Abstract
Improving symbiotic nitrogen fixation would reduce reliance on synthetic fertilizers, but establishment of effective bacterial strains is hampered due to competition by indigenous, less effective rhizobia. This study investigated the origins, diversity, and competitiveness of Sinorhizobium meliloti (syn. Ensifer meliloti) strains isolated from root nodules of alfalfa (lucerne; Medicago sativa L.) grown in soils that had not been in alfalfa cultivation for over 30 years. Sets of PCR primers were developed to identify Sinorhizobium spp. and to identify strains with virD 4 , the defining gene for a type IV secretion system (T4SS), which has been implicated in increasing strain competitiveness for nodule colonization of M. truncatula, an annual species closely related to alfalfa. A collection of S. meliloti isolates was made from nodules of field-grown plants and from seeds used for establishing field plots that had been inoculated with rhizobia by the manufacturer prior to packaging. Diversity among strains was examined by repetitive element palindromic PCR (rep-PCR) DNA fingerprinting using the BOXA1R primer. Strains originating from the seed inoculant could not be detected in nodules, even in the first year of alfalfa establishment, which were occupied exclusively by indigenous rhizobia originating from soil. Field strains were very diverse within and among field sites, and genetically distinct from inoculant strains. Approximately 33% of field strains were positive for virD 4 , a component of the T4SSb gene cluster, which putatively mobilizes effector proteins across the bacterial cell envelop into the host cell. Strains with virD 4 , the presence of which suggests that they have a functional T4SS, had more rapid nodulation kinetics than did those lacking virD 4 . These results provide additional support for the role of the S. meliloti T4SS in competitiveness for nodule occupancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perennial forage legumes are an important source of nitrogen (N) in cropping systems worldwide. Prior cultivation of alfalfa (lucerne; Medicago sativa L.) can offset the need for N-amendments in rotational cash crops, including first-year corn (Zea mays L.) and, in some cases, second-year corn in the Upper Midwestern U.S. (Yost et al. 2012, 2014). The majority of the N returned to the soil is the product of biological dinitrogen (N2) fixation, which occurs due to the symbiotic association between the alfalfa plant host and the microsymbiont, Sinorhizobium meliloti (syn. Ensifer meliloti). However, alfalfa plants are flexible in N acquisition and preferentially take-up soil N instead of utilizing symbiotic N2 fixation if soil N is sufficient for metabolic requirements (Dayoub et al. 2017; Heichel et al. 1981; Lamb et al. 1995). Over the life of the alfalfa stand, reliance on fixed N increases, likely due to biomass production and progressive depletion of available soil N. By the fourth year of forage production, plants were reported to obtain about 80% of required N from fixation (Burity et al. 1989; Heichel et al. 1984; Heichel and Henjum 1991). In N poor soils, plants rely on fixation, and microsymbiont strains introduced at planting as a soil drench, granular inoculant, or as a treatment on seeds may improve plant productivity under low soil fertility conditions (Thies et al. 1991a).
Overall improvements in N2 fixation capacity in alfalfa and other forage legumes would be beneficial for enhancing plant dry matter production, soil N fertility, and subsequent cash crop production. Significant increases in alfalfa forage dry matter have been obtained by modifying the copy number of nifA and dctABD, genes important in symbiotic N2 fixation in S. meliloti (Bosworth et al. 1994). However, such improved bacteria introduced into the environment via commercial seed treatments, or by granular or liquid inoculants, must compete with the indigenous populations of rhizobia for plant infection and subsequent nodule occupancy (Triplett and Sadowsky 1992). Often, such rhizobial strains must be applied repeatedly and/or in high inoculum concentrations for establishment in soil and nodules (Amarger and Lobreau 1982; Dunigan et al. 1984). While there are reports of high nodule occupancy of inoculated rhizobia strains in sites with low indigenous populations (Bosworth et al. 1994), as few as 100 cells per gram of soil can prevent establishment of introduced strains (Thies et al. 1991b).
Competitiveness for nodule formation is a complex trait involving both the plant host and microbial symbiont (Vlassak and Vanderleyden 1997), and is dependent on multiple environmental factors (Streeter 1994). However, competitiveness has been strongly correlated with speed of nodulation in many host-rhizobial interactions as a means of identifying more competitive strains (Lupwayi et al. 1996; de Oliveira and Graham 1990; Stephens and Cooper 1988; Sugawara and Sadowsky 2014). A greater understanding of the molecular determinants of competitiveness would provide insight into how nodule occupancy changes temporally with respect to alfalfa N acquisition and could possibly aid in selecting or developing more effective rhizobial inoculants.
Recent publication of genome sequences from multiple strains of S. meliloti, and the closely related species S. medicae, have enabled comparative genomic studies to identify the core and accessory genomes, as well as to investigate genetic diversity among strains. Alfalfa and M. truncatula can be nodulated by S. meliloti and S. medicae. A study of 48 complete genome sequences by Sugawara et al. (2013) revealed that S. meliloti and S. medicae strains vary in the presence of gene clusters for the type III secretion system (T3SS) and type IV secretion system (T4SS), which are of interest in host-symbiont and host-pathogen interactions (Büttner and Bonas 2006; Christie et al. 2014). Proteins secreted by the T3SS have been demonstrated to have key roles in symbioses between plants and microsymbionts including Rhizobium etli, Bradyrhizobium elkanii, B. fredii, B. japonicum, Mesorhizobium loti, and S. fredii (reviewed by Nelson and Sadowsky 2015). In the study by Sugawara et al. (2013), none of the S. medicae strains and only a few strains of S. meliloti had a T3SS, and none of the T3SS gene clusters included nop genes, encoding T3SS-dependent surface appendage or effector proteins. In contrast, seven types of T4SS gene clusters were found in S. meliloti. While the T4SSa gene cluster was found in all strains, the T4SSb gene cluster occurred in only a limited number of strains. The T4SSa and T4SSb gene clusters contain virB 1 -virB 11 , that encode proteins putatively involved in forming the secretion apparatus. The T4SSb gene cluster also has virG, virF, and a gene with similarity to virD 4 from Agrobacterium and M. loti. Presence of virD 4 defines the T4SSb gene cluster in S. meliloti and is indicative of the presence of the complete T4SSb gene cluster (Sugawara et al. 2013). VirD4, a coupling protein, mobilizes effector proteins and DNA-protein complexes across the bacterial cell envelop into the host cell (Cascales and Christie 2004; Redzej et al. 2017). The M. loti T4SS has been shown to be important in symbiosis, transporting effector proteins from the bacterium to host plant that are involved in symbiosis (Hubber et al. 2004). Mutations in the vir gene cluster (virD 4 , virB 1–11 , virG, or effector genes) result in delayed nodulation kinetics and reduced competitiveness. In S. meliloti and S. medicae, deletion of virB 6–9 in the T4SSb gene cluster significantly reduced nodule number and nodule dry mass in M. truncatula (Sugawara et al. 2013). Furthermore, deletion of virB 6–9 reduced competiveness for nodulation in M. truncatula, and the T4SSb gene cluster was shown to transport the effector protein TfeA from bacterial cells into host plant cells (Nelson et al. 2017). These results led us to investigate the role of the T4SSb in S. meliloti-alfalfa interactions. Although the T4SS has been identified in well-characterized Sinorhizobium strains, the frequency of the T4SSb gene cluster in field populations is unknown. In this study, we report the frequency of virD 4 , indicative of the T4SSb gene cluster, in S. meliloti strains isolated from alfalfa root nodules under field conditions, and provide additional evidence that the T4SS plays an important role in symbiosis.
