Abstract
The symbiosis of Leguminosae with arbuscular mycorrhizal fungi (AMF) and N2-fixing nodulating bacteria (NFNB) can occur simultaneously, forming a tripartite symbiosis. In particular, AMF can colonize root nodules, although this interaction is not yet well elucidated, especially with regard to nodule activity and to the influence of external factors, such as biostimulants. In this study, we hypothesized that the application of the flavonoid formononetin, used to stimulate root colonization by native AMF, increases the AMF colonization of soybean (Glycine max) root nodules, especially under low availability of phosphorus (P). To test this hypothesis, we performed a field experiment in randomized blocks in a 4 × 3 factorial design, with 4 treatments of formononetin (0, 0.46, 0.92 and 1.84 g per kg seed) and 3 of P (0, 60 and 120 kg ha−1) with 5 replicates. Nodules and roots were collected during the R2 stage (full flowering) and evaluated with respect to AMF colonization. Formononetin stimulated mycorrhizal fungi colonization of active nodules, especially when no P was applied, as also observed for AMF root colonization; however, it had no effect with 60 and 120 kg P ha−1. Thus, the application of formononetin increases surface AMF colonization of active nodules and roots, but its effect disappears with an increase in P and the inactivity of the nodule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Leguminosae plants can establish tripartite symbioses with arbuscular mycorrhizal fungi (AMF) and N2-fixing nodulating bacteria (NFNB). Through their hyphae, AMF broaden the soil exploration area of the roots, increasing the absorption of nutrients that are less mobile in the soil, especially phosphorus (P) (Smith and Read 2008). The bacteria, in turn, provide N that is accessible to the plant. The simultaneous occurrence of both symbioses can have synergistic, neutral or antagonists effects on plant growth, depending on the identity of the symbionts (Azcón et al. 1991; Ahmad 1995; Xavier and Germida 2002; Franzini et al. 2010; Xiao et al. 2010). The factors that determine the functional compatibility in the tripartite symbiosis are not yet understood, with both nutritional (e.g. enhanced fitness of the common host plant due to increased nutrient uptake, specially P by AMF and nitrogen by symbiotic N2 fixation but also a possible competition for host photosyntates) (Mortimer et al. 2008; Kaschuk et al. 2009) and non-nutritional (production of signaling molecules that elicitate root infection by both symbionts) interactions (Xie et al. 1995; Catford et al. 2003, 2006) being reported. Besides, it is possible that nodule colonization by AMF plays a role in the compatibility among those symbionts (Vidal-Dominguez et al. 1994; Scheublin and van der Heijden 2006). However, this interaction is yet poorly understood.
Root nodules are highly specialized structures with many specific physiological and anatomical specific features that make them unique when compared to other parts of the root (Udvardi and Poole 2013). However, they can be colonized by AMF as demonstrated by microscopy techniques in Phaseolus vulgaris (Baird and Caruso 1994), Trifolium repens, and Medicago sativa (Vidal-Dominguez et al. 1994) and in three species of Leguminosae (Lotus corniculatus, Trifolium repens, and Ononis repens) naturally found in dunes (Scheublin et al. 2004). If nodule colonization by AMF could direct supply P to the nodule, the tripartite symbiosis would increase nitrogen fixation because this process requires large quantities of P, as shown by Vadez et al. (1997) who reported that P concentration in the nodule is usually about three times higher than that found in other parts of the root.
Although inoculation with NFNB strains is an established and widely used technique in agricultural systems (Moreira and Siqueira 2006), the agricultural application of mycorrhizal symbiosis is limited, mainly due to the difficulty in producing inoculants (Powell and Bagyaraj 1984; Douds et al. 2006) because of the obligatory symbiotrophy of AMF, and because little is known regarding the performance of different AMF isolates under varying edaphic and climatic conditions. Thus, alternative techniques have been developed, such as those based on the stimulation of root colonization by AMF native to soil through the application of biostimulants, which are signaling molecules that play a natural role in the communication between the plant and AMF during the colonization process and whose primary example is formononetin (Nair et al. 1991; Siqueira et al. 1991).
