Abstract
As key soil microorganisms of terrestrial ecosystems, arbuscular mycorrhiza fungi (AMF) play a key role in vegetation succession and mediation and stabilizing ecosystems. This study investigated the structures of AMF communities at the roots and rhizosphere soil of Vetiveria zizanioides of different ages (6-, 10-, 14-, and 17-year-old) in coal gangue heaps in Liupanshui City, Guizhou Province using high-throughput sequencing. The factors that affected the structures are also discussed. The results demonstrated that the roots and rhizosphere soil of V. zizanioides contained 109 and 173 AMF operational taxonomic units (OTUs). At the generic level, AMF communities in the roots were different from those in the rhizosphere soil. The roots of AMF communities mainly included Glomus, Dominikia and Rhizophagus, while the mainly included Glomus, Dominikia, Rhizophagus, Septoglomus and Paraglomus, Glomus and Dominikia were the dominant AMF communities in the roots and rhizosphere soil of V. zizanioides. The Shannon and Simpson diversity indexes of AMF communities in the roots and rhizosphere soil did not significantly change with V. zizanioides planting years. Redundancy analysis (RDA) revealed that the soil available phosphorus and pH were the main factors affecting AMF communities in the rhizosphere soil and roots. Our study provided references for the remedy of coal gangue heaps via mycorrhiza inoculation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to its abundant reserves, coal has been the primary energy source in China for an extended time (Chao et al., 2010; Qiu et al., 2018). However, while coal mining drives China’s economic development, it also causes severe adverse effects on the environment and society (Kompała-Bąba et al., 2019). Coal gangues are solid by-products inevitably produced during coal mining and washing (Bell et al., 2000). The accumulation of coal gangues consumes considerable land resources and destroys the landscape. In addition, toxic and harmful substances in coal gangues are discharged into the air, soil, and waterbodies by weathering and leaching, thus posing a substantial threat to ecosystems and human health (Qiu et al., 2011; Zhang et al., 2015). The ecological issues caused by coal gangue heaps have been a major hidden danger threatening sustainable economic development and regional ecological security (Liu et al., 2017). Hence, efficient yet cost-effective remediation techniques for coal gangue heaps are urgently needed.
Vetiveria zizanioides is a perennial herb in the Gramineae family. It has fast growth and reproduction characteristics, a developed root system, wide adaptability, and strong resistance. It is widely used in soil and water conservation and soil reforestation (Nero et al., 2019; Kiamarsi et al., 2020; Mondal and Patel, 2020) and is one of the main species in vegetation reforestation of coal gangue hills (Meyer et al., 2016). V. zizanioides roots form mycorrhizal symbionts with arbuscular mycorrhizal fungi (AMF) (Wong 2003; Khan 2009). The symbiont explores the soil pores that cannot be touched by the root hair and establish material transport channels between the root and the soil, promoting the absorption of nutrients by the plants (Neumann and George 2004; Gloria et al., 2012; Smith and Smith 2012; Chitarra et al., 2016). Studies have shown that arbuscular mycorrhizae secrete H+ and organic acids (such as citric acid and oxalic acid) to activate insoluble phosphate, thereby increasing the available phosphorus content in soil (Hong et al., 2002). The soil’s acid phosphatase activity increased after plant inoculation with AMF, and P uptake by plants was positively correlated with acid phosphatase content (Ma et al., 2021). In plants that form arbuscular mycorrhizae, AMF can provide up to 100% P for plants (Smith et al., 2004), and can directly absorb NH4+ (Tanaka and Yano 2010), NO3− (Bago et al., 1996), and small molecular organic nitrogen (Barrett et al., 2011) from the soil to promote plant growth. In addition, AMF improves the stress resistance of host plants by maintaining the Na+ and Cl− contents balance, increasing the SOD, POD, and CAT activities and the soluble sugar and proline contents (Garg and Aggarwal 2012; He and Huang 2013; Grümberg et al., 2015; Zhu et al., 2015).
AMF belonging to the phylum Glomeromycota are an important part of natural and agricultural ecosystems widely found in soil and can establish a mutualistic symbiotic relationship with more than 80% of land plant roots (Smith and Read, 2008; Zu et al., 2019; Zhang et al., 2020). AMF diversity affects plant community structure, diversity, and productivity in different ecosystems and plays a vital role in vegetation succession and restoration (Heijden et al., 2010). AMF community composition and diversity are affected by those of the host plant and biological and abiotic factors, which often interact (Niall and Alison 2016). Johnson et al. (2004) found that grassland plant communities had a significant impact on AMF diversity, and different host plants have their unique AMF community (Hausmann and Hawkes 2010). Compared with saprophytic soil microorganisms, the AMF abundance in soil has a more pronounced effect on the composition and diversity of plant communities (Deyn et al., 2011), and the abundance of AMF in the roots of plants with higher community richness is relatively higher (Vanessa et al., 2016).
Soil physical and chemical properties are important factors affecting AMF diversity. Recent studies on karst ecosystems have shown that AMF abundance is sensitive to N addition, while diversity is sensitive to P addition (Xiao et al., 2019). Moreover, AMF abundance and diversity are negatively correlated with total phosphorous (TP) and available phosphorus (AP) in soil organic matter, and positively correlated with K (Lin et al., 2019; Wang et al., 2021). However, other studies showed that AMF richness was positively correlated with pH, TP, and AP and negatively correlated with N (Yang et al., 2015a; Xiao et al., 2019). In addition, AMF community composition changes with seasons (Wang et al., 2021), altitude (Zhao et al., 2020), root age (Kil et al., 2014), and spatio-temporal distribution (Kezia et al., 2020). Although soil properties (soil C, N, P, C: N, C: P, N: P) greatly influence AMF diversity in the rhizosphere (Zhao et al., 2020), there is no fixed model for the effect of soil properties on AMF community. At present, there are many studies on AMF community composition and diversity, but there are few reports on AMF community structure and diversity in vetiver roots and rhizosphere soil of different planting years in mining areas.