2 Materials and methods
2.1 Plant growth conditions
Long-term agricultural research network (LTARN) plots were established at the University of Minnesota Southern Research and Outreach Center in Waseca, MN and the Southwestern Research and Outreach Center in Lamberton, MN. Experimental plots were established at each site on uniform research land representative of regional-scale soil and climate conditions that had been in an annual soybean-corn rotation for at least 30 years. The soil in Lamberton is a Normania loam/Webster clay loam and the Waseca site has a Webster/Nicollet clay loam. Experimental plots were 24.4 m × 24.4 m, and bordered by 7.6 m grassed alleyways.
At each LTARN site, a randomized complete block experimental design was used, with four replications and six cropping systems. In 2013, LTARN plots were planted uniformly with oat (Avena sativa L.). The oat crop was grown to maturity, grain and stover were harvested, and plants were terminated with glyphosate in early September. Plots were tilled with a field cultivator in early November. In the spring of 2014, plots were field cultivated once, and a Brillion packer was used to firm up the seedbed. Plots were planted with the alfalfa variety DKA44-16RR (DEKALB), using a 5-ft Brillion seeder at a rate of approximately 16.8 kg seed/ha in four plots per site, hereafter referred to as two-year-old alfalfa (Y2). The seed had been treated with a rhizobial inoculant of unknown composition by the manufacturer prior to packaging. Roundup ULTRA® MAX was applied (1746 ml/ha) for weed control in June. In 2015 four additional plots of alfalfa were established at each site with the same variety, hereafter referred to as one-year-old alfalfa (Y1), as described above.
2.2 Chemical and biological characterization of soil samples
In October 2015, intact soil cores containing alfalfa roots and soil approximately 12 cm in diameter and 20 cm deep were removed from alfalfa plots Y1 and Y2 from the two LTARN sites (eight plots per site). Cores were transported on ice to the laboratory, and were stored overnight in a cold room at 4 °C prior to processing. Cores were broken-up by hand and subsamples of bulk soil were collected, air dried, and sieved (2 mm × 2 mm) prior to chemical analyses.
Soil pH was determined in a 1:1 (v/v) soil:water mixture. Organic matter was determined by ashing 2 g of soil for 2 h at 360 °C. Nitrate was extracted from soil using 0.01 M CaSO4 and was measured by using a Latchat Quikchem 8500 Flow Injection Analyzer. Available potassium (K+) was determined using a 1 M NH4OAc extraction and filtrates were measured by atomic emission spectrometry (Perkin Elmer Analyst 100). Available P was extracted using the Bray method for 0.5 M NaHCO3 and filtrates were measured using the molybdate-blue method using a Brinkmann PC 900 probe colorimeter (Frank et al. 1998). All soil analyses were done at the University Minnesota Research Analytical Laboratory, St. Paul, MN.
Differences in soil characteristics across sites and between years were assessed using one-way ANOVA for each soil property using the lm function in R (R core team 2014). All data were tested for normality and homogeneity of variances. If necessary, data were log transformed prior to statistical analyses. Significance was determined at α = 0.05.
In order to estimate numbers of indigenous Sinorhizobium in field soil, soil samples from LTARN plots were sieved (2 mm × 2 mm), homogenized, and soil dilutions were used to inoculate alfalfa seedlings cv. Agate in CYG growth pouches (Mega International, Newport, MN). Population size was determined using the most-probable-number method as described by Somasegaran and Hoben (1994).
2.3 Nodule sampling and isolation of rhizobial strains
Up to 24 effective nodules, identified based on their pinkish coloring, were collected from each soil core described above for isolation of rhizobia. Nodules were surface sterilized with 5% sodium hypochlorite for 3 min and rinsed five times with sterile deionized water. Each nodule was placed into 50 μl of sterile water in individual wells of 96-well plates (Martínez-Hildago et al. 2014) and was crushed with a sterile glass rod. Nodule bacteria were streaked onto the surface of yeast extract–mannitol agar with 0.5% Congo red (Vincent 1970) and plates were incubated at 23 °C for 3 to 5 days. Pure cultures were obtained by repeatedly streaking single colonies on the same medium.
To obtain rhizobia originating from commercially inoculated seeds that were used to establish LTARN plots, remnant seeds were planted in sterilized vermiculite in 3.8 cm × 21 cm plastic cell “cone-tainers” (Stuewe & Sons, Tangent, OR) and placed in a growth chamber with a 16 h photoperiod at 23 °C. Alfalfa seeds without an inoculant were used as controls to ensure that contaminant rhizobia were not introduced from the environment. Plants were watered daily with nitrogen-free 0.5× Hoagland’s nutrient solution (Hoagland and Arnon 1950). After 21 days, plants were harvested, roots were rinsed briefly in sterile deionized water, nodules removed, and processed as detailed above to obtain pure cultures of the inoculant strains. Attempts to isolate bacteria directly from seeds were not successful.
