Abstract
The fungal root endophytes Chaetomium globosum, Epicoccum nigrum and Piriformospora indica have value as biocontrol and biofertilising organisms in barley, but have not been well tested at low temperatures. This study assessed the efficacy of the endophytes on barley varieties grown under low temperature stress with variable nutrient input. Seed from three cultivars of spring barley were inoculated with one of the three fungal root endophyte isolates – C. globosum, E. nigrum or P. indica - and grown in low temperature under higher and lower nutrient input regimes. Compared with the control, for P.indica-inoculated plants with the higher nutrient input, flowering was earlier and grain dry weight significantly greater for all barley varieties by a mean of 22 %. The nitrogen and carbon content of the grains did not differ significantly between treatments. Chaetomium globosum and Epicoccum nigrum conferred no significant benefits under either nutrient regime. Piriformospora indica is amenable to axenic culture, sporulates readily and can be multiplied rapidly, suggesting that it could be developed as an effective crop treatment in low temperature stressed barley and may have the potential to increase crop yield in colder growing conditions provided that adequate nutrients are supplied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Barley (Hordeum vulgare L.) is the world’s fourth most important cereal crop, grown on 56 Mha with a 2005 – 2008 mean production of 1.43 × 1011 kg (Newton et al. 2011). It can be grown profitably on marginal, stress-prone land and is more cold-tolerant than most other cereal crops (Visioni et al. 2013). High inputs of chemical fertilisers, pesticides and fungicides are required to maintain barley yields in less than optimal growing conditions (Powell and Jutsum 1993; Underwood 2000), so ways of reducing the economic and ecological costs associated with chemical use are needed. Biocontrol and biofertilisation treatments using fungal root endophytes may help to reduce these costs while still maintaining yield (Murphy et al. 2013).
Fungal root endophytes are non-mycorrhizal associates that spend most or part of their lives within plant root tissue without inducing pathogenic symptoms (Stone et al. 2004; Schulz and Boyle 2006; Weiss et al. 2011). They are known to infect a wide variety of plants, including many important crops (Qiang et al. 2011). Benefits to barley and other plants colonised by endophytic root fungi include increased yield (Achatz et al. 2010; Fávaro et al. 2012), enhanced resistance to pathogens and herbivores (Waller et al. 2008; Cheplick and Faeth 2009; Felle et al. 2009; Rahnamaeian et al. 2009) and increased abiotic stress tolerance (Waller et al. 2005; Baltruschat et al. 2008; Redman et al. 2011).
One particular fungal root endophyte, Piriformospora indica, discovered in north-west India in 1997 (Verma et al. 1998), has been extensively studied and reviewed (Singh et al. 2003; Ghahfarokhi and Goltapeh 2010; Qiang et al. 2011; Ansari et al. 2013; Unnikumar et al. 2013) and has become the model experimental organism for the study of fungal root endophyte interactions (Oelmüller et al. 2009). The complete genome of P. indica has also recently been described (Zuccaro et al. 2011). The barley-P. indica relationship has also been well studied, and improvements in yield (Schäfer et al. 2009, Achatz et al. 2010), salt tolerance (Waller et al. 2005; Baltruschat et al. 2008) and pathogen resistance (Waller et al. 2008; Felle et al. 2009; Rahnamaeian et al. 2009) induced by the fungal colonization have all been demonstrated. At least one commercial product containing P. indica as the active component has been developed as a plant treatment (Varma et al. 2012), and products using other endophytes have also been marketed (Soytong et al. 2001; Rolston and Agee 2007). None of these products have managed to establish a firm reputation for efficacy in all situations, due in most part to confounding differences in growth conditions (anecdotal evidence), but there are signs of significant promise in a recent commercial launch (Jones 2013).
While Achatz et al. (2010) showed that there was no relationship between nitrogen (N) input and P. indica colonisation, Lahrmann et al. (2013) demonstrated that lower nitrogen (N) input was associated with greater colonisation of barley roots by P. indica. A reduction in N fertilisation has also been shown to increase endophyte concentration in the leaves of the forage and amenity grass, Lolium perenne (Rasmussen et al. 2007). Piriformospora indica is known to survive and have beneficial effects on vegetables grown in cold, arid desert conditions (Murugan 2011), but studies in central European field experiments using winter wheat and overwintering inoculants of P. indica produced no significant increases in yield (Serfling et al. 2007). We aimed to determine if any of these effects translate to barley grown under controlled low temperature and nutrient limitation stress. A recent review of the fungal root endophytes of barley emphasised that more work needs to be done on endophyte-induced cold tolerance and nutrient limitation in barley (Murphy et al. 2013). Our study partly addressed that need by examining how P. indica and two other fungal root endophytes (Chaetomium globosum and Epicoccum nigrum) interact with different barley cultivars at low growing temperature and nutrient limitation.
2 Materials and methods
2.1 Isolation of endophytes
Spring barley root samples from 16 random selections of the cultivars ‘Overture’, ‘Propino’ and ‘Sy Taberna’ were collected prior to harvest from the Department of Agriculture, Forestry and the Marine (DAFM) trials site at Backweston, Co. Kildare, Ireland (53.348 N, 6.488 W). Roots were washed in running tap water and surface sterilised with 70 % ethanol for 1 min, soaking for 4 mins in 5 % NaClO then rinsing three times in sterile ultra-pure water. 28 root pieces of 5 mm length were cut from each sample and distributed evenly between two 900 mm culture dishes in half-strength Fluka modified malt extract agar (Fluka 38954). Inoculated plates were incubated in the dark at 27 C in ambient humidity and inspected daily for any emergent fungal hyphae. Individual hyphal extension growths were removed, and separately subcultured on the same medium. Isolate cultures were incubated in the dark at 27 C in ambient humidity for 28 days.