This isoflavonoid can stimulate root colonization by AMF, especially under low P availability (Davies et al. 2005a, b). If AMF nodule colonization is controlled by the same factors that operate in AMF root colonization, it is possible that formononetin also increases AMF nodule colonization and that its effect is dependent on P availability to the plant. Therefore, we hypothesized that the application of the flavonoid formononetin on soybean (Glycine max) increases the AMF colonization of root nodules, especially under low availability of phosphorus (P).
2 Material and methods
2.1 Study area characterization
A field experiment was performed from December 2010 to April 2011 at an experimental farm located in the southeast of Minas Gerais, Brazil, at 21°12’17” S, 44°58’49” W, with an altitude of 957 m and an annual average rainfall of 1411 mm. The area where the experiment was performed was kept as fallow since 2009, then it was used for maize production until April 2010. From April to December 2010, the area was again kept in fallow with the growth of spontaneous vegetation, predominantly Brachiaria sp. Before 2009, this area has been used mainly for the production of maize and soybean. The soil of the area is classified as Red-Yellow Latosol (Oxisol according USDA classification), and its chemical and physical characteristics were the following: pH in H2O = 6.50; H + Al = 2.3 cmolc dm−3; Al = 0.0 cmolc dm−3; Ca = 3.8 cmolc dm−3; Mg = 1.5 cmolc dm−3; CTC = 7.9 cmolc dm−3; K = 0.26 mg dm −3; P = 10.3 mg dm −3; soil organic matter =27 g kg−1; clay =570 g kg−1; silt =80 g kg−1; sand =350 g kg−1. Soil pH was measured in a soil/H2O suspension (1:2.5 w/v). Exchangeable Al, Ca and Mg were extracted with 1 mol l−1 KCl solution. Al was measured by titration and both Ca and Mg by atomic-absorption spectrophotometry. Available potassium and phosphorus were extracted with Mehlich I solution (Mehlich 1953). Then, K was determined by flame photometry and P by colorimetry. Organic carbon (Corg) was determined by titration with a solution of ferrous ammonium sulfate after oxidation of the carbon by potassium dichromate (Walkley and Black 1934). The soil texture was determined by using the hydrometer method (Bouyoucos 1951).
2.2 Experimental design
The experimental design was randomized blocks with two crossed factors: 3 treatments of phosphorus (0, 60, and 120 kg ha−1 of P2O5, the last two corresponding to 50 and 100 % of the recommendation based on soil analysis); and 4 treatments of the biostimulant formononetin (Myconate®, Plant Health Care Inc.) (0, 0.46, 0.92 and 1.84 g per kg seed, corresponding to 0, 25, 50, and 100 % of the recommend by the manufacturer), with 5 replicates for a total of 60 plots. Each plot had 6 rows with 10 m spaced by 0.45 m.
Soil preparation was performed with a plowing and a harrowing cycle followed by the opening of furrows. Fertilization was performed manually before sowing by applying P2O5 corresponding to each treatment along with 40 kg ha−1 of K2O in the furrows. The variety of soybean used was FAVORITA RR (provided by the Departamento de Agricultura, Universidade Federal de Lavras), which is recommended for the region. The seeds were inoculated with a commercial peat inoculant containing Bradyrhizobium japonicum (SEMIA 5079 and 5080), in the proportion 100 g of inoculant (5 × 109 cells g−1) per 50 kg of seed. Sowing was manually performed in December 2010 by uniformly distributing 15 seeds per linear meter, and the harvest was performed in April 2011. Culture treatments were performed uniformly on all experimental plots according to the need of the culture.
2.3 Collection of nodules and roots
During the R2 stage, indicated by the full flowering of soybean plants, random collection of nodules and roots was performed on three plants from each of the 60 plots by carefully taking a volume of approximately 1 dm3 of soil with the root system from each plant. Nodules attached to the root or in the soil were manually collected. The R2 stage was chosen because it is when the peak of nitrogenase activity in nodules occurs and it also coincides with the peak of nutrients demand by plants. From the total number of nodules collected, fifteen nodules from each of the three plants were randomly pooled in order to obtain a total of 45 nodules per plot.
