Nod-/Fix-actinomycete. Can J Microbiol 44(2):140–148
Russo RO, Gordon JC, Berlyn GP (1993) Evaluating Alder-Endophyte (Alnus acuminata-Frankia-Mycorrhizae) Interactions: Growth Response of Alnus acuminata Seedlings to Inoculation with Frankia Strain Ar13 and Glomus intra-radices, Under Three Phosphorus Levels. J Sustain for 1(1):93–110
Roy M, Pozzi AC, Gareil R, Nagati M, Manzi S, Nouioui I et al (2017) Alder and the Golden Fleece: high diversity of Frankia and ectomycorrhizal fungi revealed from Alnus glutinosa subsp. barbata roots close to a Tertiary and glacial refugium. PeerJ 18:e3479. https://doi.org/10.7717/peerj.3479
Sanginga N, Danso SKA, Bowen GD (1989) Nodulation and growth response of Allocasuarina and Casuarina species to phosphorus fertilization. Plant Soil 118:125–132
Sayed WF (2011) Improving Casuarina growth and symbiosis with Frankia under different soil and environmental conditions. Folia Microbiol 56(1):1–9
Sempavalan J, Wheeler CT, Hooker JE (1995) Lack of competition between Frankia and Glomus for infection and colonization of roots of Casuarina equisetifolia (L.). New Phytol 130(3):429–436
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):76–85
Smolander A, Sundman V (1987) Frankia in acid soils of forests devoid of actinorhizal plants. Physiol Plant 70(2):297–303
Stewart WDP, Bond G (1961) The effect of ammonium nitrogen on fixation of elemental nitrogen in Alnus and Myrica. Plant Soil 14(4):347–359
Singleton PW, Stockinger KR (1983) Compensation against ineffective nodulation in soybean 1. Crop Sci 23(1):69–72
Singleton PW, Tavares JW (1986) Inoculation response of legumes in relation to the number and effectiveness of indigenous Rhizobium populations. Appl Environ Microbiol 51(5):1013–1018
Vendan RT, Rajeshwari T, Thamizh R, Narayanan R (1999) Studies on strain specificity of Frankia in Alnus nepalensis. Trop Agric Res Ext 2:124–125
Vemulapally S, Guerra T, Hahn D (2019) Localization of typical and atypical Frankia isolates from Casuarina sp. in nodules formed on Casuarina equisetifolia. Plant Soil 435(1):385–393
Weber A, Sarsa ML, Sundman V (1989) Frankia-Alnus incana symbiosis: effect of endophyte on nitrogen fixation and biomass production. Plant Soil 120:291–297
Wheeler CT, McLmighlin ME, Steele P (1981) A comparison of symbiotic nitrogen fixation in Scotland in Alnus giutinosa and Alnus rubra. Plant Soil 61:169–188
Yamanaka T, Okabe H, Kawai S (2016) Growth and nodulation in Alnus sieboldiana in response to Frankia inoculation and nitrogen treatments. Trees 30(2):539–544
Yasuda M, Miwa H, Masuda S, Takebayashi Y, Sakakibara H, Okazaki S (2016) Effector-triggered immunity determines host genotype-specific incompatibility in legume–Rhizobium symbiosis. Plant Cell Physiol 57(8):1791–1800
Zhang Z, Lopez MF, Torrey JG (1984) A comparison of cultural characteristics and infectivity of Frankia isolates from root nodules of Casuarina species. Plant Soil 78:79–90
Zhong C, Zhang Y, Wei Y, Meng J, Chen Y, Bush D, Bogusz D, Franche C (2019) The role of Frankia inoculation in casuarinas plantations in China. Anton Leeuw 112:47–56
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghodhbane-Gtari, F., Saadaoui, M., Mohamed, I.B. et al. Alnus glutinosa seedlings grown following co-inoculation with Frankia torreyi strain CpI1 and Frankia asymbiotica strain NRRL B-16386. Symbiosis 86, 273–279 (2022). https://doi.org/10.1007/s13199-022-00845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-022-00845-0