2.2 Endophyte selection
Endophyte isolates were grouped according to their colony characteristics and 14 representative morphotypes were identified by using a combination of morphological and DNA characters. For the DNA analysis, 20 mg of fungal material was scraped from the agar surface and placed into shaker tubes. DNA was extracted using a Qiagen DNeasy mini kit, following the Qiagen protocol, producing 200 μl of DNA extract for each isolate. PCR was carried out on the DNA extracts using the ribosomal DNA (rDNA) internal transcribed spacer (ITS) primers ITS4 and ITS5 (White et al. 1990). PCR products were cleaned up using Exonuclease (New England Biolabs) and Shrimp Alkaline Phosphatase (ExoSAP (Roche)). Purified PCR products underwent cycle sequencing using the reverse ITS4 primer (4 pmol) or forward ITS5 primer (4 pmol) in separate reactions with the ABI BigDye 3.1 kit (Foster City, CA). The products were further purified using a BigDye XTerminator purification kit and protocol. DNA was sequenced using an ABI Hitachi 3130xL Genetic Analyzer. The isolate sequences were compared with GenBank accessions using the Basic Local Alignment Search Tool (BLAST), and identified using morphological and DNA characters. Two of the fungal species isolated have been reported as beneficial endophytes, Chaetomium globosum 13ENDPP12 (our GenBank accession: KF018413) (Soytong et al. 2001; Dhingra et al. 2003; Soytong and Ratanacherdchai 2005) and Epicoccum nigrum 13ENDPP3 (our GenBank accession: KF018414) (Hashem and Ali 2004; Fávaro et al. 2012). These two species and a laboratory strain of Piriformospora indica (P. indica-DSM11827 from Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany), were chosen as experimental treatments for this study.
2.3 Endophyte purification
Cultures for the experimental treatments were grown from single spores. To obtain pure isolate cultures from which spores could be harvested and in order to maintain and/or stabilise important endophyte biochemical and physiological characteristics, a cultivation cycle was followed. Fungal spores were collected from the initial isolate culture by rolling a sterilised, moistened cocktail stick over the culture surface and swirling it in a 2 ml microcentrifuge tube filled with pure water. Fungal mycelium was regrown from single spores obtained by swirling a sterilised metal probe in the spore solution and streaking it on the half-strength Fluka 38954 medium. After incubation in the dark at 25 C, any plates that had contaminants were discarded and pieces of the remaining pure cultures were inoculated on sterilised barley seed in agar dishes (see below for details). After germination and 1 week of growth, root pieces were harvested and sterilised then cultured on fresh agar dishes. Only endophyte cultures which were confirmed as the original inoculant were used to harvest further spores as treatments or to start the single spore storage cycle again. This cycle provides a way of conserving the desirable characteristics of the endophytes in the long term.
2.4 Barley cultivar selection
Untreated freshly collected seeds of 3 spring barley cultivars, ‘Frontier’, ‘Propino’ and ‘Soldo’, were used (Goldcrop Seeds, Cork, Ireland). Seeds were stored in cool, dry, dark conditions in sealed paper bags. Three barley cultivars were used in order to obtain both cultivar-specific and combined results for all cultivars. These cultivars were chosen as they are popular, proven performers at low temperatures and they have different characteristics, particularly for Pyrenophora teres (net blotch) resistance. Frontier is of Danish origin (Cross: (Tavern) × [Annabell × (Lux × Ferment)]) and is widely grown. It is a fully recommended cultivar, suitable for feed, and has high yield potential and short straw with moderate susceptibility to mildew and Rhynchosporium secalis infection. It is, however, resistant to net blotch. Propino is a British cultivar (Cross: (Quench × NFC Tipple)) suitable for both malting and feed, with very high yield potential and good resistance to both Rhynchosporium and net blotch. Soldo is of German origin ((NFC Tipple × Braemar) × NFC 401-17) and is new to the market in 2013. It has high, reliable and early yield, and is suitable for use as feed, with malting potential. In contrast to Frontier and Propino, it is not known for good net blotch resistance.
2.5 Experimental procedure
Seeds were surface-sterilised by soaking in 5 % NaClO for 2 h, rinsing 3 times with 70 % ethanol and then rinsing 5 times with pure water. The growth compost consisted of sterilised coarse vermiculite to which was added 1.43 g P4 broadleaf water absorbing polymer granules (Agricultural Polymers International Ltd.) per 1.5 l of vermiculite. The compost was dry mixed, moistened with tap water and placed into 120 × 1.5 litre washed and sterilised (soaked for 2 h in 5 % NaClO then rinsed × 5 with tap water) plastic pots.
Five seeds of each barley cultivar were sown at 30 mm depth on top of 5 mm2 endophyte culture plugs (C. globosum, E. nigrum, P. indica) or control pure agar plugs in 10 replicate pots for each treatment, giving 30 replicate plants per treatment (3 × endophyte inoculated and 1 × control). Pots were placed into two controlled environment chambers, then randomly relabelled with a single number (1–120) by a third party, to produce a double-blind setup. The environmental settings were programmed to produce a 9 h photoperiod at a compost surface illumination of 210 μmol.m−2 s−1, a constant temperature of 8 C and 70 % relative humidity. The photoperiod was extended by 1.5 h every 3 weeks until it reached 15 h (at day 84). The temperature was raised to 13 C at day 70 and to 16 C at day 84. The temperature was therefore maintained at the acclimation temperature of 8 C for the first half of the growing period. The photoperiod was lengthened and temperature raised to speed up plant development.