Nodules were removed from roots, washed in tap water, and cross-sectioned. Immediately after sectioning, they were classified according to their activity by analysis of their inner color, which varies according to the presence of leghemoglobin (Minchin et al. 2008). Nodules were considered active only when their internal color was clearly reddish, with larger proportion of magenta than the 0/60/20/0 color on the % scale cyan/magenta/yellow/black (CYMK), compared to a gradient of colors in a printed color chart in the CYMK scale under the same light source. Nodules with a lower proportion of magenta and a larger proportion of yellow than the 0/40/40/0 color on the same scale were classified as inactive. Nodules with intermediate colors were discarded to avoid ambiguity in the classification.
Five other plants were also collected per treatment as above to sample fine roots for assessing the percentage of AMF colonization. The fine roots were randomly taken from each plant and pooled to form a composite sample for each plot.
2.4 Evaluation of surface colonization of active and inactive nodules and the percentage of AMF colonization of soybean roots with different treatments of P and formononetin
Nodules selected as previously described were heated for 1 h in KOH (5 %) for clearing, washed in distilled water, immersed in HCl (5 %) for 1 h at room temperature, and then immersed in acid fuchsin for 1 h at 90 °C. Permanent slides were then mounted with nodule sections for microscopic observation. The sections were placed on microscope slides on a solution of polyvinyl-lacto-glycerol (PVLG) and gently crushed with a coverslip. A total of 1200 nodules collected from 60 plots were used to prepare 240 slides with 5 nodules in each; for each plot, a total of 4 slides were prepared, with 2 slides containing active nodules and 2 slides containing inactive nodules. Once prepared, the slides were examined under an optical microscope (Nikon Labophot) for the identification of fungal structures such as hyphae, spores, and vesicles, which are indicative of AMF colonization. The analysis was performed by scanning the entire surface of all nodule sections. Hyphae from AMF were distinguished from other hyphae by the presence of entry points and by the criteria defined by Steinberg and Rillig (2003). The slides were photographed with a NIKON EFD-3 optical microscope coupled to a Canon A630 digital camera. Nodule colonization was calculated for each plot as the proportion of nodules where AMF structures were detected.
Plant roots were depigmented with KOH (10 %) and colored with trypan blue according to the method of Phillips and Hayman (1970) modified by Koske and Gemma (1989), and the percentage of root length colonized was assessed according to the method used by Giovannetti and Mosse (1980).
For scanning electron microscopy analysis, six sections of nodules classified as active or inactive were randomly chosen. The sections were removed from the modified Karnovsky fixing solution (2.5 % glutaraldehyde, 2.0 % paraformaldehyde, 0.05 M cacodylate buffer, pH 7.2) in which they were immersed, transferred to cryo-protecting solution (30 % glycerol) for 30 min, and cross-sectioned in liquid nitrogen. The fragments were transferred to a sufficient amount of 1 % osmium tetroxide solution (3 drops) and water to cover them for an hour, washed three times in distilled water, subsequently dehydrated in an acetone series (25, 50, 75, 90, and 100 %, each three times), and taken to the critical point apparatus. Specimens were mounted on aluminum stubs, covered with gold, and observed under a LEO EVO 40 XVP scanning electron microscope, where images were generated for analysis.
2.5 Statistical analysis
Data exploration following the protocol described in Zuur et al. (2010) was performed before the analysis. For the proportion of nodules colonized by AMF, a generalized linear mixed-effects model was used with a binomial distribution in which the proportion of nodules colonized (dependent variable) was modeled by the P, formononetin, and the activity of nodules (independent variables) and their interactions (fixed effects). Due to the block design and because nodule activity was nested within the combinations of P and formononetin treatments, random intercepts for blocks and for the main plots were used. For comparing alternative models, likelihood ratio tests were performed to select the best model following Zuur et al. (2009).
For the AMF colonization of roots, a generalized linear model with a binomial distribution was used, with the percentage of AMF colonization as the dependent variable and P and formononetin treatments as independent variables. Due to the overdispersion detected in this model, the “quasi” correction was applied. The alternative models were compared by likelihood ratio tests as described by Zuur et al. (2009). Data were analyzed using the lme4 package on R 2.15 (R Development Core Team 2012).