2.4 Development and use of PCR primers for identification of Sinorhizobium species and detection of the T4SSb gene cluster
Nodulation specific genes mined from the genome sequences of 33 strains of S. meliloti and 13 strains of S. medicae were aligned using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) to identify conserved sequences. Those regions were then used in a BLASTN search of the NCBI, JGI, and Genoscope databases to identify sequences unique to Sinorhizobium. This search returned a putative ROK family transcriptional regulator upstream of nodP and nodQ that was used to design PCR primers M2SM-F (5′-AGCGGCACCAATCAGG-3′) and M2SM-R (5′-CGGCTGATCGGCTCGGC-3′) using ApE (http://biologylabs.utah.edu/jorgensen/wayned/ape/). The primer sequences were validated using 20 strains of S. meliloti, five strains of S. medicae, as well as single strains of S. fredii, S. saheli, and S. terangae. Other bacteria potentially associated with alfalfa, Agrobacterium rhizogenes, A. tumefaciens, Clavibacter michiganensis subsp. insidiosus, Pseudomonas syringae pv. syringae, and Xanthomonas alfalfae, were also tested for amplification with these primers. The PCR templates were composed of whole cells prepared by removing a portion of a single colony from the culture plate of each strain using a 1-μl sterile loop and suspending the cells in 50 μl sterile deionized water. PCR cycling was performed in a total volume of 15 μl containing 7.5 μl of 2× GoTaq Green Master Mix (Promega, Madison, WI), 0.3 μl M2SM-F (40 pmol/μl), 0.3 μl M2SM-R (40 pmol/μl), 2.9 μl of nuclease-free water, and 4 μl of whole cells. The PCR reactions were initiated with a denaturation step of 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 1 min, and extension at 72 °C for 1 min, with a final elongation step for 5 min at 72 °C. Agarose gels (1%) were run to confirm the presence of the 1142 bp product. The amplicon from S. medicae and S. meliloti was purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and sequenced.
The T4SSb gene cluster contains the coding sequence for the coupling protein VirD4 (Sugawara et al. 2013). The virD 4 nucleotide sequence in Sinorhizobium was compared to other known virD 4 sequences, regions specific to Sinorhizobium were identified, and primers VirD4-F (5′- CTCACAGCCTCATCGTTG -3′) and VirD4-R (5′- TCTCAATCGCCTCGAGCT-3′) were designed from this conserved sequence. Whole cell PCR templates were prepared as described above and tested with strains previously characterized for the T4SSb gene cluster as well as 16 additional S. meliloti strains not previously assayed for the T4SS. PCR reactions were initiated with a denaturation step of 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min, with a final elongation step at 72 °C for 5 min. Agarose gels (1%) were run to confirm the presence of the 807 bp PCR product. The amplicon was purified from S. medicae and S. meliloti strains and sequenced.
Bacterial isolates from nodules were tested first with the Sinorhizobium primers M2SM-F and M2SM-R using a whole cell template and the PCR protocol described above. Strains that produced the expected amplicon were subsequently tested for virD 4 using primers VirD4-F and VirD4-R.
2.5 16S rRNA gene sequencing
The 53 strains isolated from nodules that developed on plants from commercially inoculated seeds and 156 field strains identified as Sinorhizobium based on the M2SM PCR assay were selected at random for 16S rRNA amplification and sequencing using primers 27F and 1492R (Polz and Cavanaugh 1998). PCR cycling was performed in a total volume of 50 μl containing 25 μl of 2× GoTaq Green Master Mix (Promega), 1 μl 27F primer (40 pmol/μl), 1 μl 1492R primer (40 pmol/μl), 11 μl of nuclease-free water, and 12 μl of whole cell template prepared as described above. The PCR reactions were initiated with a denaturation step of 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min, with a final elongation step for 5 min at 72 °C. After verification of amplification by gel electrophoresis, PCR products were sent to GENEWIZ (South Plainfield, NJ) for DNA purification and sequencing of both strands. Forward and reverse DNA sequences were assembled with Phred/Phrap (Ewing et al. 1998; de la Bastide and McCombie 2007). The resulting sequences were used as queries in a BLASTN analysis to the NCBI RefSeq database (https://www.ncbi.nlm.nih.gov/refseq/).
2.6 Rep-PCR DNA fingerprinting
Repetitive element palindromic PCR (rep-PCR) DNA fingerprinting (Schneider and de Bruijn 1996) was done using the BOXA1R primer (Versalovic et al. 1994) on the subset of S. meliloti strains used for 16S rRNA sequencing. A whole cell PCR template was prepared as previously described. PCR amplifications were carried out in a 25 μl reaction mixture containing 6 μl of whole cells, 12.5 μl of 2× GoTaq Green Master Mix (Promega), 1 μl BOXA1R primer (1.6 pmol), and 5.5 μl of nuclease-free water. The PCR reactions were initiated with a denaturation step of 95 °C for 2 min followed by 30 cycles of denaturation at 94 °C for 3 s and 92 °C for 30 s, annealing at 50 °C for 1 min, and extension at 65 °C for 8 min, with a final elongation step for 8 min at 65 °C (Johnson et al. 2004). The amplification products were separated by electrophoresis in 1.5% (w/v) Seakem LE agarose gels for 17.5 h at 70 V with constant buffer recirculation in a cold room at 4 °C. The electrophoresed gels were stained for 20 min in a solution of 0.5 μg/ml ethidium bromide in 0.5 X Tris-acetate-EDTA buffer. Gel images were captured using a FOTO/Analyst Archiver electronic documentation system (Fotodyne Inc., Hartland, WI).
Gel images were analyzed using BioNumerics v.3.5 software (Applied Maths, Sint-Martens-Latem, Belgium) and were converted to 8-bit gray-scale tagged image file format. Gel lanes were normalized using the 1 kbp ladder from 253 to 10,000 bp (Promega) as an external reference standard. DNA fingerprints were compared using the following BioNumerics settings: 5% minimum profiling and zero gray zone, minimum area, and shoulder sensitivity. Similarities of the DNA fingerprints were calculated using the curved-based Pearson’s product-moment correlation coefficient with zero optimization. Similarity matrices were clustered using the unweighted group method with arithmetic means. Maximum similarity Jackknife analysis was used to determine how accurately the known source of the isolate was represented by the genetic relatedness of each group (Johnson et al. 2004). Isolates were manually assigned to their correct source groups (Lamberton Y1, Lamberton Y2, Waseca Y1, Waseca Y2, seed inoculant) and then each isolate was individually removed from the database for jackknife analysis. Maximum similarity is represented as the percentage of isolates from each source group having been correctly assigned to the group of origin.