Three agar-filled covered culture dishes containing 5 sterilised seeds of each cultivar of barley were kept in the growth chambers during the experimental period to monitor any seed-produced endophyte growth. Further, agar-filled covered culture dishes containing 5 split sterilised and unsterilised seeds of each cultivar of barley were incubated in the dark at 25 C.
The seedlings were thinned to 3 plants per pot 7 days after germination. Plants were given a liquid fertiliser (Bayer Phostrogen®) at each watering after germination. Half of the plants were given lower nutrient inputs (LO) and half were given higher (HI) nutrients; for the HI nutrient treatments, the total nutrient input per pot was: ammoniacal N = 0.04728 g, ureic N = 0.2836 g, Total N = 0.3308 g, P = 0.208 g, K = 0.5292 g, Mg = 0.0344 g, S = 0.0714 g, Ca = 0.0338 g and traces of Boron, Copper, Iron, Manganese, Molybdenum and Zinc; for LO nutrient treatments, the total nutrient input per pot was halved for all elements. These treatments were shared among the three plants per pot. The HI treatment contained the recommended input of fertiliser for hydroponically-grown plants (2 g Bayer Phostrogen® per 5 l water, see http://www.phostrogen.co.uk/gardenerscorner/guides). To ensure healthy growth, the plants were sprayed with plain water once a week and the aggregate washed through with plain water monthly to remove any accumulation of nutrient salts.
2.6 Measurements and data analysis
The number of days to reach each selected Zadoks stage (Zadoks et al. 1974) was recorded for each plant. The height of each plant was recorded prior to the increase in incubator temperature from 8 to 13 C. Any disease or physiological stress symptoms were recorded, and suspected diseases were identified both during and at the end of the experiment. There were no visible sporulating structures produced on the plants during the experiment so there was little, if any, cross-contamination.
Plants were grown for 147 days (21 weeks) from date of sowing (the approximate time required to reach maturity in northern temperate field plantings), then harvested and processed over a period of 3 days. Pots were selected for processing in random order, 40 pots per day. All plants had at least reached flowering stage (Zadoks stage 61), and the most advanced plants were at the soft dough stage (Zadoks stage 85). Fresh weights and measurements were recorded for grains and above-ground parts, then all plant parts (including roots) were separately dried in ovens for 1 week at 65 C, and dry weights recorded. Measurements were made per pot of 3 plants and, in addition to fresh and dry weights, included the number of tillers, number of heads, maximum number of nodes per stem, height of plant to tip of highest awn and number of grains.
The nitrogen (N) and carbon (C) content of the grains was measured using an Elementar vario EL Cube. Approximately 7 mg of crushed and homogenised grain from each sample was used to determine proportions of total N and C.
Treatment identity was only revealed after all measurements had been made.
Four 5 mm pieces of mid-section root from each plant were surface-sterilised and incubated on half-strength MEA at 25 C in the dark to test for endophyte presence.
Data analysis by ANOVA was performed using the Data Analysis modules provide by Microsoft Excel 2010®.
3 Results
3.1 Endophyte identities
We isolated 33 individual fungal cultures from the root pieces of the 16 barley plants sampled. From these, we chose 14 representative fungal morphotypes. Of the 14 chosen isolates, ten fungal species, representing 6 orders (Diaporthales, Eurotiales, Helotiales, Pleosporales, Sordariales, Xylariales) and 8 genera (Cadophora, Chaetomium, Epicoccum, Gaeumannomyces, Leptodontidium, Microdochium, Ophiosphaerella, Penicillium), were identified using a combination of BLAST searches with ITS DNA sequences and morphological characteristics (Table 1). Five of these isolate sequences which were of good quality were deposited in GenBank (accessions KF018413 – KF018417). Mean ITS sequence length for the GenBank deposits was 604 bp. While most of the isolates have been reported as plant pathogens, only Chaetomium globosum 13ENDPP12 (GenBank accession: KF018413) and Epicoccum nigrum 13ENDPP3 (GenBank accession: KF018414) are recognised biocontrol organisms.
3.2 Early growth and development measurements
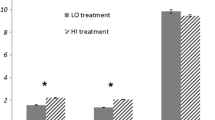
We compared early growth and development of the plants by measuring the germination date, number of seeds germinated, the height of the plant from compost surface to tip of topmost leaf and the number of days from seed sowing to first flower. Though there was very little difference in germination success between treatments, the HI nutrient input plants flowered significantly later than the LO nutrient input (2-way ANOVA, F1,48 = 6.6, P = 0.013). Piriformospora indica-inoculated plants subsequently performed better for nearly all early growth and development parameters for both LO and HI nutrient input (Table 2). A strong interaction was indicated between nutrient input and number of days to flowering (2-way ANOVA, F1,48 = 5.11, P = 0.028), where for the HI nutrient input P. indica-inoculated plants flowered 5 days earlier than the control. Piriformospora indica-inoculated plants reached Zadoks stage 71 (kernel watery ripe) 7 days earlier than the control for HI nutrient input (ANOVA, F1,28 = 7.59, P = 0.01), but 2 days later for LO (data not shown). The best performing barley cultivar-endophyte combination was Propino with P. indica; under both nutrient regimes, this combination was greater in height at temperature change and flowered earlier (Supplementary Table S1). Different barley cultivars have different growth and development characteristics, but we found mostly minor differences between cultivars (Table 3), though the net-blotch resistant cultivars Frontier and Propino both had observably less early disease load than the more susceptible Soldo.