Since 2000, our research group has planted V. zizanioides in the coal gangue hill of the Dahe Coal Mine, Zhongshan District, Liupanshui City, Guizhou Province for ecological restoration. In the previous study, we discussed the effects of vetiver planting on the physicochemical properties of heavy metals (Cu, Zn, Cd, Pb, As) in the coal gangue matrix, showing that AMF might play an important role in vetiver’s adaptation to coal gangue environment. Therefore, samples of V. zizanioides with different planting years (2002, 2005, 2009, and 2013) were collected, and the following aspects were investigated via spatiotemporal substitution: (1) the AMF communities in the roots and rhizosphere soil of V. zizanioides; (2) the effects of age on the diversity and community structure of AMF in the roots and rhizosphere soil of V. zizanioides; (3) the soil factors that affect the diversity and community structure of AMF in V. zizanioides.
2 Materials and methods
2.1 Sample collection
The sample sites were in the Dahe Mine, Liupanshui City, Guizhou Province, China (see Fig. 1). This area is characterized by a warm and humid northern subtropical monsoon climate. The annual average temperature is 14 °C, the annual precipitation is 1182.8 mm, and the frost-free period is 230-298 days. The abundant coal resources have become the local main economic pillar. Due to long-term over-exploitation, many coal gangues are exposed, resulting in a severe negative impact on the surrounding environment.
The Dahe Mine has been mined for a long time. In 2000, our research group initiated V. zizanioides mediated biological remediation of coal gangue heaps. V. zizanioides was planted on the gangue hills where coal was mined that same year. Seedlings were planted with topsoil soil and a small amount of water, and the failed seedlings were replanted after a month. After their survival, V. zizanioides and coal gangue mountain were no longer managed, and each V. zizanioides community grew in its natural state. To date, V. zizanioides communities of seven different ages are available. This study selected four vetiver communities from different planting years (2002, 2005, 2009, and 2013) as research objects. All samples were collected in March 2019. Three samples were collected from different planting years, and each sample consisted of three randomly selected V. zizanioides in good conditions. Large sand and other debris on the ground were removed, and fine root and soil samples were collected at 0-30 cm of the soil layer. The soil samples were divided into two groups: one group was stored at −80 °C for DNA extraction, and the other was naturally dried, screened by a 2-mm sieve for physicochemical properties determination. The roots were rinsed with clean water and stored at −80 °C for DNA extraction. After repeatedly rinsed with distilled water, the root samples were divided into two parts. One part was stored in a − 80 °C for DNA extraction, and the other was placed in a glass bottle containing FAA fixative solution (5 mL formaldehyde, 5 mL acetic acid and 90 mL 70% ethanol) for AMF infection rate determination.
2.2 Detection of physicochemical properties of soils
The pH value, organic matter (OM), total nitrogen (TN), total phosphorous (TP), total potassium (TK), available potassium (AK), and alkaline phosphatase (AP) contents of the soil were respectively measured using the pH acidity method, high-temperature external heat potassium dichromate oxidation capacity method, Kjeldahl method, molybdenum antimony resistance colorimetric method, flame spectrophotometry, and the NaHCO3 method (Saunders and Williams 1955; Dormaar 1964; Steward and Oades, 2006).
2.3 AMF spore density and mycorrhizal colonization rate
2.3.1 AMF spore extraction
AMF spores in the rhizospheric soil were separated by wetsieving and decanting-sucrose centrifugation (Gerdemann and Nicolson 1963). The rhizospheric soil (10 g per sample) was suspended in distilled water and filtered through two sieves (upper 500 μm; middle 106 μm; lower 45 μm). The residue in the lower sieve was transferred to a 100 mL centrifuge tube and centrifuged (3000 rpm, 3 min). After discarding the supernatant, a sucrose solution was added to the centrifuge tube and mixed well. Then the mixture was centrifuged (1500 rpm, 1.5 min). The supernatant was sifted immediately through a clean sieve (45 μm). The residue on the sieve was washed by distilled water to ensure that no sucrose solution remained in the residue, then washed into a petri dish and examined with a dissecting microscope. The spore density was as:
2.3.2 AMF colonization rate
The AMF colonization rate was determined using the acid fuchsin stain (Phillips and Hayman 1970). V. zizanioides roots stored in FAA fixating solution were taken out, washed with distilled water and cut into 1-cm-long segments, which were put in 5% w/v KOH solution and heated at 90 °C for 30 min in a water bath until they were completely transparent. Hydrochloric acid at 2% v/v was then added to neutralize the KOH. The root segments were placed in the acid fuchsin solution and heated at 90 °C for 20 min in a water bath. After samples were washed in distilled water, they were placed in a lactic acid–glycerin solution to decolorize the tissue. The decolorized root segment was observed under a microscope on a slide, and the infection rate was determined by root segment method (Biermann and Linderman 1981). The mycorrhizal colonization rate was determined as:
Percentage of mycorrhizal colonization % = (number of mycorrhizal root segments observed) / (total number of root segments observed) × 100.
2.4 Detection of AMF molecule diversity of roots and rhizosphere soil
2.4.1 DNA extraction, PCR, and sequencing
The total genomic DNA from the roots and rhizosphere soil samples was extracted using the cetyltrimethylammonium bromide (CTAB) method. DNA concentration and purity were monitored on 1% agarose gels. According to the concentration, DNA was diluted to 1 ng/μL using sterile water.