3 Results and discussion
3.1 Structural aspects of soybean nodules colonized by mycorrhizal fungi through photomicrographs and optical scanning electron microscopy
Surface colonization of nodules by AMF occurred in both active and inactive nodules, as observed by the presence of cenocytic hyphae (aseptate), spores (except for active nodules), and vesicles (Fig. 1). Because nodule colonization was evaluated by using crushed nodules, the cases when the nodules are only surface colonized or when both surface and internal colonization occurred could not be clearly distinguished, thus the term surface colonization is used herein. Appressorium-like structures were common in both active and inactive nodules, being characterized by the presence of a swelled hypha on nodule surface (Fig. 1a). The occurrence of appressoria on the surface of active nodules indicates internal AMF colonization in these nodules, although these structures can be occasionally aborted without penetrating the roots (Giovannetti et al. 1993). With regard to the fungal structures found, hyphae predominated in both active and inactive nodules, with a minor presence of vesicles (Fig. 1b), whereas spores only occurred in inactive nodules (Fig. 1c). There were no apparent differences in the shape and size of vesicles corresponding to the activity of the nodule. Spores and vesicles were present in an insignificant amount (less than 0.1 % of the total nodules analyzed, 1200). This low sporulation in the nodules differs from the results reported by Vidal-Dominguez et al. (1994) by studying Glomus fasciculatum in two Leguminosae species (Trifolium repens and Medicago sativa). Differences in the legume species as well as experimental conditions could be responsible for the discrepancy between our results and those of Vidal-Dominguez et al. (1994).
Optical and scanning electron microscopy images of Glycine max (L.) root nodules colonized by arbuscular mycorrhizal fungi. a) Surface of active nodule showing fungal hyphae (black arrows) and entry point (gray arrow); b) Vesicle (black arrow) in an active nodule; c) Surface of inactive nodule with fungal hyphae (black arrows) and spores (gray arrow); d) Surface of active nodule showing fungal hyphae (white arrows); e) Cross section showing inactive nodule with fungal hyphae (white arrows)
Arbuscules were not observed in colonized nodules, although they were abundantly present in roots. With the exception of Baird and Caruso (1994), who reported the occurrence of structures similar to degenerated arbuscules in common bean nodules, there are no other reports on the occurrence of arbuscules in nodules.
3.2 Evaluation of surface colonization of active and inactive nodules and the percentage of AMF colonization of soybean roots after treatment with different treatments of P and formononetin
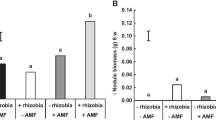
The selected models for AMF colonization of roots and nodules are shown in Table 1. The response of AMF colonization to formononetin and P indicated similar trends in roots and active nodules, as shown in Fig. 2. In both cases, there was a negative interaction between treatments of P and formononetin on AMF colonization of roots and nodules (Table 1). Without P, the estimated coefficients for formononetin were positive and significant at the 5 % level for both roots and active nodules (Fig. 3), indicating that AMF colonization increased linearly with increasing doses of formononetin within the studied interval when no P fertilizer was applied. At 60 and 120 kg P ha−1, the estimated coefficients for formononetin were non-significant at the 5 % level for roots and active nodules. For inactive nodules, the effect of formononetin on AMF colonization was negative and significant at the 5 % level at 120 kg P ha−1. Fitted values for all models for the combination phosphorus and formononetin are presented in Table S1.
Coefficients for the effect of formononetin on AMF colonization derived from the models shown in Table 1. The horizontal bars indicates the 95 % confidence interval. Coefficients are not statistically significant when their confidence interval includes zero (vertical line)
The positive effect of formononetin on the AMF colonization of roots has been widely reported (Siqueira et al. 1991; Davies et al. 2005a, b; Antunes et al. 2006; Catford et al. 2006; Novais and Siqueira 2009); however, this is the first report to describe the effect of this isoflavonoid on the colonization of nodules. The mechanisms of the effect of formononetin on root AMF colonization were not yet elucidated, but it is known that exogenous application of formononetin can reduce peroxidase activity (Fries et al. 1998) and induce the catalase activity (Lambais et al. 2003), which are enzymes associated with the control of plant defense during AMF root colonization. Formononetin also directly stimulates AMF spores germination and hyphae growth (Nair et al. 1991).