All sterilised control seeds on agar in the growth chambers had no external fungal growth after 7 days, and all germinated, indicating that surface sterilisation was successful. The control surface-sterilised split seeds produced mycelia (less than 10 mm diameter) of only one fungus, Pyrenophora teres, attached to the seed after 7 days but the split unsterilised seeds had produced at least 6–7 different types of fungal growth.
3.3 Harvest measurements
We compared final harvest characteristics of the plants by measuring the height of the plant from compost surface to the tip of the highest awn, number of tillers, the number of heads, the number of grains, the fresh and dry weight of grains, the fresh and dry weight of shoots and the dry weight of roots. The number of tillers and heads per plant at harvest was similar for all treatments (Table 4), but there were some differences in the other harvest parameters. Compared with all other treatments, mean root dry weight was greater and mean shoot dry weight lower (though not significantly) in control plants with LO nutrient inputs. Compared with the P. indica treatment, control plants also had the greatest mean dry weight of grains with LO nutrient input (ANOVA, F1,24 = 3.27, P = 0.08). In contrast, the mean height and shoot dry weight were both greater than the control for all endophytes. However, under the HI nutrient input regime, the P. indica-inoculated plants performed better in almost all respects compared with all other treatments, despite having a lower mean height than the control (ANOVA, F1,28 = 6.64, P = 0.015). For HI nutrient input, comparison of harvest parameters for P. indica treatments and controls indicated a strong interaction between nutrient input and grain dry weight (2-way ANOVA, F1,48 = 7.59, P = 0.008), where P. indica inoculated plants had significantly greater grain dry weight (ANOVA, F1,24 = 4.75, P = 0.039) than the control. Shoot dry weight for the P.indica-inoculated plants was also greater than the other treatments, though not significantly. The barley cultivar-endophyte combination of Soldo with P. indica had the greatest dry weight of grains and the combination Propino-P. indica had the greatest dry shoot weight (Supplementary Table S1).
While cultivar comparison between the HI and LO treatments followed the expected pattern (greater values for the HI treatments), the Soldo cultivar had a slightly lower grain dry weight for the HI treatment (Table 5).
The nitrogen and carbon content of the grains did not differ significantly between treatments (Table 6) or cultivars (a series of 2-way ANOVAs with interaction statistics ranging from: F1,48 = 0.25, P = 0.62 to F1,48 = 0.11, P = 0.74), but overall the controls differed slightly for N (15 % greater), C (5 % greater) and C/N (8 % lower) compared with the endophyte treatments for both nutrient regimes.
Slight net-blotch infection was observed on 41 plants at 69 days from germination, rising to 74 plants (62 % of total plants) at final harvest. However, the symptoms were not severe, with the worst-affected plants having less than 50 % of leaves with visible signs of infection. Net-blotch infection symptoms were not significantly different for any of the treatments (ANOVA, F1,118 = 0.56, P = 0.45). All of the root pieces from the endophyte inoculated plants produced growth from root endophytes at the end of the experiment, which matched the morphology of the original inoculants.
4 Discussion
Two of the most important parameters used to determine the effectiveness of any crop treatments are the time taken to reach flowering and maturity and the grain dry weight at harvest. Our results have shown that the improvements in these factors due to colonization by the fungal root endophyte Piriformospora indica in low temperature stressed barley are positively related to nutrient input. These results suggest that P. indica, despite its origin in hot desert conditions –and contrary to earlier reports (Serfling et al. 2007) - may persist and have significant beneficial effects in barley grown in cool temperate conditions, provided adequate nutrients are supplied. This suggests that treatments based on P. indica inoculation of barley crops may even have the potential to extend the growing season in cooler climates. In contrast, colonization by C. globosum and E. nigrum had no beneficial effects, with barley plants having a neutral to negative response. Both of these organisms have been associated with reduced pathogen infection in other crop species (Soytong et al. 2001; Hashem and Ali 2004; Soytong and Ratanacherdchai 2005; Istifada and McGee 2006; Fávaro et al. 2012), but under our experimental conditions, these benefits were not shown for barley.
The diversity among the fungal isolates identified from the field collected barley roots was striking for such a small number of individuals, and more extensive sampling may lead to the discovery of even greater diversity among the fungal root endophytes of barley. Although some of the isolates are known barley pathogens (Cadophora sp., Gaeumannomyces graminis, Microdochium nivale), the biocontrol/biofertilisation potential of several other isolates has not been extensively studied. Of these, Penicillium glabrum may hold the greatest promise. In unpublished work, we have found this fungus to aggressively supress barley pathogens in vitro, and it is extremely easy to culture and propagate, and sporulates readily on several different media. Another interesting feature of P. glabrum is high cellulolytic activity (Sukumaran et al. 2005; Karboune et al. 2008; de Castro et al. 2010) and it’s remarkable efficiency in extracting fermentable sugars from oil palm empty fruit bunch (Cabezas et al. 2012).