Using diluted genomic DNA as template, the nuclear ribosomal internal transcribed spacer region (ITS rDNA gene) was amplified by polymerase chain reaction (PCR) using the fungal primer set for ITS1-1F-F (5’-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS1-1F-R (5’-GCTGCGTTCTTCATCGATGC-3′) (Liang et al., 2020; Wang et al., 2020). All PCR reactions were carried out in a 30 μL total volume with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of forward and reverse primers, and 10 ng of template DNA. Thermal cycling consisted of initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, elongation at 72 °C for 30 s, and final extension at 72 °C for 5 min. The same volume of 1X loading buffer (SYBR green) was mixed with PCR products, and electrophoresis was performed on 2% agarose gel for detection. Then, the mixed PCR products were purified with a Gene JET Gel Extraction Kit (Thermo Scientific).
Sequencing libraries were generated using an Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific) following the manufacturer’s recommendations. The library quality was assessed on a Qubit® 2.0 Fluorometer (Thermo Scientific). Finally, the library was sequenced on an Ion S5TM XL platform, and 400 bp/600 bp single-end reads were generated.
2.4.2 Processing of sequencing data
Single-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Quality filtering on the raw reads was performed under specific conditions to obtain high-quality clean reads according to the Cut adapt quality-controlled process. The reads were compared with the reference database using the UCHIME algorithm to detect chimera sequences, then removed. The clean reads ultimately obtained by the sequencing analysis were analyzed by UPARSE software. Sequences with ≥97% similarity were assigned to the same OTU. A representative sequence for each OTU was screened for further annotation. The UNITE Database was used to annotate taxonomic information for each representative sequence based on the Blast algorithm, calculated by QIIME software (version 1.9.1). Multiple sequence alignments were conducted using MUSCLE software (version 3.8.31) to study the phylogenetic relationships of different OTUs and the differences of the dominant species in different samples (groups). The OTU abundance information was normalized using a standard sequence number corresponding to the sample with the fewest sequences. All subsequent alpha and beta diversity analyses were performed based on this output normalized data. Alpha diversity was applied to analyze the species diversity complexity for a sample via 5 indices, namely Chao1, Shannon, Simpson, ACE, and Good’s coverage. All indices were calculated with the QIIME software (version 1.7.0) and displayed with R software (version 2.15.3).
2.5 Statistical analysis
SPSS 18.0 was used for statistical data analysis. The single-factor analysis of variance and Duncan’s test were used to determine the significance of differences in soils’ physicochemical properties and diversity indices in different planting years. Mantel-test was used to test the significant difference of AMF spore density and infection rate with planting years and soil physical and chemical properties. The Alpha diversity index was calculated using QIIME (version 1.7.0) software. The bar chart of the community compositions, which was generated using SigmaPlot 12.5, reflected the abundances of AMF communities in different plots. Non-metric multidimensional scaling (NMDS) using R software reflected the differences in the structures of AMF communities in samples of different ages. The relationships between the structures and diversity of AMF communities and the physicochemical properties of soils were investigated using redundancy analysis (RDA).
3 Results
3.1 Changes in soil properties across different planting years
The main properties of the soil samples collected from all selected sites are presented in Table 1. The soil samples were all slightly alkaline, with the pH of all study sites ranging from 7.21 (6 years) to 8.48 (17 years). The soil’s OM, TN, and TP were maximized in the 14- year-old samples and minimized 17-year-old samples. The TK and AK of the soil decreased and then increased as the sample age. The AP in the soil decreased with planting time was significantly lower inthe17-year-old soil sample than those in the 6- and 10-year-old soil samples (P < 0.05).
3.2 The AMF spore density and mycorrhizal colonization
Many AMF spores were isolated from V. zizanioides rhizosphere soils, ranging from 104.33 (10 years) to 462.67 (17 years) spores per 10 g of soil. The spore density was significant in each planting year (P < 0.05) (Fig. 2), and significantly decreased and increased with increasing OM and pH, respectively (P < 0.01). However, no significant correlation could be found between spore density and soil TP, TK, AP levels (P>0.05) (Table 2). All the samples obtained were colonized by AMF and formed typical arbuscular structures, ranging from 48.66% (16 years) to 65.43% (10 years) (Fig. 2). Furthermore, no significant correlation could be found between mycorrhizal colonization and soil properties (P>0.05). Overall, AMF spore density first decreased and then increased with planting years, and mycorrhizal colonization was opposite to spore density. In addition, planting years were significantly correlated with spore density (P < 0.05) and no significantly correlated with mycorrhizal colonization (P>0.05) (Table 2).
3.3 Analysis of sequencing results and dilution curves
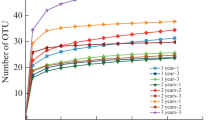
High-throughput sequencing of the roots and rhizosphere soil of V. zizanioides in the four plots generated 79,487 valid data points after quality control averaging. The sequences were clustered into OTUs with 97% similarity, and 178 AMF OTUs were obtained. The rhizosphere soil contained 173 OTUs in total, with 112, 92, 131, and 103 OTUs in the 6-, 10-, 14-, and 17-year-old samples, respectively. The roots contained 109 OTUs in total, with 86, 78, 61, and 100 OTUs in the 6-, 10-, 14-, and 17-year-old samples, respectively (Fig. 3). Data of a specific sequencing volume were randomly extracted from samples. In the dilution curves presented in Fig. 4, the x-axis refers to the number of sequences extracted, and the y-axis refers to the quantity of OTUs that can be established based on this sequence quantity. The curve was saturated as the sequence quantity increased, suggesting that the sequencing volume was sufficient. This sequencing reflected the diversity of AMF in the roots and rhizosphere soil of V. zizanioides of all ages, and the population abundance of the rhizosphere soil was higher than that of the roots.