2.7 Assessment of nodulation rate
The speed of nodulation of 12 randomly selected S. meliloti strains isolated from nodules at the Waseca field site was compared as described by Nelson et al. (2015). Six of 12 field isolates were positive for virD 4 while the remaining six were lacking virD 4 , as determined by the PCR assay. Additionally, S. meliloti strain KH46c and strain KH46cΔvirB 6–9 , which is unable to form a type IV secretion apparatus, were used for inoculation as controls that differ only by utilization of type IV secretion. Briefly, alfalfa seeds (cv. Agate) were surface sterilized and germinated on water agar plates for 36 h at room temperature. The seedlings were placed in clear plastic CYG growth pouches (Mega International) with nitrogen-free Fahraeus nutrient solution and placed in a growth chamber with a 16 h photoperiod at 23 °C. Each S. meliloti strain was grown for 24 h in YM medium at 25 °C with shaking, pelleted, washed with sterile 0.85% NaCl, and resuspended at OD600 = 0.1, corresponding to approximately 108 colony forming units/ml. When seedlings were 5 days old, roots were inoculated with 1 ml of S. meliloti cell suspension. Plants were observed daily and the appearance of each nodule was recorded. After 21 days, plants were removed from the growth pouches, the total number of nodules counted, and plant dry weight (90 °C for 48 h) was determined. For each S. meliloti strain there were five replications with three plants in each replication. Differences in nodule number and plant dry weight were assessed using one-way ANOVA and significance determined at α = 0.05 using Prism 7 (GraphPad Software, Inc.). To assess nodulation kinetics, the nodule number ratio (Hubber et al. 2004) was calculated at 4, 6, 8, and 21 days after inoculation by dividing the total number of nodules formed on plants inoculated with strains lacking virD 4 by the total number of nodules on plants inoculated with field strains containing virD 4 . Nodulation kinetics were determined in the same manner for the wild type KH46c and T4SS mutant KH46cΔvirB 6–9 . The mean nodule number ratio at each time point was compared to the theoretical mean = 1, indicating no difference in speed of nodulation, by two-tailed student’s t-test with significance determined at α = 0.05.
3 Results
3.1 Soil chemical properties
Within each site, soil chemical characteristics were not significantly different between the Y1 and Y2 plots. The Waseca soil had significantly greater soil pH (F = 4.32; P = 0.046), available P (F = 17.66; P = 0.0002), extractable K (F = 37.76; P < 0.0001), and organic matter (F = 23.06; P < 0.0001) concentrations compared to the Lamberton site (Table 1). In contrast, concentrations of available N were not significantly different between sites (P = 0.9).
3.2 Primers specific for Sinorhizobium species and detection of T4SSb gene cluster
PCR assays using the primers M2SM-F/R designed to a conserved region in both S. meliloti and S. medicae encoding an ROK family transcriptional regulator sequence upstream of nodP and nodQ amplified the expected 1142 bp product from the S. meliloti and S. medicae strains tested but no product was produced from other Sinorhizobium species or the alfalfa-associated bacteria tested (Table 2). The amplified DNA sequences obtained from S. meliloti KH46c, and field isolates WA101W4 and WA200E4 (GenBank accessions MG030683, MG030679, MG030680) and S. medicae strains M58 and WSM419 (GenBank accessions MG030684, MG030685) matched the conserved genome sequence used for primer design.
The primers designed to amplify the virD 4 nucleotide sequence in the T4SSb gene cluster produced the expected 807 bp PCR product from S. meliloti and S. medicae strains known to have the T4SSb gene cluster (Table 2). Analysis of 16 additional S. meliloti strains from the USDA collection identified five strains positive for virD 4 . All strains containing virD 4 had the same amplicon size, although there were several single nucleotide polymorphisms (SNPs) identified in the amplicons that were sequenced; S. medicae WSM419 with 5 SNPs (GenBank accession MG030682), S. meliloti KH46c with 3 SNPs (GenBank accession MG030681), and S. meliloti WA200E4 with 2 SNPs (GenBank accession MG030678) compared to WA101W4 (GenBank accession MG030677).
3.3 Rhizobial collection and characterization
Population densities of indigenous Sinorhizobium in soil determined by the most probable number plant-infection assay were similar from Waseca and Lamberton. At Waseca the mean population was 993 Sinorhizobium cells/g soil (ranging from 1.8 × 102 to 1.4 × 103 cells/g) and the Lamberton soil averaged 327 Sinorhizobium cells/g soil (ranging from 8.0 × 101 to 7.2 × 102 cells/g).
Abundant pink effective nodules were observed on alfalfa roots collected from the Y1 and Y2 plots at both locations. A total of 615 Sinorhizobium strains were isolated in pure culture, each from an individual alfalfa root nodule. Out of 280 Sinorhizobium strains obtained from alfalfa root nodules grown in Lamberton, 162 and 118 strains were isolated from the nodules of Y1 and Y2 alfalfa plots, respectively. Of the 282 Sinorhizobium strains from Waseca, 174 and 108 were from Y1 and Y2 plots, respectively. In addition, 53 Sinorhizobium strains were isolated from nodules that developed on plants from commercially inoculated seeds that were grown in sterile vermiculite in a growth chamber; 24 strains from seeds used to plant alfalfa stands in 2014 (Y2) and 29 strains from seeds used in 2015 (Y1). Amplification and sequencing of 16S rRNA was done for 209 Sinorhizobium strains and the identical 1346 bp sequence was obtained from each strain (GenBank accession MF682265) that had 100% identity to the 16S rRNA gene sequence of S. meliloti USDA1021 (CP021800.1), as well as numerous additional S. meliloti strains.