Previous studies which examined the effects of different nutrient regimes on the efficacy of P. indica in barley have found either no significant consistent relationship (Achatz et al. 2010) or a diametrical relationship (Lahrmann et al. 2013). Achatz et al. (2010) showed that higher grain yield in barley was induced by P. indica colonization, independently of markedly different phosphate (P) and nitrogen (N) fertilisation levels. Lahrmann et al. (2013) found that P. indica colonisation of Arabidopsis thaliana was increased due to lower N input. Increased colonisation and activity by P. indica due to lower N input has also been shown in the related grassy species Lolium perenne (Rasmussen et al. 2007). Though we did not quantify absolute levels of P. indica root colonization, the fungus was present in the associated root samples tested at the end of the experiment. We found similar effects as other studies regarding P. indica-induced earlier flowering and higher biomass (Achatz et al. 2010; Das et al. 2012), but, again, only for the HI nutrient input.
Proportions of total N and C in the grains did not differ significantly between treatments, although the controls did accumulate slightly higher proportions of both N and C in the grains. The relatively high N content of the grain under both nutrient regimes for the 3 cultivars we used suggests that under our particular growth conditions all three are unsuitable for malting. The United Kingdom malt industry (http://www.ukmalt.com/barley-requirements) requires a crude protein content of between 10 and 10.9 % (equivalent to 1.6 – 1.75 % total N), and our higher values for N (a mean of 2.05 %) indicates a below-optimum starch content in the grains (Broadbent and Palmer 2001). Under field conditions, the increased light available for photosynthesis and starch synthesis would be expected to produce an altered (and potentially more favourable for the malting industry) C/N ratio.
Our results clearly demonstrate a positive relationship between total nutrient input and the beneficial effects due to P. indica colonization of barley grown in low temperature. Clues as to the elemental limiting factor may be found in the fertiliser ‘recipe’ because Bayer Phostrogen® plant food is remarkable for its relatively low N (14 %) and relatively high P (10 %) and K (27 %) contents. In general, agricultural fertilisers would normally contain at least the same proportion of N as K, and often more, so even at our HI nutrient input the amount of N available to the plants was relatively low. As we have shown that P. indica colonization only produces beneficial effects for the barley with a higher nutrient input, it would seem that there is minimum amount of N that is needed for the fungus to produce a beneficial effect on barley grown at low temperature, and that this value lies somewhere between a total N input of 0.055 g and 0.11 g per plant. This translates to between 4.1 × 10−4 and 8.2 × 10−4g N per plant per day.
But in which partner is N limiting? Mycorrhizal fungal hyphae have been shown to absorb N more efficiently than plant roots (Chalot and Brun 1998; Hodge et al. 2001; Phillips et al. 2012), but this may not be true for endophytes such as P. indica which have relatively fewer scavenging hyphae external to the plant root (Deshmukh et al. 2006; Schafer and Kogel 2009). With LO nutrient input, the increased grain yield for the control plants relative to the endophyte treated plants indicates that the plant is sequestering N at the expense of the endophyte.
With low levels of N entering the system, the metabolic interdependence between host metabolism and fungal nutrient uptake (Lahrmann et al. 2013) combined with low temperatures may lead to reduced optimal metabolic processes in the endophyte, particularly affecting P. indica-induced increases in nitrate reductase activity (Sherameti et al. 2005). Low levels of N and reduced metabolic efficiency may also compromise the ability of the fungus to manufacture the amino acid tryptophan. Tryptophan is a precursor for the manufacture of indole acetic acid (IAA), which has been shown to be a key factor in the establishment of the barley-P. indica symbiosis (Hilbert et al. 2012; Waqas et al. 2012). At low temperature, P. indica can only increase barley grain yield above a certain threshold level of available nitrogen. Zuccaro et al. (2011) reported on the lack of genes for nitrogen metabolism in the genome of P. indica, and the activity of the associated proteins may be limited by low temperature and low N. With N input below the threshold level, P. indica colonization is slightly detrimental for barley yield, which contradicts the widely-held belief that endophytes, and particularly P. indica, are always beneficial, or at least neutral, for the host. In recent reviews (Murphy 2013; Murphy et al. 2013) evidence is presented to support the view that endophytes can be either a ‘friend or foe’ to barley, depending on prevailing circumstances, and our results seem to support that position. The neutral response of the plants to the proven biocontrol fungi C. globosum and E. nigrum also supports the conclusion that the particular combination of determining factors (e.g. genotypes, environment) necessary to promote benefits to the plants was not present.
Photosynthetic capacity rises with increasing nitrogen content but the temperature optimum for protein synthesis is related to acclimation temperature. For wheat grown at an acclimation temperature similar to ours (8 C) protein synthesis is optimal at 27.5 C (Larcher 2003), so the related N utilisation was also unlikely to be optimal at our maximum growth temperature of 16 C. Phostrogen® contains approximately 6 times the amount of ureic N than ammoniacal N, and any detrimental effects due to a high ratio of N supplied as ammonium are probably not present (Kaldorf et al. 2005). Even though N is strongly implicated as the limiting element for P. indica efficacy, a role for deficiencies of P and K (or even a micronutrient) cannot be ruled out.
Paradoxically, despite performing best in almost all respects with HI nutrient input, the P. indica inoculated plants had a lower mean height than all other treatments. The growth promoting effects related to P. indica colonization are well documented (Oelmüller et al. 2009; Franken 2012), and this is reflected in our result of greater biomass for the P. indica-inoculated plants. The lower height may result from an earlier cessation of apical growth due to earlier flowering and maturation induced by the endophyte (Achatz et al. 2010).
