Abstract
We provide a preliminary report of the mycobionts found within four Monotropoideae (Ericaceae) species from China: Monotropa uniflora, Hypopitys monotropa, Monotropastrum humile and Monotropastrum sciaphilum (a rare endemic species never previously studied for mycorrhizae). Such achlorophyllous Monotropoideae plants obtain their carbohydrates from mycorrhizal fungi linking them to surrounding trees, on which these fungi form ectomycorrhizae. Since Monotropoideae were rarely studied in continental Asia, the root systems of the four species sampled in Yunnan were examined using morphological and molecular methods. All the roots of these four species exhibit a typical monotropoid mycorrhizal morphology, including a fungal mantle, a Hartig net and hyphal pegs. In M. uniflora and M. humile mycorrhizae, cystidia typical of Russula symbionts covered the fungal mantle. ITS barcoding revealed that Russulales were the most frequent colonizers in all species, but Hypopitys monotropa displayed various additional mycorrhizal taxa. Moreover, a few additional ectomycorrhizal and saprotrophic Basidiomycota taxa were identified in the three other species, challenging that these four Monotropoideae species are as strictly fungal specific as the other Monotropoideae species hitherto studied. Moreover, a comparison with accompanying fungus sporocarps revealed that the fruiting fungal community significantly differed from that associated with the Monotropoideae roots, so that a clear fungal preference was evident. Finally, four fungal species were found on more than one Monotropoideae species: this contrasted with previous reports of sympatrically growing mycoheterotrophic plants, which did not reveal any overlap. This again challenges the idea of strict fungal specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over 400 achlorophyllous plant species receive carbon nutrition from their mycorrhizal fungi, in a form of heterotrophy called ‘mycoheterotrophy’ (MH) (Leake 1994; Leake et al. 2004; Smith and Read 2008) and have attracted considerable research in the two last centuries (Selosse et al. 2011). In the Ericaceae family, MH species occur in the subfamily Monotropoideae (Leake 1994; Leake et al. 2004; Tsukaya et al. 2008) that is divided into three tribes (Kron et al. 2002): Monotropeae and Pterosporeae encompass fully MH species, while Pyroleae are usually green (see references in Hashimoto et al. 2012) with the exception of the MH Pyrola aphylla Sm. (Hynson and Bruns 2009). Monotropeae and Pterosporeae consist of 50 species in 14 genera (Tucker 2009) from the Northern Hemisphere (Leake 1994), many of which grow in old-growth forests and are thus endangered because of habitat disturbance (United States Department of Agriculture 1993).
The structure of mycorrhizae has been previously described in several MH Monotropeae and Pterosporeae (Duddridge and Read 1982; Robertson and Robertson 1982; Snetselaar and Whitney 1990; Leake et al. 2004; Massicotte et al. 2005, 2010; Yamada et al. 2008). As in ectomycorrhizae, a fungal mantle covers the roots, and some hyphae penetrate between cortical cells, forming the Hartig net. However, a particular feature is a fungal peg inserted in some epidermal cells, perhaps transfering carbon from the fungus to the plant. These mycorrhizae have thus been placed in a separate mycorrhizal type, the ‘monotropoid’ mycorrhiza (Duddridge and Read 1982; Young et al. 2002).
Björkman (1960) first established how the MH Hypopitys monotropa Crantz (syn.: Monotropa hypopitys L.) obtains carbon from nearby trees by way of shared mycorrhizal fungi, and that these fungi ectomycorrhizal on trees formed monotropoid mycorrhizae on H. monotropa. In the past decade, many studies have confirmed that MH plants and surrounding trees share fungi in temperate forests (Selosse et al. 2002; Leake et al. 2004; Smith and Read 2008). Together with other MH plants, Monotropeae and Pterosporeae provide the most outstanding evidence that mycelial networks linking plants can transfer carbon between plant individuals (Selosse et al. 2006; Selosse and Roy 2009).
The ectomycorrhizal symbiosis displays variable specificity levels, but many ectomycorrhizal fungi associate with diverse host tree species and vice-versa (Smith and Read 2008; Douhan et al. 2011). In strong contrast, MH Monotropeae and Pterosporeae engage in highly specialized interactions. The fungal species forming monotropoid mycorrhizae were first identified by pioneering morphological and biochemical works of J.-F. Martin, who identified Tricholoma spp. as symbionts on H. monotropa (Martin 1985) and Russula spp. as symbionts of Monotropa uniflora L. on herbarium samples (Martin 1986). More identification was undertaken in diverse MH Monotropeae and Pterosporeae species after molecular tools were developed for fungal identification (Cullings et al. 1996; Bidartondo and Bruns 2001, 2002, 2005; Young et al. 2002; Yokoyama et al. 2005; Matsuda et al. 2011; Yang and Pfister 2006; Yamada et al. 2008; Dowie et al. 2011). All these studies showed that MH plant species have extremely specific associations with well-delimited fungal clades. The associated fungi of MH Monotropeae and Pterosporeae belong to the Russulales (Russulaceae), Boletales (Suillaceae), Thelephorales (Thelephoraceae), Phallales (Gautieriaceae) or Agaricales (Tricholomataceae), i.e. in every case, lineages that form ectomycorrhizae on autotrophic trees and shrubs. These lineages are common in temperate forest ecosystems from the Northern Hemisphere and Tropical Asia (Smith and Read 2008) where MH Monotropeae and Pterosporeae occur.