3.4 Occurrence of virD 4 in field isolates
The virD 4 gene was identified in 33% of field isolates by PCR amplification using the VirD4-F/R primers. Based on previous comparative genome sequence analysis of 48 Sinorhizobium strains, the presence of virD 4 in S. meliloti indicates the presence of the T4SSb gene cluster. Out of 280 Sinorhizobium strains obtained from alfalfa root nodules grown in Lamberton, 38% (106 strains) contained virD 4 , 30% (49 of 162 strains) from Y1 plots and 48% (57 of 118 strains) from Y2 plots. From the Waseca field site, of the 282 total isolates, 29% (82 strains) were identified with virD 4 , 29% (51 of 174 strains) from Y1 and 29% (31 of 108 strains) for Y2 alfalfa plots. None of the 53 S. meliloti strains recovered from nodules on plants from commercially coated seeds were positive for virD 4 . These results suggest that the T4SSb gene cluster occurs in widely in indigenous field populations of S. meliloti.
3.5 Molecular diversity of S. meliloti strains
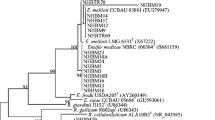
The rep-PCR DNA fingerprints of S. meliloti strains showed a high degree of genetic diversity among field isolates (Fig. 1). Nodules from the Lamberton field site harbored strains that were more genetically diverse than those isolated from Waseca (Fig. 2). For either site, Lamberton or Waseca, strains from alfalfa plants that were 6-months-old (Y1) and 18-months-old (Y2) were not significantly different (data not shown). None of the strains from inoculated seeds were identified in nodules of field grown plants based on rep-PCR banding patterns. Strains isolated from the commercially inoculated seeds differed from those obtained from field samples and grouped in three distinct clusters (Fig. 3). Strains with virD 4 , suggesting the presence of the T4SSb gene cluster, were distributed in all clusters of field isolates.
Principal Component Analysis (PCA) shows significant genetic differences among Sinorhizobium meliloti strains isolated from field sites and inoculated seeds regardless of year. This PCA analysis is based on rep-PCR DNA fingerprint patterns generated by the gel analysis software BioNumerics v.3.5 (Applied Maths, Sint-Martens-Latem, Belgium). PC1 = principal component 1, PC2 = principal component 2
Dendrogram illustrating genetic diversity of Sinorhizobium meliloti based on rep-PCR fingerprinting. Similarity matrices were clustered using the unweighted group method with arithmetic means. Field strains do not cluster by location. Strains originating from seeds treated with a commercial inoculant form three clusters distinct from field strains
Jackknife maximum similarity analysis demonstrated that a great number of strains overall were assigned to the correct source group (Lamberton, Waseca, seed inoculant), such that 87% of S. meliloti strains from Lamberton and 67% of strains from Waseca were assigned to the correct source (Table 3). By contrast, all seed inoculant strains (100%) were correctly assigned to the correct source.
3.6 Strains with virD 4 have more rapid nodulation kinetics
Nodulation kinetics of 12 field strains that differed for virD 4 were evaluated in growth chamber assays. The first nodules appeared 4 days post-inoculation (dpi) and by 8 dpi almost all plants had developed nodules. As a group, the strains positive for virD4 had more rapid nodulation kinetics than plants lacking virD 4 . As shown in Fig. 4, the mean nodule number ratio for field strains at 4 dpi was significantly less than 1, indicating that plants inoculated with the strains lacking virD 4 formed nodules at a slower rate than the plants inoculated with strains positive for virD 4 . Similarly, the plants inoculated with strain KH46cΔvirB 6–9 , which is unable to transport effectors through a T4SS, nodulated at a slower rate than did the wild type strain KH46c. The mean number of nodules/plant and mean plant dry weight at 21 dpi were not significantly different among the field strains (Supplementary Table 1).
Nodulation kinetics of alfalfa plants inoculated with Sinorhizobium meliloti strains. White bars are mean nodule number ratios calculated after inoculation with six field strains without virD 4 compared to six field strains with virD 4 (±SE). Hatched bars are mean nodule number ratios calculated after inoculation with S. meliloti KH46c ΔvirB 6–9 lacking a functional Type IV secretion system compared to the wild type KH46c (± SE). n = 15 plants per strain. * = significantly different from the theoretical mean = 1 by two-tailed student’s t-test with significance determined at α = 0.05
4 Discussion
In this study we evaluated a collection of Sinorhizobium strains obtained from root nodules of alfalfa plants grown in two locations in Minnesota that had not been cultivated to alfalfa for >30 years. We developed, validated, and used novel PCR primers to distinguish Sinorhizobium strains from other bacteria isolated from nodules and to identify the Sinorhizobium strains with virD 4 , indicative of presence of the T4SSb gene cluster. The T4SS was hypothesized to play a role in host-determination and/or symbiotic efficiency. We found the T4SSb gene cluster, defined by the presence of virD 4 , to occur in about 33% of the field isolates. Randomly selected strains with virD 4 formed nodules at a faster rate than those lacking virD 4 . Moreover, a mutant lacking Type IV secretion (KH46cΔvirB 6–9 ) also had slower nodulation kinetics compared to the wild type strain. These results provide additional evidence that the T4SSb gene cluster, which encodes proteins for transfer of effector proteins into the host cell, is involved in competitiveness for nodulation on alfalfa.
Two closely related species of Sinorhizobium, S. meliloti and S. medicae, form effective nodules on alfalfa (de Lajudie et al. 1994; Rome et al. 1996). Although S. medicae has been reported in Europe, North Africa, the Middle East, and Mexico (Bailly et al. 2006; Silva et al. 2007; Talebi et al. 2008; Trabelsi et al. 2010), it has not been reported to be native to North America. In contrast, S. meliloti appears to be found in most temperate soils worldwide as a free-living bacterium, forming nodules on species of Medicago, Melilotus, and Trigonella, and increasingly identified as an endophyte in dicots and monocots (Chi et al. 2005; Pini et al. 2012; Sprent 2001). PCR primers for identifying both species have been lacking although PCR primers are available for specifically identifying S. meliloti (Sánchez-Contreras et al. 2000; Trabelsi et al. 2009). Sequencing recA (Silva et al. 2007) or MsaI digestion of 16S rDNA (Zribi et al. 2005) is required to differentiate S. medicae from S. meliloti. The availability of a large collection of S. meliloti and S. medicae genome sequences allowed us to develop novel primers that amplified conserved sequences in both S. meliloti and S. medicae. The M2SM-F/R primers may be useful in further studies to investigate the biogeography of the two species from plant or soil samples. In our studies, we followed the Sinorhizobium PCR assay with 16S rDNA sequencing and found only S. meliloti in alfalfa nodules. Alternatively, after amplification with M2MS-F/R primers, a second PCR could be done with primers for amplifying rpoE or nodC to identify S. meliloti (Trabelsi et al. 2009).