3.4 The structures of AMF communities
3.4.1 Compositions of AMF communities in roots and rhizosphere soil of V. zizanioides
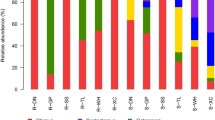
Figure 5 illustrates the compositions of AMF communities in the roots and rhizosphere soil of V. zizanioides of different planting years at the genus level. In the four plots, the roots’ AMF communities mainly consisted of Glomus, Dominikia, and Rhizophagus. Those in rhizosphere soil mainly consisted of Glomus, Dominikia, Rhizophagus, Septoglomus, and Paraglomus. Dominikia was the dominant genus in the roots (96.38%) and rhizosphere soil (95.77%) in the 17-year-old V. zizanioides sample. Glomus was the dominant genus in the roots and rhizosphere soil of the other samples. Septoglomus was only observed at the root of the 17-year-old sample (0.18%); Diversispora was only observed at the rhizosphere soil of the 10-year-old sample (0.38%).
3.4.2 Diversity of AMF communities in roots and rhizosphere soil of V. zizanioides
Calculations of the AMF communities’ diversity in the roots and rhizosphere soil of V. zizanioides in the four plots revealed that the Shannon and Simpson indices of the rhizosphere soil were minimized in the 17- and 10-year-old samples, respectively, and maximized in the 6-year-old sample. Additionally, samples of different ages exhibited negligible differences (P > 0.05). The Chao1 and ACE indices values decreased with sample age, while those of the 17-year-old sample were significantly lower than those of the 6- and 10-year-old samples (P < 0.05). The Shannon, Simpson, Chao1, and ACE indices of the root samples of different ages exhibited negligible differences. Additionally, the coverage indices of roots and rhizosphere soil of all samples were above 97% (Table 3).
3.5 NMDS analysis of AMF communities in roots and rhizosphere soil of V. zizanioides of different planting years
PERMANOVA analysis revealed that the AMF communities in the roots and rhizosphere soil of V. zizanioides were significantly different (P < 0.01), but that there were no significant differences among planting years (P > 0.05). NMDS analysis demonstrated that the AMF communities in the roots and rhizosphere soil varied along the first axis from the 6- to the 17-year-old sample. For samples of different planting years, compositions of the AMF communities in the roots and rhizosphere soil were different, and these communities were distributed in different locations (Fig. 6).
3.6 Diversity of AMF communities and physicochemical properties of soils
Spearman’s correlation analysis (Table 4) indicated that the Simpson index of the rhizosphere soil of V. zizanioides was significantly positively related to the TP content (P < 0.05). The Chao1 and ACE indices were significantly positively related to the AP content (P < 0.05). The Shannon and Simpson indices were significantly negatively related to the pH value of soil (P < 0.05). The four diversity indices of the roots were negatively related to the OM and TN contents and positively related to TP and TK contents.
Due to the different structures of the AMF communities in the rhizosphere soil and roots of V. zizanioides (Fig. 5), the correlations between the AMF and the physicochemical properties of the soil were investigated by RDA (Fig. 7). As presented in Fig. 7a, the cumulative interpretation rate for both axes was 61.08%. The first axis (RDA1) had an interpretation rate of 34.95% and was significantly positively related to the TP, AP, OM, and TN. The second axis (RDA2) had an interpretation rate of 26.13% and was significantly negatively related to the OM, TN, and TP content. The soil’s pH value, AP, and OM were found to have the most significant effects on the diversity of AMF communities in the rhizosphere soil. As shown in Fig. 7b, the accumulated interpretation rate of the two axes was 78.72%. The first axis (RDA1) had an interpretation rate of 45.54% and was significantly positively related to the pH value of the soil. The second axis (RDA2) had an interpretation rate of 33.18% and was significantly negatively related to the TP content. The pH value of the soil, TP and AP contents were found to have the most significant effects on the diversity of AMF communities in the roots.
4 Discussion
In this study, the structures of AMF communities in the roots and rhizosphere soil of V. zizanioides of different planting years (6-, 10-, 14-, and 17-year-old) in coal gangue heaps were investigated using high-throughput sequencing. A total of 178 AMF OTUs of Dominikia, Diversispora, Glomus, Paraglomus, Rhizophagus, Septoglomus, Rhizophagus, and unidentified_Glomeromycota were obtained. In the 6-, 10-, and 14-year-old roots and rhizosphere soil samples of V. zizanioides, Glomus was dominant with contents between 34.37% and 94.95%. Yang et al. (2015) showed that Glomus was the dominant genus in the Robinia pseudoacacia AMF communities in the roots and rhizosphere soil of in a lead-zinc mine. Mehrotra (1998) investigated AMF communities in an Indian coal mine via morphological methods and claimed that the Glomus genus was dominant. The present study further demonstrated that the Glomus genus was widely observed in coal mines in the Karst areas in China, suggesting that it has strong adaptability in coal gangue heaps. Indeed, this genus can reproduce directly through hypha and mycorrhiza and has resistance to various adverse environments (Hassan et al., 2011). Compared with other AMF species, the reproduction strategy of Glomus is more suitable for survival in coal gangue heaps. Interesting finding in our analyses was that the number of Dominikia genus increases with planting age and became dominant genus in the roots and rhizosphere soil of V. zizanioides of the age of 17-year-old. Colombo et al. (2020) studied arbuscular mycorrhizal fungi in soils heavily contaminated with heavy metals in the Riachuelo River basin and found that Dominikia genus is one of the most representative arbuscular mycorrhizal fungi with a relative abundance of 26.5%. Dominikia genus may be an AMF with strong tolerance to heavy metals. Therefore, the reproduction pattern of the Dominikia genus in coal gangues and its effect on V. zizanioides requires further investigation. The changes of relative abundance between roots and rhizosphere soil in different planting years demonstrated that the sample age had a significant effect on the dominant genus of AMF communities the in roots and rhizosphere soil of V. zizanioides. However, Herrmann et al. (2016) claimed that the sample age has negligible effects on the dominant genus of AMF in the soil of a rubber tree plantation in Thailand. These different conclusions may be attributed to multiple factors such as the type of host plant, the physicochemical properties of the soils, and the climate.