According to Vidal-Dominguez et al. (1994), nodules growing in non-mycorrhizal roots can be directly colonized by external hyphae. Consequently, nodule colonization does not only occur from the internal root mycelium. For that reason, formononetin may stimulate AMF colonization of the nodule in a manner similar to its action on root. Furthermore, the increase in AMF colonization of the roots caused by the formononetin increases the likelihood of colonization of nodules by hyphae originated from colonized roots.
The similarity between the increasing rates of AMF colonization in response to decreasing dosages of P and increasing doses of formononetin in roots and active nodules may likewise be explained; it has been widely reported that with high availability of P, the plant negatively regulates AMF colonization of roots (Siqueira et al. 1984; Siqueira and Colozzi-Filho 1986; Sena et al. 2004; Nogueira and Cardoso 2006, 2007; Sheng et al. 2012) even in the presence of biostimulants (Rodríguez and Gómez 2011).
Our results reopen the discussion of whether or not the colonization of active nodules by AMF directly affects nitrogen fixation. This subject has not been addressed since 2006. This interaction could be synergistic, neutral or competitive. The fact that we did not observe arbuscules in the colonized nodules supports the hypothesis that this interaction is neutral. In addition, the fact that nodules remain active after colonized by AMF supports the neutrality of the interaction.
4 Conclusions
Increasing doses of formononetin linearly increase the colonization of nodules and roots of Glycine max by AMF when no P was applied; however, this effect is reduced or eliminated by 60 and 120 kg P ha−1, respectively, or when nodules are inactive. Soybean active nodules under field conditions were more colonized by AMF than inactive nodules.
References
Ahmad MH (1995) Compatibility and coselection of vesicular-arbuscular mycorrhizal fungi and Rhizobia for tropical legumes. Crit Rev Biotechnol 15:229–239. doi:10.3109/07388559509147410
Antunes PM, Rajcan I, Goss MJ (2006) Specific flavonoids as interconnecting signals in the tripartite symbiosis formed by arbuscular mycorrhizal fungi, Bradyrhizobium japonicum (Kirchner) Jordan and soybean (Glycine max [L.] Merr.). Soil Biol Biochem 38:533–543. doi:10.1016/j.soilbio.2005.06.008
Azcón R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol 117:399–404. doi:10.1111/j.1469-8137.1991.tb00003.x
Baird LM, Caruso KJ (1994) Development of root nodules in Phaseolus vulgaris inoculated with Rhizobium and mycorrhizal fungi. Int J Plant Sci 155:633–639. doi:10.1086/297203
Bouyoucos GJ (1951) A recalibration of the hydrometer method for making analysis of soils. Agric J 43:433–437. doi:10.2134/agronj1951.00021962004300090005x
Catford JG, Staehelin C, Lerat S, Piche Y, Vierheilig H (2003) Suppression of arbuscular mycorrhizal colonisation and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with nod factors. J Exp Bot 54:1481–1487. doi:10.1093/jxb/erg156
Catford JG, Staehelin C, Larose G, Piché Y, Vierheilig H (2006) Systemically suppressed isofavonoids and their stimulating effects on nodulation and mycorrhization in alfalfa split-root systems. Plant Soil 285:257–266. doi:10.1007/s11104-006-9012-8
Davies JFT, Calderón CM, Huaman Z, Gómez R (2005a) Influence of a flavonoid (formononetin) on mycorrhizal activity and potato crop productivity in the highlands of Peru. Sci Hortic 106:318–329. doi:10.1016/j.scienta.2005.04.013
Davies JFT, Calderón CM, Huaman Z (2005b) Influence of Arbuscular Mycorrhizae indigenous to Peru and a flavonoid on growth, yield, and leaf elemental concentration of ‘Yungay’ potatoes. Hortscience 40:381–385