Previous research indicated that the T4SSb gene cluster is involved in host specificity or symbiotic efficiency and was identified in 27% of strains used for complete genome sequencing (Sugawara et al. 2013). We identified virD 4 , indicative of the T4SSb gene cluster, in an additional five of 16 well-characterized strains from the USDA collection and in 33% of our field strains. However, virD 4 was lacking in all strains isolated from commercially inoculated seeds. The T4SS is well characterized in Agrobacterium and functions to translocate T-DNA and effector proteins into plant cells (Wallden et al. 2010). Seven T4SS subfamilies were identified based on gene structure and evolution. Of particular interest to symbiosis is the T4SSb gene cluster that encodes VirB1-VirB11, which forms the secretion apparatus, and a VirD4-like protein that may function to recruit proteins for secretion. Secretion of effectors by a T4SS to promote symbiosis was first demonstrated in R. loti (Hubber et al. 2004) and more recently in S. meliloti and S. medicae (Nelson and Sadowsky 2015). Deletion of virB 6–9 in S. meliloti decreases competitive ability for nodule occupancy in M. truncatula and deletion of the effector gene tfeA results in reduced nodule number (Nelson et al. 2015, 2017). We found that the field strains with virD 4 had more rapid nodulation kinetics compared to the field strains lacking virD 4 . These data further support the importance of the T4SS in symbiosis in S. meliloti. Strains with the T4SS may be more competitive for nodulation when used as seed or soil inoculants.
Alfalfa plants grown in two locations that had not been used for alfalfa cultivation for >30 years were found to be well nodulated with genetically diverse S. meliloti strains indigenous to the soils at each site. Lamberton and Waseca soil samples were significantly different in terms of soil chemical characteristics known to influence soil microbial communities such as pH, organic matter, and available macronutrients (Fierer and Jackson 2006). Differences in soil properties may have contributed to the differences in genetic identity and diversity of S. meliloti among sites, as others have previously reported (Pafetti et al. 1996). A previous study using RAPD markers found high levels of genetic diversity in S. meliloti strains in Italian field soils and that single plants were nodulated by many different strains (Carelli et al. 2000). Genetic diversity in S. meliloti has been associated with field locations in a study using AFLP markers (Donnarumma et al. 2014) and with environmental conditions in a study using rep-PCR (Talebi et al. 2008). The accessory plasmids in S. meliloti likely contribute greatly to diversity because they are important in environmental adaptation, and a high level of diversity occurs in pSymA and in accessory plasmids (Lagares et al. 2014). Whether the populations in our study were maintained from original soil colonists or were augmented from strains that dispersed from alfalfa fields in the vicinity remains an open question. The indigenous populations were clearly sufficient for effective nodulation of alfalfa in the study sites, and were more competitive for nodule occupancy than strains used for commercial seed inoculation. Soils without recent alfalfa cultivation should not be assumed to have low S. meliloti populations. Strains inoculated onto seeds could not be recovered from field-grown plants and may not have competed with indigenous strains for nodulation or did persist in field soils.
References
Amarger N, Lobreau JP (1982) Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol 44(3):583–588
Bailly X, Oliveri I, De Mita S, Cleyet-Marel J-C, Béna G (2006) Recombination and selection shape the molecular diversity pattern of nitrogen-fixing Sinorhizobium sp. associated to Medicago. Mol Ecol 15(10):2719–2734. https://doi.org/10.1111/j.1365-294X.2006.02969.x
de la Bastide M, McCombie WR (2007) Assembling genomic DNA sequences with PHRAP. Curr Protoc bioinformatics 17: 11.4: 11.4.1–11.4.15
Bosworth AH, Williams MK, Albrecht KA, Kwiatkowski R, Beynon J, Hankinson TR, Ronson CW, Cannon F, Wacek TJ, Triplett EW (1994) Alfalfa yield response to inoculation with recombinant strains of Rhizobium meliloti with an extra copy of dctABC and/or modified nifA expression. Appl Environ Microbiol 60(10):3815–3832
Burity HA, Ta TC, Faris MA, Coulman BE (1989) Estimation of nitrogen fixation and transfer from alfalfa to associated grasses in mixed swards under field conditions. Plant Soil 114(2):249–255. https://doi.org/10.1007/BF02220805
Büttner D, Bonas U (2006) Who comes first? How plant pathogenic bacteria orchestrate type III secretion. Curr Opin Microbiol 9(2):193–200. https://doi.org/10.1016/j.mib.2006.02.006
Carelli M, Gnocchi S, Fancelli S, Mengoni A, Paffetti D, Scotti C, Bazzicalupo M (2000) Genetic diversity and dynamics of Sinorhizobium meliloti populations nodulating different alfalfa cultivars in Italian soils. Appl Environ Microbiol 66(11):4785–4789. https://doi.org/10.1128/AEM.66.11.4785-4789.2000
Cascales E, Christie PJ (2004) Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304(5674):1170–1173. https://doi.org/10.1126/science.1095211
Chi F, Shen S-H, Cheng H-P, Jing Y-X, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. App Environ Microbiol 71(11):7271–7278. https://doi.org/10.1128/AEM.71.11.7271-7278.2005