Higher yield associated with P.indica colonization in stressed plants is partly due to the endophyte-induced increase in antioxidant activity (Waller et al. 2005; Kumar et al. 2009; Sun et al. 2010; Ansari et al. 2013; Harrach et al. 2013). Photodamage of PS1, and a consequent reduction of photosynthetic efficiency and growth/yield, has been observed in low light illumination at chilling temperatures, resulting from reduced activity of active oxygen-scavenging enzymes (Tjus et al. 1998). Piriformospora indica associated stimulation of antioxidant activity may be a contributory factor to stress response in our study, though perhaps somewhat attenuated by the low temperature.
The increase in shoot biomass and grain yield associated with P. indica colonization was not reflected in root biomass, where there was a relative decrease in root biomass over shoot biomass. An endophyte-induced relative increase in root biomass over shoot biomass has been demonstrated in some grass species (Czarnoleski et al. 2012) including rice (Redman et al. 2011). Under our specific conditions, resources seem to have been preferentially allocated to above-ground parts for P. indica-colonized plants.
Seed-borne pathogens are particularly difficult to control, and we found that over half of the plants developed symptoms of Pyrenophora teres (net blotch) infection even though the seeds were sterilised before sowing. Although research using related cereal crops (Poling et al. 2008) has demonstrated an endophyte-mediated resistance to Pyrenophora teres (net blotch) and other seed-borne infections, protection against these pathogens related to endophyte colonization in barley has not yet been well studied. Endophyte-induced antimicrobial metabolites similar to those described in Poling et al. (2008) have been detected in barley (Nukina et al. 1979). Further work using controlled infection by P. teres may reveal endophyte-induced pathogen resistance in barley.
The challenge now is to extend research under these conditions into more realistic field experiments, particularly with regard to the potential contribution of N-fixing soil bacteria to any deliberate shortfalls in fertiliser application. Reducing agricultural inputs of N is of critical importance in the development of future strategies for more sustainable and environmentally friendly farming (Dobermann and Nelson 2013) and our results suggest that tailoring the nutrient and endophyte treatment combination may provide part of the solution. Gan et al. (2012) make the point that the key to lowering the carbon footprint of barley is to increase grain yield, reduce N inputs and improve N use efficiency. Discovering and developing potentially beneficial endophyte treatments through experimentation such as ours may make a significant contribution towards reducing the carbon footprint of barley.
We have determined that under low temperature and nutrient stressed growing conditions, there is a threshold level of N input above which P. indica colonization will be beneficial for barley. Future global climate change will result in local alterations in growing conditions, and the contribution of fungal root endophytes in enabling successful cultivation of barley in normally unsuitable situations may become crucial.
Some of the results from our study which contradict the findings from previous work may be directly due to the environmental conditions, particularly low temperature and nutrient status, and represent an important contribution to the growing body of knowledge regarding the Piriformospora indica-barley symbiosis.
Further experiments, including field testing, which involve fungal root endophyte colonization of barley using these organisms and others will give new insight into these results. Any further development of fungal root endophytes as inoculants for barley would have to demonstrate their ability to persist in the plant over the long term under natural conditions. The discovery of previously unrealised benefits associated with these fungi holds great future promise for developing economically and ecologically viable crop treatments for barley.
References
Achatz B, Rüden S, Andrade D, Neumann E, Pons-Kühnemann J, Kogel K-H, Franken P, Waller F (2010) Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil 333:59–70
Ansari MW, Trivedi DK, Sahoo RK, Gill SS, Tuteja N (2013) A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol Biochem: PPB/Société française de physiologie végétale 70:403–410
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel K-H, Schäfer P, Schwarczinger I et al (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180:501–510
Broadbent RE, Palmer GH (2001) Relationship between B-amylase activity, steeliness, mealiness, nitrogen content and the nitrogen fractions of the barley grain. J Inst Brewing 107:349–354
Cabezas L, Calderon C, Medina LM, Bahamon I, Cardenas M, Bernal AJ, Gonzalez A, Restrepo S (2012) Characterization of cellulases of fungal endophytes isolated from Espeletia spp. J Microbiol (Seoul, Korea) 50:1009–13
Chalot M, Brun A (1998) Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiol Rev 22:21–44
Cheplick GP, Faeth S (2009) Ecology and evolution of the grass-endophyte symbiosis. Oxford University Press USA, New York
Czarnoleski M, Olejniczak P, Górzyńska K, Kozłowski J, Lembicz M (2012) Altered allocation to roots and shoots in the endophyte-infected seedlings of Puccinellia distans (Poaceae). Plant Biol (Stuttgart, Germany) 15:1–10
Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M, Tuteja N, Johri AK, Varma A (2012) The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal Behav 7:103–112
de Castro A, de Albuquerque de Carvalho M, Leite S, Pereira N (2010) Cellulases from Penicillium funiculosum: production, properties and application to cellulose hydrolysis. J Indust Microbiol Biotechnol 37:151–158
Deshmukh S, Hückelhoven R, Schäfer P, Imani J, Sharma M, Weiss M, Waller F, Kogel K-H (2006) The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. PNAS 103:18450–18457
Dhingra OD, Mizubuti ESG, Santana FM (2003) Chaetomium globosum for reducing primary inoculum of Diaporthe phaseolorum f. sp. meridionalis in soil-surface soybean stubble in field conditions. Biocontrol 26:302–310
Dobermann A, Nelson R (2013) Solutions for Sustainable Agriculture and Food Systems: Technical Report for the Post-2015 Development Agenda. United Nations Sustainable Development Solutions Network. http://unsdsn.org/files/2013/09/130919-TG07-Agriculture-Report-WEB.pdf
Fávaro LCDL, Sebastianes FLDS, Araújo WL (2012) Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PloS One 7:e36826
Felle HH, Waller F, Molitor A, Kogel K-H (2009) The mycorrhiza fungus Piriformospora indica induces fast root-surface pH signaling and primes systemic alkalinization of the leaf apoplast upon powdery mildew infection. MPMI 22:1179–1185
Franken P (2012) The plant strengthening root endophyte Piriformospora indica: potential application and the biology behind. Appl Microbiol Biotechnol 96:1455–1464
Gan Y, Liang C, May W, Malhi SS, Niu J, Wang X (2012) Carbon footprint of spring barley in relation to preceding oilseeds and N fertilization. Int J Life Cycle Assess 17:635–645
Ghahfarokhi RM, Goltapeh ME (2010) Potential of the root endophytic fungus Piriformospora indica, Sebacina vermifera and Trichoderma species in biocontrol of take-all disease of wheat Gaeumannomyces graminis var. tritici in vitro. J Agric Technol 6:11–18
Harrach BD, Baltruschat H, Barna B, Fodor J, Kogel K-H (2013) The mutualistic fungus Piriformospora indica protects barley roots from a loss of antioxidant capacity caused by the necrotrophic pathogen Fusarium culmorum. MPMI 26:599–605
Hashem M, Ali E (2004) Epicoccum nigrum as biocontrol agent of Pythium damping-off and root-rot of cotton seedlings. Arch Phytopathol Plant Protect 37:283–297
Hilbert M, Voll LM, Ding Y, Hofmann J, Sharma M, Zuccaro A (2012) Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol 196:520–534
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297e299
Istifadah N, McGee P (2006) Endophytic Chaetomium globosum reduces development of tan spot in wheat caused by Pyrenophora tritici-repentis. Aust Plant Pathol 35:411
Jones N (2013) Food fuelled with fungi. Nature 504:199
Kaldorf M, Koch B, Rexer K-H, Kost G, Varma A (2005) Patterns of interaction between Populus Esch5 and Piriformospora indica: a transition from mutualism to antagonism. Plant Biol (Stuttgart, Germany) 7:210–218
Karboune S, Geraert PA, Kermasha S (2008) Characterization of selected cellulolytic activities of multi-enzymatic complex system from Penicillium funiculosum. J Agricult Food Chem 56:903–909
Kumar M, Yadav V, Tuteja N, Johri AK (2009) Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology (Reading, England) 155:780–790
Lahrmann U, Ding Y, Banhara A, Rath M, Hajirezaei MR, Döhlemann S, von Wirén N, Parniske M, Zuccaro A (2013) Host-related metabolic cues affect colonization strategies of a root endophyte. PNAS 110:13965–13970
Larcher W (2003) Physiological plant ecology. Springer-Verlag Berlin Heidelberg, Berlin
Murphy BR (2013) Fungal Infection in Barley Roots - Friend and Foe. In: Schneider C, Leifert C, Feldman F (eds) Endophytes for plant protection: The state of the art. Deutsche Phytomedizinische Gesellschaft, Braunschweig, pp 102–116
Murphy BR, Doohan FM, Hodkinson TR (2013) Fungal endophytes of barley roots. J Agric Sci. doi:10.1017/S0021859613000348
Murugan P (2011) Enhancing winter vegetable production in greenhouse of cold arid desert of Ladakh by inoculation of Piriformospora indica. DRDO Technol. Spectr.164–170
Newton AC, Flavell AJ, George TS, Leat P, Mullholland B, Ramsay L, Revoredo-Giha C, Russell J, Steffenson BJ, Swanston JS et al (2011) Crops that feed the world 4. Barley: a resilient crop? Strengths and weaknesses in the context of food security. Food Secur 3:141–178
Nukina M, Sassa T, Oyama H, Ikeda M (1979) Structures and biological activities of fungal macrolides, pyrenolide and resorcylide. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 36:2–9 (in Japanese)
Oelmüller R, Sherameti I, Tripathi S, Varma A (2009) Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 49:1–17
Phillips RP, Meier IC, Bernhardt ES, Grandy S, Wickings K, Finzi AC (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15:1042e1049
Poling SM, Wicklow DT, Rogers KD, Gloer JB (2008) Acremonium zeae, a protective endophyte of maize, produces dihydroresorcylide and 7-hydroxydihydroresorcylides. J Agr Food Chem 56:3006–3009
Powell KA, Jutsum AR (1993) Technical and commercial aspects of biocontrol products. Pestic Sci 37:315–321
Qiang X, Weiss M, Kogel K-H, Schäfer P (2011) Piriformospora indica-a mutualistic basidiomycete with an exceptionally large plant host range. Mol Plant Pathol 13:508–518