Douds DD Jr., Nagahashi G, Pfeffer PE, Reider C, Kayser WM (2006) On-farm production of AM fungus inoculum in mixtures of compost and vermiculite. Bioresour Technol 97:809–818. doi:10.1016/j.biortech.2005.04.015
Franzini VI, Azcón R, Latanze-Mendes F, Aroca R (2010) Interaction between Glomus species and rhizobium strains affect the nutritional physiology of drought stressed legume hosts. J Plant Physiol. doi:10.1016/j.jplph.2009.11.010
Fries LLM, Pacovsky RS, Safir GR (1998) Influence of phosphorus and formononetin on isozyme expression in the Zea mays–Glomus intraradices symbiosis. Physiol Plant 103:172–180. doi:10.1034/j.1399-3054.1998.1030204.x
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. doi:10.1111/j.1469-8137.1980.tb04556.x
Giovannetti M, Avio L, Sbrana C, Citernesi AS (1993) Factors affecting appressorium development in the vesicular arbuscular mycorrhizal fungus Glomus mosseae (Nicol & Gerd.) Gerd. and Trappe. New Phytol 123:114–122
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem 41:1233–1244. doi:10.1016/j.soilbio.2009.03.005
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488. doi:10.1016/S0953-7562(89)80195-9
Lambais MR, Ríos-Ruiz WF, Andrade RM (2003) Antioxidant responses in bean (Phaseolus vulgaris) roots colonized by arbuscular mycorrhizal fungi. New Phytol 160:421–428. doi:10.1046/j.1469-8137.2003.00881.x
Mehlich A (1953) Determination of P, Ca, Mg, K, Na and NH4. North Carolina Soil Testing Laboratories, Raleigh
Minchin FR, James EK, Becana M (2008) Oxygen diffusion, production of reactive oxygen and nitrogen species, and antioxidants in legume nodules. In: Dilworth MJ, James EK, Sprent JI, Newton WE (eds) Nitrogen-fixing leguminous symbioses. Springer, Dordrecht, pp. 321–353
Moreira FMS, Siqueira JO (2006) Microbiologia e bioquímica do solo. Editora UFLA, Lavras
Mortimer PE, Pérez-Fernández MA, Valentine AJ (2008) The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol Biochem 40:1019–1027. doi:10.1016/j.soilbio.2007.11.014
Nair MG, Safir GR, Siqueira JO (1991) Isolation and identification of vesicular-arbuscular mycorrhiza-stimulatory compounds from clover (Trifolium repens) roots. Appl Environ Microbiol 57:434–439
Nogueira MA, Cardoso EJBN (2006) Plant growth and phosphorus uptake in mycorrhizal rangpur lime seedlings under different levels of phosphorus. Pesq Agrop Brasileira 41:93–99. doi:10.1590/S0100-204X2006000100013
Nogueira MA, Cardoso EJBN (2007) Phosphorus availability changes the internal and external endomycorrhizal colonization and affects symbiotic effectivenes. Sci Agric 64:295–300. doi:10.1590/S0103-90162007000300013
Novais CB, Siqueira JO (2009) Formononetin application on colonization and sporulation of arbuscular mycorrhizal fungi in Brachiaria. Pesq Agropec Brasileira 44:496–502. doi:10.1590/S0100-204X2009000500009
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Powell CL, Bagyaraj DJ (1984) VA mycorrhiza. CRC Press, Boca Raton
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rodríguez NF, Gómez R (2011) Asociación simbiótica entre hongos micorrízicos arbusculares y el sistema radicular de plántulas de cacao (Theobroma cacao L.): efecto de la formononetina y la disponibilidad de fósforo en el suelo. Rev Corpoica Cienc Tecnol Agropec 12:77–85
Scheublin TR, van der Heijden MGA (2006) Arbuscular mycorrhizal fungi colonize nonfixing root nodules of several legume species. New Phytol 172:732–738. doi:10.1111/j.1469-8137.2006.01858.x
Scheublin TR, Karyn PR, Young JPW, van der Heijden MGA (2004) Non-legumes, legumes, and root nodules harbour different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70:6240–6246. doi:10.1128/AEM.70.10.6240-6246.2004