Christie PJ, Whitaker N, Gonzáles-Rivera C (2014) Mechanism and structure of the bacterial type IV secretion systems. Biochim Biophys Acta 1843(8):1578–1591. https://doi.org/10.1016/j.bbamcr.2013.12.019
Core Team R (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org/. Accessed 21 Dec 2016
Dayoub E, Naudin C, Piva G, Shirtliffe SJ, Fustec J, Corre-Hellou G (2017) Traits affecting early season nitrogen uptake in nine legume species. Heliyon 3(2):e00244. https://doi.org/10.1016/j.heliyon.2017.e00244
Donnarumma F, Bazzicalupo M, Blazinkov M, Mengoni A, Sikora S, Babic KH (2014) Biogeography of Sinorhizobium meliloti nodulating alfalfa in different Croatian regions. Res Microbiol 165(7):508–516. https://doi.org/10.1016/j.resmic.2014.06.001
Dunigan EP, Bollich PK, Hutchinson RL, Hicks PM, Zaunbrecher FC, Scott SG, Mowers RP (1984) Introduction and survival of an inoculant strain of Rhizobium japonicum in soil. Agron J 76(3):463–466. https://doi.org/10.2134/agronj1984.00021962007600030023x
Ewing B, Hillier L, Wendl M, Green P (1998) Basecalling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8(3):175–185. https://doi.org/10.1101/gr.8.3.175
Fierer N, Jackson RB (2006) The diversity and biogeorgraphy of soil bacterial communities. Proc Natl Acad Sci U S A 103(3):626–631. https://doi.org/10.1073/pnas.0507535103
Frank K, Beagle D, Denning J (1998) Phosphorus. In: Recommended chemical soil test procedures for the north central region. North central regional research publication no. 221 (revised). Missouri agricultural Experiment Station SB 1001, pp 21–29. http://msue.anr.msu.edu/uploads/234/68557/Rec_Chem_Soil_Test_Proce55c.pdf
Heichel GH, Henjum KI (1991) Dinitrogen fixation, nitrogen transfer, and productivity of forage legume-grass communities. Crop Sci 31(1):202–208. https://doi.org/10.2135/cropsci1991.0011183X003100010045x
Heichel GH, Barnes DK, Vance CP (1981) Nitrogen fixation of alfalfa in the seeding year. Crop Sci 21(2):330–335. https://doi.org/10.2135/cropsci1981.0011183X002100020032x
Heichel GH, Barnes DK, Vance CP, Henjum KI (1984) N2 fixation, and N and dry matter partitioning during a 4-year alfalfa stand. Crop Sci 24:811–815
Hoagland DR, Arnon DI (1950) The water-culture method of growing plants without soil. Calif Agr Expt Sta Circ 347:1–32
Hubber A, Vergunst AC, Sullivan JT, Hooykaas PJJ, Ronson CW (2004) Symbiotic phenotypes and translocated effector proteins of the Mesorhizobium loti strain R7A VirB/D4 type IV secretion system. Mol Microbiol 54(2):561–574. https://doi.org/10.1111/j.1365-2958.2004.04292.x
Johnson LK, Brown MB, Carruthers EA, Ferguson JA, Dombek PE, Sadowsky MJ (2004) Sample size, library composition, and genotypic diversity among natural populations of Escherichia coli from different animals influence accuracy of determining sources of fecal pollution. Appl Environ Microbiol 70(8):4478–4485. https://doi.org/10.1128/AEM.70.8.4478-4485.2004
Lagares A, Sanjuan J, Pistorio M (2014) The plasmid mobilon of the model plant-symbiont Sinorhizobium meliloti: coming up with new questions and answers. Microbiol Spectrum 2(5):PLAS-0005-2013
de Lajudie P, Willems A, Pot B, Dewettinck D, Maestrojuan G, Neyra M, Collins MD, Dreyfus B, Kersters K, Gillis M (1994) Polyphasic taxonomy of rhizobia: emendation of the genus Sinorhizobium and description of Sinorhizobium meliloti comb. nov., Sinorhizobium saheli sp. nov., and Sinorhizobium teranga sp. nov. Int J Syst Bacteriol 44(4):715–733. https://doi.org/10.1099/00207713-44-4-715
Lamb JFS, Barnes DK, Russelle MP, Vance CP, Heichel GH, Henjum KI (1995) Ineffectively and effectively nodulated alfalfas demonstrate biological nitrogen fixation continues with high nitrogen fertilization. Crop Sci 35(1):153–157. https://doi.org/10.2135/cropsci1995.0011183X003500010028x
Lupwayi NZ, Stephens PM, Noonan MJ (1996) Relationship between timing of infection and nodulation competitiveness of Rhizobium meliloti. Symbiosis 21:233–248
Martínez-Hildago P, Galindo-Villardón P, Trujillo ME, Igual JM, Martínez-Molina E (2014) Micromonospora from nitrogen fixing nodules of alfalfa (Medicago sativa L.). A new promising plant probiotic bacteria. Sci Rep 4:6389
Nelson MS, Sadowsky MJ (2015) Secretion systems and signal exchange between nitrogen-fixing rhizobia and legumes. Front Plant Sci 6:491
Nelson MS, Chun CL, Sadowsky MJ (2015) Evaluation of nodulation speed by Sinorhizobium strains. Bio-protocol 5(15):e1554
Nelson MS, Chun CL, Sadowsky MJ (2017) Type IV effector proteins involved in the Medicago-Sinorhizobium symbiosis. Mol Plant-Microbe Interact 30(1):28–34. https://doi.org/10.1094/MPMI-10-16-0211-R
de Oliveira LA, Graham PH (1990) Speed of nodulation and competitive ability among strains of Rhizobium leguminosarum bv phaseoli. Arch Microbiol 153(4):311–315. https://doi.org/10.1007/BF00248998
Pafetti D, Scotti C, Gnocchi S, Fancelli S, Bazzicalupo M (1996) Genetic diversity of an Italian Rhizobium meliloti population from different Medicago sativa varieties. Appl Environ Microbiol 62:2279–2285
Pini F, Frascella A, Santopolo L, Bazzicalupo M, Biodi EG, Scotti C, Mengoni A (2012) Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol 12(1):78. https://doi.org/10.1186/1471-2180-12-78
Polz MF, Cavanaugh CM (1998) Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol 64(10):3724–3730
Redzej A, Ukleja M, Connery S, Trokter M, Felisberto-Rodrigues C, Cryar A, Thalassinos K, Hayward RD, Orlova E, Waksman G (2017) Structure of a VirD4 couling protein bound to a VirB type IV secretion machinery. EMBO J 36(20):3080–3095. https://doi.org/10.15252/embj.201796629