In general, AMF communities were generally not characteristic of individual plant species, but those associated with ecological groups of plant species – habitat generalists and forest specialists – were nonrandom subsets of the available pool of fungal taxa (Davison et al., 2011). In this study, the OTU quantity of the rhizosphere soil was higher than the roots, and the AMF communities’ compositions in the roots and rhizosphere soil were significantly different, demonstrating that AMF colonizing the roots of V. zizanioides may be a subgroup of AMF communities in rhizosphere soil. This is consistent with previous studies (Sheng et al., 2017), although other researchers have claimed that the diversity of AMF communities in rhizosphere soil is no greater than that of AMF communities in roots (Verbruggen et al., 2012; Saks et al., 2014; Deepika and Kothamasi, 2015). This may be attributed to three reasons. First, the identification of AMF communities in the roots of V. zizanioides has excluded dormant spore propagules, extra rhizomes, and dead mycorrhiza segments (Liu et al., 2015). Secondly, host plants prefer some AMF genera (De Souza and Santos, 2018). The V. zizanioides in this study may have preferred AMF genera in the rhizosphere soil, resulting in a low diversity of AMF communities in the roots. Finally, the relative abundances of the internal or external structures of AMF groups are different, and spore generation is seasonal (Bever et al., 2001; Jansa et al., 2002).
The time scale and physicochemical properties of soils are the dominant factors that affect the structures of AMF communities (Helgason et al., 2014; Alguacil et al., 2015). Cui et al. (2016) reported that the vegetation succession period affects the diversity and abundancy indices of AMF. In this study, the AMF diversity index in the V. zizanioides rhizosphere soil decreased, while in the roots increased as with planting years, demonstrating that the AMF diversity is affected by the planting years. The Simpson index of the rhizosphere soil was significantly positively related to the TP content and significantly negatively related to the pH value of soil. Chao1 and ACE indices were significantly positively related to the AP content, suggesting that the pH value of soil, TP and AP contents have significant effects on the structures of AMF communities in the rhizosphere soil of V. zizanioides. Previous studies have revealed that a high P content and low pH value of soil would inhibit spore germination and mycelial growth, thus hindering AMF growth and development. Hence, these factors directly or indirectly affect the construction of AMF communities (Bever et al., 2001; Hart et al., 2001). Additionally, heavy metal content is also a key factor that affects the structures of AMF communities (Faggioli et al., 2019). Our previous study revealed that Cu and Zn were the main heavy metals in the coal gangue heap. Yang et al. (2015) believed that Zn was the dominant factor that affects the structure of AMF communities in the roots and rhizosphere soil of R. pseudoacacia. Therefore, to fully understand the relationship between the local environment of coal gangue heaps and AMF communities, the relationship between the heavy metal content and diversity of AMF communities in coal gangues will be investigated in future research. RDA also demonstrated that the available phosphorus in the soil and the pH value of soil have the most significant effects on the diversity of AMF communities in the rhizosphere soil and roots. Be Enhouwer et al. (2015) studied AMF communities in soils under Arabica coffee trees in Ethiopia and demonstrated that the soil’s AP in the soil and the pH value significantly affect AMF communities’ diversity.
In summary, this study discussed the structures and diversity of AMF communities in the roots and rhizosphere soil of V. zizanioides in coal gangue heaps in Liupanshui, China. The results demonstrated that the diversity of AMF communities in rhizosphere soil was superior to that in the roots. Glomus and Dominikia were found to be the dominant genera of AMF communities in the rhizosphere soil and roots of V. zizanioides in coal gangue heaps, and the available phosphorus and pH value of the soil were found to be the dominant factors that affect AMF communities in the rhizosphere soil and roots. Additionally, the heavy metal content and activity of microorganisms and enzymes in coal gangues affect AMF communities. Future studies will investigate these factors, as will the reproduction pattern of identified Dominikia in coal gangue heaps.
References
Alguacil MM, Torrecillas E, Lozano Z, Roldán A (2015) Arbuscular mycorrhizal fungi communities in a coral cay system (morrocoy, venezuela) and their relationships with environmental variables -ScienceDirect [J]. Sci Total Environ 505:805–813. https://doi.org/10.1016/j.scitotenv.2014.10.030
Bago B, Vierheilig H, Piche Y, Azcon-Aguilar C (1996) Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic culture [J]. New Phytol 133(2):273–280. https://doi.org/10.1111/j.1469-8137.1996.tb01894.x
Barrett G, Campbell CD, Fitter AH, Hodge A (2011) The arbuscular mycorrhizal fungus Glomus hoi can capture and transfer nitrogen from organic patches to its associated host plant at low temperature [J]. Appl Soil Ecol 48(1):102–105. https://doi.org/10.1016/j.apsoil.2011.02.002