Rahnamaeian M, Langen G, Imani J, Khalifa W, Altincicek B, von Wettstein D, Kogel K-H, Vilcinskas A (2009) Insect peptide metchnikowin confers on barley a selective capacity for resistance to fungal ascomycetes pathogens. J Exp Bot 60:4105–4114
Rasmussen S, Parsons AJ, Bassett S, Christensen MJ, Hume DE, Johnson LJ, Johnson RD, Simpson WR, Stacke C, Voisey CR et al (2007) High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol 173:787–797
Redman RS, Kim YO, Woodward CJD, Greer C, Espino L, Doty SL, Rodriguez RJ (2011) Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PloS One 6:e14823
Rolston MP, Agee C (2007) Delivering quality seed to specification-The USA and NZ novel endophyte experience. In: Popay AJ, Thom ER (eds) Proc. 6th Int. Symp. Fungal Endophytes Grasses. Christchurch, N.Z.: N.Z. Grassl. Assoc., Inc., Dunedin, pp 229–231
Schafer P, Kogel K-H (2009) The Sebacinoid Fungus Piriformospora Indica : An Orchid Mycorrhiza which may increase host plant reproduction and fitness. In: Deising H (ed) Plant relationships. Springer, Berlin, pp 99–112
Schäfer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, Scholz U, Pons-Kühnemann J, Sonnewald S, Sonnewald U et al (2009) Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant Signal Behav 4:669–671
Schulz B, Boyle C (2006) What are Endophytes? In: Schulz BJE, Boyle CJC, Sieber TN (eds) Microb. Root Endophytes. Springer-Verlag, Berlin, pp 1–14
Serfling A, Wirsel SGR, Lind V, Deising HB (2007) Performance of the Biocontrol Fungus Piriformospora indica on Wheat Under Greenhouse and Field Conditions. Phytopathology 97:523–531
Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R (2005) The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in. J Biol Chem 280:26241–26247
Singh AA, Kumari M, Rai MK, Varma A (2003) Biotechnological Importance of Piriformospora indica Verma et al.-A Novel Symbiotic Mycorrhiza-like Fungus : An Overview. Indian J Biotechnol 2:65–75
Soytong K, Kanokmedhakul S, Kukongviriyapa V, Isobe M (2001) Application of Chaetomium species (Ketomium) as a new broad spectrum biological fungicide for plant disease control: A review article. Fungal Divers 7:1–15
Soytong K, Ratanacherdchai K (2005) Application of mycofungicide to control late blight of potato. J Agricultural Technol 1:19–32
Stone JK, Polishook JD, White JF (2004) Endophytic fungi. In: Mueller GM, Bills GF, Foster MS (eds) Biodivers. Fungi Invent. Monit. Methods. Elsevier Inc., Amsterdam, pp 241–270
Sukumaran R, Singhania R, Pandey A (2005) Microbial cellulases: Production, applications and challenges. National Institute of Science Communication and Information Resources, New Delhi, India
Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B (2010) Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol 167:1009–1017
Tjus SE, Møller BL, Scheller HV (1998) Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures. Plant Physiol 116:755–764
Underwood AK (2000) Adjuvant Trends for the New Millennium 1. Weed Technol 14:765–772
Unnikumar KR, Sree KS, Varma A (2013) Piriformospora indica: a versatile root endophytic symbiont. Symbiosis 60:107–113
Varma A, Bajaj R, Agarwal A, Asthana S, Rajpal K, Das A, Prasad R, Kharkwal AC (2012) Memoirs of “Rootonic” - The Magic Fungus
Verma S, Varma A, Rexer K, Hassel A, Kost G, Bisen P, Bütehorn B, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90:896–903
Visioni A, Tondelli A, Francia E, Pswarayi A, Malosetti M, Russell J, Thomas W, Waugh R, Pecchioni N, Romagosa I et al (2013) Genome-wide association mapping of frost tolerance in barley (Hordeum vulgare L.). BMC Genomics 14:424
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D et al (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. PNAS 102:13386–13391
Waller F, Mukherjee K, Deshmukh SD, Achatz B, Sharma M, Schäfer P, Kogel K-H (2008) Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. J Plant Physiol 165:60–70
Waqas M, Khan AL, Kamran M, Hamayun M, Kang S-M, Kim Y-H, Lee I-J (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules (Basel, Switzerland) 17:10754–10773
Weiss M, Sýkorová Z, Garnica S, Riess K, Martos F, Krause C, Oberwinkler F, Bauer R, Redecker D (2011) Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PloS One 6:e16793
White TJ, Bruns S, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: A guide to methods and applications. Academic Press, San Diego, CA, pp 315–322
Zadoks JC, Chang TT, Konzak CF (1974) A Decimal Code for the Growth Stages of Cereals. Weed Res 14:415–421
Zuccaro A, Lahrmann U, Güldener U, Langen G, Pfiffi S, Biedenkopf D, Wong P, Samans B, Grimm C, Basiewicz M et al (2011) Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLos Pathog 7:e1002290
Acknowledgments
We thank: Goldcrop Seeds, Cork, Ireland for the generous supply of barley seeds, and for advice on suitable cultivars to use; Josephine Brennan of the Department of Agriculture, Fisheries and the Marine at the Backweston crop trials site for providing plant material; Mark Kavanagh of the Centre for the Environment in Trinity College Dublin for expert advice and help in elemental analyses; Helena Murphy for proofreading and the de-cluttering of technical terms. Trinity College Dublin provided financial support through a PhD studentship grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Murphy, B.R., Doohan, F.M. & Hodkinson, T.R. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis 62, 29–39 (2014). https://doi.org/10.1007/s13199-014-0268-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-014-0268-0