Rome S, Fernandez MP, Brunel B, Normand P, Cleyet-Marel JC (1996) Sinorhizobium medicae sp.nov., isolated from annual Medicago spp. Int J Syst Bacteriol 46(4):972–980. https://doi.org/10.1099/00207713-46-4-972
Sánchez-Contreras M, Lloret J, Martín M, Villacieros M, Bonilla I, Rivilla R (2000) PCR use of highly conserved DNA regions for identification of Sinorhizobium meliloti. Appl Environ Microbiol 66(8):3621–3623. https://doi.org/10.1128/AEM.66.8.3621-3623.2000
Schneider M, de Bruijn FJ (1996) Rep-PCR mediated genomic fingerprinting of rhizobia and computer–assisted phylogenetic pattern analysis. World J Microb Biot 12(2):163–174. https://doi.org/10.1007/BF00364681
Silva C, Kan FL, Martínez-Romero E (2007) Population genetic structure of Sinorhizobium meliloti and S. medicae isolated from nodules of Medicago spp. in Mexico. FEMS Microbiol Ecol 60(3):477–489. https://doi.org/10.1111/j.1574-6941.2007.00301.x
Somasegaran P, Hoben HJ (1994) Handbook for rhizobia: methods in legume-Rhizobium technology. Springer-Verlag, New York. https://doi.org/10.1007/978-1-4613-8375-8
Sprent JL (2001) Nodulation in legumes. Royal botanic Gardens, Kew, London
Stephens PM, Cooper JE (1988) Variation in speed of infection of “no root hair zone” of white clover and nodulating competitiveness among strains of Rhizobium trifolii. Soil Biol Biochem 20(4):465–470. https://doi.org/10.1016/0038-0717(88)90059-4
Streeter JG (1994) Failure of inoculant rhizobia to overcome the dominance of indigenous strains for nodule formation. Can J Microbial 40(7):513–522. https://doi.org/10.1139/m94-084
Sugawara M, Sadowsky MJ (2014) Enhanced nodulation and nodule development by nolR mutants of Sinorhizobium medicae on specific Medicago host genotypes. Mol Plant-Microbe Interact 27(4):328–335. https://doi.org/10.1094/MPMI-10-13-0312-R
Sugawara M, Epstein B, Badgley BD, Unno T, Xu L, Reese J, Gyaneshwar P, Denny R, Mudge J, Bharti AK, Farmer AD, May GD, Woodward JE, Médigue C, Vallenet D, Lajus A, Rouy Z, Martinez-Vaz B, Tiffin P, Young ND, Sadowsky MJ (2013) Comparative genomics of the core and accessory genomes of 48 Sinorhizobium strains comprising five genospecies. Genome Biol 14(2):R17. https://doi.org/10.1186/gb-2013-14-2-r17
Talebi MB, Bahar M, Saeidi G, Mengoni A, Bazzicalupo M (2008) Diversity of Sinorhizobium strains nodulating Medicago sativa from different Iranian regions. FEMS Microbiol Lett 288(1):40–46. https://doi.org/10.1111/j.1574-6968.2008.01329.x
Thies JE, Singleton PW, Bohlool BB (1991a) Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl Environ Microbiol 57(1):19–28
Thies JE, Singleton PW, Bohlool BB (1991b) Modeling symbiotic performance of introduced rhizobia in the field by use of indexes of indigenous population-size and nitrogen status of the soil. Appl Environ Microbiol 57(1):29–37
Trabelsi D, Pini F, Aouani ME, Bazzicalupo M, Mengoni A (2009) Development of a real-time PCR assay for detection and quantification of Sinorhizobium meliloti in soil and plant tissue. Lett Appl Micrbiol 48(3):355–361. https://doi.org/10.1111/j.1472-765X.2008.02532.x
Trabelsi D, Mengoni A, Aouani ME, Bazzicalupo M, Mhamdi R (2010) Genetic diversity and salt tolerance of Sinorhizobium populations from two Tunisian soils. Ann Microbiol 60(3):541–547. https://doi.org/10.1007/s13213-010-0084-6
Triplett EW, Sadowsky MJ (1992) Genetics of competition for nodulation of legumes. Annu Rev Microbiol 46(1):399–428. https://doi.org/10.1146/annurev.mi.46.100192.002151
Versalovic J, Schneider M, de Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Method. Mol Cell Biol 5:25–40
Vincent JM (1970) A manual for the practical study of the root nodule bacteria. IBP handbook no. 15. Blackwell, Oxford
Vlassak KM, Vanderleyden J (1997) Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci 16(2):163–229. https://doi.org/10.1080/07352689709701948
Wallden K, Rivera-Calzada A, Waksman G (2010) Type IV secretion systems: versatility and diversity in function. Cell Microbiol 12(9):1203–1212. https://doi.org/10.1111/j.1462-5822.2010.01499.x
Yost MA, Coulter JA, Russelle MP, Sheaffer CC, Kaiser DE (2012) Alfalfa nitrogen credit to first-year corn: potassium, regrowth, and tillage timing effects. Agron J 104(4):953–962. https://doi.org/10.2134/agronj2011.0384
Yost MA, Morris TF, Russelle MP, Coulter JA (2014) Second-year corn after alfalfa often requires no fertilizer nitrogen. Agron J 106(2):659–669. https://doi.org/10.2134/agronj2013.0362
Zribi K, Mhamdi R, Huguet T, Aouani ME (2005) Diversity of Sinorhizobium meliloti and S. medicae nodulating Medicago truncatula according to host and soil origins. World J Microb Biot 21(6-7):1009–1015. https://doi.org/10.1007/s11274-004-7653-4
Acknowledgements
This work was supported by the State Scholarship Fund of China Scholarship Council, USDA-ARS 5062-12210-002-00D, and University of Minnesota LTARN. We thank Matt Bickell and Kara Anderson for excellent LTARN plot support. Mention of any trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture. USDA is an equal opportunity provider and employer. This paper is a joint contribution from the USDA-ARS-Plant Science Research Unit and the Minnesota Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Cao, Y., Miller, S.S., Dornbusch, M.R. et al. Widespread occurrence of Sinorhizobium meliloti strains with a type IV secretion system. Symbiosis 75, 81–91 (2018). https://doi.org/10.1007/s13199-018-0547-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-018-0547-2