Be Enhouwer MD, Muleta D, Peeters B, Van Geel M, Lievens B, Honnay O (2015) DNA pyrosequencing evidence for large diversity differences between natural and managed coffee mycorrhizal fungal communities [J]. Agron Sustain Dev 35:241–249. https://doi.org/10.1007/s13593-014-0231-8
Bell FG, Stacey TR, Genske DD (2000) Mining subsidence and its effect on the environment: some differing examples [J]. Environ Geol 40(1-2):135–152. https://doi.org/10.1007/s002540000140
Bever JD.; Schultz P A.; Pringle A.; Morton J B. Arbuscular Mycorrhizal Fungi: More Diverse than Meets the Eye, and the Ecological Tale of Why. (cover story) [J]. BioScience, 2001, 51, 923-931. Doi: https://doi.org/10.1641/0006-3568(2001)051[0923:AMFMDT]2.0.CO;2
Biermann B, Linderman RG (1981) Quantifying vesicular-arbuscular mycorrhizae: a proposed method towards standardization [J]. New Phytol 87(1):63–67. https://doi.org/10.1111/j.1469-8137.1981.tb01690.x
Chao L, Wan J, Sun H, Li L (2010) Investigation on the activation of coal gangue by a new compound method [J]. Journal Of Hazardous Mater 179:515–520. https://doi.org/10.1016/j.jhazmat.2010.03.033
Chitarra W.; Pagliarani C.; Maserti B.; Lumini E.; Siciliano I & Ca Scone P.; Schubert A.; Gambino G.; Balestrini R.; Guerrieri E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress [J]. Plant physiology, 2016: pp. 00307. 2016. Doi:https://doi.org/10.1104/pp.16.00307
Colombo RP, Benavidez ME, Bidondo LF, Silvani VA, Bompadre MJ, Statello M, Scorza MV, Scotti A, Godeas AM (2020) Arbuscular mycorrhizal fungi in heavy metal highly polluted soil in the Riachuelo river basin – ScienceDirect [J]. Rev Argent Microbiol 52(2):145–149. https://doi.org/10.1016/j.ram.2019.05.001
Cui XC, Hu JL, Wang JH, Yang JS, Lin XG (2016) Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in eastern China as revealed by Illumina sequencing [J]. Appl Soil Ecol 98:140–149. https://doi.org/10.1016/j.apsoil.2015.10.008
Davison J, Opik M, Daniell TJ, Moora M, Zobel M (2011) Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages: selectivity in AMF-plant associations [J]. FEMS Microbiol Ecol 78:103–115. https://doi.org/10.1111/j.1574-6941.2011.01103.x
De Souza TAF, Santos D (2018) Effects of using different host plants and long-term fertilization systems on population sizes of infective arbuscular mycorrhizal fungi [J]. Symbiosis 76:139–149. https://doi.org/10.1007/s13199-018-0546-3
Deepika S, Kothamasi D (2015) Soil moisture-a regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake [J]. Mycorrhiza 25:67–75. https://doi.org/10.1007/s00572-014-0596-1
Deyn GBD, Quirk H, Bardgett RD (2011) Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility [J]. Biol Lett 7(1):75–78. https://doi.org/10.1098/rsbl.2010.0582
Dormaar JF (1964) Evaluation of methods for determination of total organic phosphorus in chernozemic soils of southern Alberta [J]. Can J Soil Sci 44(3):265–271. https://doi.org/10.4141/cjss64-040
Faggioli V, Menoyo E, Geml J, Kemppainen M, Pardo A, Salazar MJ, Becerra AG (2019) Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities [J]. Mycorrhiza 29:363–373. https://doi.org/10.1007/s00572-019-00895-1
Garg N, Aggarwal N (2012) Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. Genotypes grown in cadmium and lead contaminated soils [J]. Plant Growth Regulation: An International Journal on Natural and Synthetic Regulators 66(1):9–26. https://doi.org/10.1007/s10725-011-9624-8
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting [J]. Trans Br Mycol Soc 46(2):235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Gloria B, Ricardo A, Antonio PJ, François C, Carmen MBM, Micaela C, Manuel RLJ (2012) Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions [J]. Ann Bot 5:1009–1017. https://doi.org/10.1093/aob/mcs007
Grümberg BC, Urcelay C, Shroeder MA, Vargas-Gil S, Luna MC (2015) The role of inoculum identity in drought stress mitigation by arbuscular mycorrhizal fungi in soybean [J]. Biol Fertil Soils 51(1):1–10. https://doi.org/10.1007/s00374-014-0942-7
Hart MM, Reader RJ, Klironomos JN (2001) Life-history strategies of arbuscular mycorrhizal fungi in relation to their successional dynamics [J]. Mycologia 93:1186–1194. https://doi.org/10.2307/3761678
Hassan SD, Boon E, St-Arnaud M, Hijri M (2011) Molecular biodiversity of arbuscular mycorrhizal fungi in trace metal-polluted soils [J]. Mol Ecol 20:3469–3483. https://doi.org/10.1111/j.1365-294x.2011.05142.x
Hausmann NT, Hawkes CV (2010) Plant neighborhood control of arbuscular mycorrhizal community composition [J]. New Phytol 183(4):1188–1200. https://doi.org/10.1111/j.1469-8137.2009.02882.x
He ZQ, Huang Z (2013) Expression analysis of LeNHX1 gene in mycorrhizal tomato under salt stress [J]. J Microbiol 51(1):100–104. https://doi.org/10.1007/s12275-013-2423-3
Heijden M, Ba Rdgett RD, Straalen N (2010) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems [J]. Ecol Lett 11(3):296–310. https://doi.org/10.1111/j.1461-0248.2007.01139
Helgason T, Feng H, Sherlock D, Young J, Fitter A (2014) Arbuscular mycorrhizal communities associated with maples (Acer spp.) in acommon garden are influenced by season and host plant [J]. Botany-Botanique 92:321–326. https://doi.org/10.1139/cjb-2013-0263
Herrmann L, Lesueur D, Br UL, Davison J, Jairus T, Robain H, Robain A, Vasar M, Wiriyakitnateekul W, Öpik M (2016) Diversity of root-associated arbuscular mycorrhizal fungal communities in a rubber tree plantation chronosequence in Northeast Thailand [J]. Mycorrhiza 26:863–877. https://doi.org/10.1007/s00572-016-0720-5