Sena JOA, Labate CA, Cardoso EJBN (2004) Caracterização fisiológica da redução de crescimento de mudas de citros micorrizadas em altas doses de fósforo. Rev Bras Ciênc Solo 28:827–832. doi:10.1590/S0100-06832004000500005
Sheng M, Lalande R, Hamel C, Ziadi N, Shi Y (2012) Growth of corn roots and associated arbuscular mycorrhizae are affected by long-term tillage and phosphorus fertilization. Agron J 104:1672–1678. doi:10.2134/agronj2012.0153
Siqueira JO, Colozzi-Filho A (1986) Micorrizas vesículo-arbusculares em mudas de cafeeiro. II. Efeito do fósforo no estabelecimento e funcionamento da simbiose. Rev Bras Ciênc Solo 10:207–211
Siqueira JO, Hubbell DH, Valle RR (1984) Effect of phosphorus on formation of the vesicular-arbuscular mycorrhizal symbiosis. Pesq Agrop Brasileira 19:1465–1474
Siqueira JO, Safir GR, Nair MG (1991) Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytol 118:87–93. doi:10.1111/j.1469-8137.1991.tb00568.x
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, Amsterdam
Steinberg PD, Rillig MC (2003) Differential decomposition of arbuscular mycorrhizal fungal hyphae and glomalin. Soil Biol Biochem 35:191–194. doi:10.1016/S0038-0717(02)00249-3
Udvardi M, Poole PS (2013) Transport and metabolism in legume-rhizobia symbioses. Annu Rev Plant Biol 64:781–805. doi:10.1146/annurev-arplant-050312-120235
Vadez V, Beck DP, Lasso JH, Drevon J-J (1997) Utilization of the acetylene reduction assay to screen for tolerance of symbiotic N2 fixation to limiting P nutrition in common bean. Physiol Plant 99:227–232
Vidal-Dominguez MT, Azcón-Aguilar C, Barea JM (1994) Preferential sporulation of Glomus fasciculatum in the root nodules of herbaceous legumes. Symbiosis 16:65–73
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Xavier LJC, Germida JJ (2002) Response of lentil under controlled conditions to co-inoculation with arbuscular mycorrhizal fungi and rhizobia varying in efficacy. Soil Biol Biochem 34:181–188. doi:10.1016/S0038-0717(01)00165-1
Xiao TJ, Yang QS, Ran W, Xu GH, Shen QR (2010) Effect of inoculation with arbuscular mycorrhizal fungus on nitrogen and phosphorus utilization in upland rice-mungbean intercropping system. Agric Sci 9:528–535. doi:10.1016/S1671-2927(09)60126-7
Xie ZP, Staehelin C, Vierheilig H, Wiemken A, Jabbouri S, Brough-Ton WJ, Ègeli-Lange VR, Boller T (1995) Rhizobial nodulation factors stimulate mycorrhizal colonization of nodulating and nonnodulating soybeans. Plant Physiol 108:1519–1525
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. doi:10.1111/j.2041-210X.2009.00001.x
Acknowledgments
We acknowledge the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and the Ministry of Science and Technology (Ministério de Ciência e Tecnologia) (MCT/CNPq/Ct-AGRO N° 69/2009) for financial support and for a scientific initiation fellowship to Jacqueline Savana da Silva and Teotonio Soares de Carvalho, a doctoral fellowship to Jessé Valentim dos Santos, and a research productivity fellowship to Fatima Maria de Souza Moreira. We also acknowledge the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Capes) for a doctoral fellowship to Paula Rose de Almeida Ribeiro.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Table S1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Savana da Silva, J., Soares de Carvalho, T., Valentim dos Santos, J. et al. Formononetin stimulates mycorrhizal fungi colonization on the surface of active root nodules in soybean. Symbiosis 71, 27–34 (2017). https://doi.org/10.1007/s13199-016-0408-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-016-0408-9