Hong S, Yan X, Ming Z, Zheng S, Wang X (2002) Exudation of organic acids in common bean as related to mobilization of aluminum- and iron-bound phosphates [J]. Environ Exp Bot 48(1):1–9. https://doi.org/10.1016/S0098-8472(02)00009-6
Jansa J, Mozafar A, Anken T, Ruh R, Sanders I, Frossard E (2002) Diversity and structure of AMF communities as affected by tillage in a temperate soil [J]. Mycorrhiza 1:225–234. https://doi.org/10.1007/s00572-002-0163-z
Johnson D, Vandenkoornhuyse PJ, Leake JR, Gilbert L, Booth RE, Grime JP, Young JPW, Read DJ (2004) Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms [J]. New Phytol 161(2):503–515. https://doi.org/10.1046/j.1469-8137.2003.00938.x
Kezia G, Run SB, Sandra K, Kathleen MR, Anna H, Markus F, Daniel P, Hans-Peter P, Doreen B, Sven M, Ellen K, Francois B, Tesfaye W (2020) Unraveling spatiotemporal variability of arbuscular mycorrhizal fungi in a temperate grassland plot [J]. Environ Microbiol 22(3):873–888. https://doi.org/10.1111/1462-2920.14653
Khan AG (2009) Role of vetiver grass and arbuscular mycorrhizal Fungi in improving crops against abiotic stresses [M]. Springer Netherlands 44:111–116. https://doi.org/10.1007/978-1-4020-9065-312
Kiamarsi Z, Kafi M, Soleimani M, Nezami A, Lutts S (2020) Conjunction of Vetiveria zizanioides L. and oil-degrading bacteria as a promising technique for remediation of crude oil-contaminated soils [J]. J Clean Prod:253. https://doi.org/10.1016/j.jclepro.2019.119719
Kil YJ, Eo JK, Lee EH, Eom AH (2014) Root age-dependent changes in arbuscular mycorrhizal fungal communities colonizing roots of Panax ginseng [J]. Mycobiology 42(4):416–421. https://doi.org/10.5941/MYCO.2014.42.4.416
Kompała-Bąba A, Bierza W, Błońska A, Sierka E, Magurno F, Chmura D, Besenyei L, Radosz L, Woźniak G (2019) Vegetation diversity on coal mine spoil heaps -how important is the texture of the soil substrate [J]. Biologia 74:419–436. https://doi.org/10.2478/s11756-019-00218-x
Liang M, Johnson D, Burslem DFRP, Yu S, Fang M, Taylor JD, Taylor AFS, Helgason T, Liu X (2020) Soil fungal networks maintain local dominance of ectomycorrhizal trees [J]. Nat Commun 11(1):2636. https://doi.org/10.1038/s41467-020-16507-y
Lin Y, He YJ, He MH, Wu CY, Fang Z, Han X, Xu XY, Wang S (2019) Species diversity of soil arbuscular mycorrhizal fungi in karst vegetation succession process [J]. Acta Ecologica Sinica in Chinese 11:4127–4137. https://doi.org/10.5846/stxb201807061475
Liu B, Tang Z, Dong S, Wang L, Liu D (2017) Vegetation recovery and groundwater pollution control of coal gangue field in a semi-arid area for a field application [J]. Int Biodeterior Biodegradation 128:134–140. https://doi.org/10.1016/j.ibiod.2017.01.032
Liu YJ, Johnson NC, Mao L, Shi GX, Jiang SJ, Ma XJ, Du GZ, An LZ, Feng HY (2015) Phylogenetic structure of arbuscular mycorrhizal community shifts in response toincreasing soil fertility [J]. Soil Biol Biochem 89:196–205. https://doi.org/10.1016/j.soilbio.2015.07.007
Ma J, Ma Y, Wei Z, Wu J, Sun C, Yang J, Liu L, Liao H, Chen T, Huang J (2021) Effects of arbuscular mycorrhizal fungi symbiosis on microbial diversity and enzyme activities in the rhizosphere soil of Artemisia annua [J]. Soil Sci Soc Am J 85(3):703–716. https://doi.org/10.1002/saj2.20229
Mehrotra VS (1998) Arbuscular mycorrhizal associations of plants colonizing coal mine spoil in India [J]. J Agric Sci 130:125–133. https://doi.org/10.1017/S0021859697005091
Meyer E, Londoño DMM, Armas RDD, Giachini AJ, Rossi MJ, Stoffel SC, Soares CR (2016) Arbuscular mycorrhizal fungi in the growth and extraction of trace elements by Chrysopogon zizanioides (vetiver) in a substrate containing coalmine wastes [J]. International Journal of Phytoremediation 19:113–120. https://doi.org/10.1080/15226514.2016.1207596
Mondal S.; Patel P P. Implementing Vetiver grass-based riverbank protection programmes in rural West Bengal, India [J]. Natural Hazards, 2020(1). Doi: https://doi.org/10.1007/s11069-020-04025-5
Nero B F.; Amponsah P.; Antobre O O.; Owusu-Prempeh N.; Acquah E. (2019). Phytoremediation of petroleum hydrocarbon-contaminated soils with Jatropha curcas and [C]. Conference on International Research on Food Security, Natural Resource Management and Rural Development, 2019
Neumann E, George E (2004) Colonisation with the arbuscular mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.) enhanced phosphorus uptake from dry soil in Sorghum bicolor (L.) [J]. Plant & Soil 261(1-2):245–255. https://doi.org/10.1023/B:PLSO.0000035573.94425.60
Niall SM, Alison EB (2016) Stressed out symbiotes: hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi [J]. Oecologia 182(3):625–641. https://doi.org/10.1007/s00442-016-3673-7
Phillips JM, Hayman DS (1970) Improved producers for clearing roots and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55(1):158–161. https://doi.org/10.1016/s0007-1536(70)80110-3
Qiu L, Bi YL, Jiang B, Wang Z, Zhang Y, Zhakypbek Y (2018) Arbuscular mycorrhizal fungi ameliorate the chemical properties and enzyme activities of rhizosphere soil in reclaimed mining subsidence in northwestern China [J]. Journal of Arid Land 11:135–147. https://doi.org/10.1007/s40333-018-0019-9
Qiu YL, Zhang XX, Liu KP, Hu XY, Guan BW (2011) Research on mechanical behaviour of cement mortar with high-volume coal gangue [J]. Adv Mater Res 261-263:685–689. https://doi.org/10.4028/www.scientific.net/amr.261-263.685
Saks Ü, Davison J, Öpik, Maarja, Vasar M, Moora M, Zobel M (2014) Root-colonizing and soil-borne communities of arbuscular mycorrhizal fungi in a temperate forest understory [J]. Botany 92:277–285. https://doi.org/10.1139/cjb-2013-0058
Saunders WMH, Williams EG (1955) Observations on the determination of total organic phosphorus in soils [J]. J Soil Sci 6:254–267. https://doi.org/10.1111/j.1365-2389.1955.tb00849.x
Sheng M, Chen X, Zhang X, Hamel C, Cui X, Chen J, Tang M, Cui X, Hamel C (2017) Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties [J]. Sci Total Environ 599–600:273–283. https://doi.org/10.1016/j.scitotenv.2017.04.199
Smith SE; Read D. Mycorrhizal symbiosis. 3rd ed. San Diego: Academic Press; 2008
Smith SE, Smith FA (2012) Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth [J]. Mycologia 104(1):1–13. https://doi.org/10.3852/11-229
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake [J]. New Phytol 162(2):511–524. https://doi.org/10.1111/j.1469-8137.2004.01039.x
Steward JH, Oades JM (2006) The determination of organic phosphorus in soils [J]. Eur J Soil Sci 23(1):38–49. https://doi.org/10.1111/j.1365-2389.1972.tb01639.x
Tanaka Y, Yano K (2010) Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied [J]. Plant Cell and Environment 28(10):1247–1254. https://doi.org/10.1111/j.1365-3040.2005.01360.x
Vanessa R.; Laura B M G.; Laura S.; Pedro M A. Composition of fungal soil communities varies with plant abundance and geographic origin [J]. AoB Plants, 7, 2016(2015-9-14), 2015. Doi: https://doi.org/10.1093/aobpla/plv110
Verbruggen E, Heijden MGAVD, Weedon JT, Kowalchuk GA, Rling WFM (2012) Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils [J]. Mol Ecol 21:2341–2353. https://doi.org/10.1111/j.1365-294x.2012.05534.x
Wang HQ; Cheng W; Hao Jun; Mao Y Y; Lu Q; Gu T Y; Li D H. Seasonal dynamic changes of community of arbuscular mycorhizal fungi (AMF) in root system and thizosphere soil of Vetiveria zizanioides in coal gangue in Guizhou of Southwest China [J]. Mycosystema in Chinese, 2021, 40(03): 514-530. Doi: https://doi.org/10.13346/j.mycosystema.200293
Wang X, Zhu X, Bi Y, Zhao R, Nie Y, Yuan W (2020) Dynamics of microbial community and changes of metabolites during production of type Ι sourdough steamed bread made by retarded sponge-dough method [J]. Food Chem 330(5):127316. https://doi.org/10.1016/j.foodchem.2020.127316
Wong C C. The role of mycorrhizae associated with Vetiveria zizaniodes and Cyperus polystachyos in the remediation of metals (lead and zinc) contaminated soils. M. Phil thesis, Hong Kong Baptist University, Hong Kong, 2003
Xiao D, Che R, Liu X, Tan Y, Yang R, Zhang W, He X, Xu Z, Wang K (2019) Arbuscular mycorrhizal fungi abundance was sensitive to nitrogen addition but diversity was sensitive to phosphorus addition in karst ecosystems [J]. Biol Fertil Soils. https://doi.org/10.1007/s00374-019-01362-x
Yang CX, Chen F, Yue YN, Yan XF (2015) Diversity characteristics of arbuscular mycorhizal fungi in the thizosphere of twenty six species of plants in Songnen saline-alkaline grassland [J]. Pratacultual Science in Chinese, 2015a, 32(12): 2008-2020. Doi: https://doi.org/10.11829/j.issn.1001-0629.2015-0232
Yang Y, Song Y, Scheller HV, Ghosh A, Ban Y, Chen H, Tang M (2015) Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils [J]. Soil Biol Biochem 86:146–158. https://doi.org/10.1016/j.soilbio.2015.03.018
Zhang RZ, Mu Y, Li XR, Li SM, Sang P, Wang XR, Wu HL, Xu N (2020) Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates [J]. Sci Total Environ 740. https://doi.org/10.1016/j.scitotenv.2020.139810
Zhang YY, Yanxia Guo YX, Cheng FQ, Yan KZ, Cao Y (2015) Investigation of combustion characteristics and kinetics of coal gangue with different feedstock properties by thermogravimetric analysis [J]. Thermochimica Acta An International Journal Concerned with the Broader Aspects of Thermochemistry & Its Applications to Chemical Problems 614:137–148. https://doi.org/10.1016/j.tca.2015.06.018
Zhao F, Feng X, Guo Y, Ren C, Wang J, Doughty R (2020) Elevation gradients affect the differences of arbuscular mycorrhizal fungi diversity between root and rhizosphere soil [J]. Agric For Meteorol. https://doi.org/10.1016/j.agrformet.2019.107894
Zhu XC, Song FB, Liu FL, Tian CJ (2015) Carbon and nitrogen metabolism in arbuscular mycorrhizal maize plants under low-temperature stress [J]. Crop and Pasture Science 66(1):62–70. https://doi.org/10.1071/CP14159
Zu Y, Ping Y, Mu L, Yang T (2019) The diversity of arbuscular mycorrhizal fungi of Rosa acicularis ‘Luhe’ in saline areas [J]. J For Res 30(04):1507–1512. https://doi.org/10.1007/s11676-018-0748-9
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (31500451), the Science and Technology Support (Agriculture) Project of Guizhou Province ([2017] 2504-2) and the Science and Technology Cooperation Support Project of Guizhou Province ([2020]1Y046) for financial support for this study. The experiment was supported by the Grassland Science Laboratory of Guizhou University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, T., Mao, Y., Chen, C. et al. Diversity of arbuscular mycorrhiza fungi in rhizosphere soil and roots in Vetiveria zizanioides plantation chronosequence in coal gangue heaps. Symbiosis 86, 111–122 (2022). https://doi.org/10.1007/s13199-022-00829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-022-00829-0