Congruently with their extreme specificity, whenever these MH plants species locally co-occur, they do not share fungal partners (but see an exception in Dowie et al. 2011). Conversely, in comparisons between conspecific MH populations originating from different regions, geographical mosaics were found, where the same plant species tends to have slightly different fungal partners (although they remain phylogenetically related; Bidartondo and Bruns 2001, 2005), or even variable levels of diversity (Yang and Pfister 2006). Such variations are interpreted as evidence that independent co-evolution processes are running between MH plants and their fungi within each site, leading to locally different outcomes (Smith and Read 2008). Whatever the cause, these geographical mosaics support the idea that multiple regions should be investigated to gain a comprehensive view of the diversity of fungal partners in each MH species.
Although several studies on MH Monotropeae and Pterosporeae already cover Europe and North America, Asia has not been thoroughly studied. Several species from Japan and Taiwan have been analyzed for mycorrhizal morphology and fungal symbionts (M. uniflora and H. monotropa: Bidartondo and Bruns 2001; Monotropastrum humile (D. Don) Hara: Matsuda and Yamada 2003; Matsuda et al. 2011; Yokoyama et al. 2005; Yamada et al. 2008), but no data is available for the large domain of continental China. Three Monotropeae genera are represented in China, represented by four species (Fang et al. 2005; taxonomy after Tsukaya et al. 2008; Fig. S1): the cosmopolitan North-temperate species M. uniflora and H. monotropa, and two endemics of the Himalayas and East Asia, M. humile and M. sciaphilum (Andres) G. Wallace (Fang et al. 2005). Monotropastrum sciaphilum is endemic to the Yunnan Province (Wallace 1987). Previous studies in Japan, North America and Europe demonstrated that M. uniflora, H. monotropa and M. humile specifically associate with different Russulales species (ectomycorrhizal Basidiomycota; Cullings et al. 1996; Yokoyama et al. 2005; Bidartondo and Bruns 2001, 2002, 2005; Young et al. 2002; Yang and Pfister 2006; Yamada et al. 2008; Matsuda et al. 2011). However, no study was conducted on MH species in China, especially on M. sciaphilum that was recently rediscovered after 91 years without report (Min et al. 2011).
This paper is a preliminary report on mycorrhizal associations of Chinese MH Monotropeae and Pterosporeae species. The aim of this study was to characterize the mycorrhizal structural features and fungal partners of the four species M. uniflora, H. monotropa, M. humile and M. sciaphilum in Yunnan (Fig. S1). In addition, we compared the fungal diversity in Monotropeae roots to that of the fungal sporocarps found on the same site to assess plant preferences in the framework of the locally available fungal diversity.
2 Materials and methods
2.1 Sampling
Four Monotropoideae species were studied in Yunnan, i.e. M. uniflora, H. monotropa (syn. Monotropa hypopitys), M. humile and M. sciaphilum (Fig. S1). The life cycle of the inflorescences is brief, lasting from September to November for M. uniflora, and from April to September for M. humile, H. monotropa and M. sciaphilum. Yunnan populations were found in 2007, and samples were collected from July to September 2009 (at most one plant per species at each survey). Ten individuals of M. uniflora, eight of H. monotropa and six of M. sciaphilum were sampled from Qiongzhusi site (25°03.885′N, 102°3738′E; elevation 2048–2186 m), and eight individuals of M. humile were sampled from Zhanyi site (26°08′N, 104°03′E, elevation 1900 m). The distance between these two sites was ca. 150 km. Within each site, the minimum and maximum distances between any two samples were 5 and 200 m, respectively. Root clusters were excavated and stored at 4 °C for up to one week until examination (as in Massicotte et al. 2005). Epigeous fungal sporocarps were collected from each site at each survey (on average, one per two weeks and site). After identification to genus or family level (since no tool is available for reliable species-level fungal identification in this area), sporocarps were frozen at −20 °C until until further molecular analyses could be performed.
2.2 Microscopy
Sampled root clusters were washed in tap water to remove soil and debris, and viewed under a dissecting microscope. Each large root-cluster was divided into smaller sections for ease of examination. At least five root tips from each root ball were carefully washed and stored separately in 1.5 ml tubes at −20 °C for subsequent molecular analysis. The remaining root clusters were kept in FAA solution (30 % formaldehyde: 50 % ethanol: acetic acid; 5:90:5 by volume) for microscopy investigations. More than 100 root tips were examined by light microscopy (LM), based on hand-made longitudinal or transverse sections. Selected roots were examined, after sections were cut using a freezing microtome (LEICA CM1100), to determine microscopic features using scanning electron microscopy (SEM). Samples were rinsed in buffer, post-fixed in 2 % aqueous osmium tetroxide for 2 h at 4 °C, rinsed with buffer, dehydrated in an ethanol series, critical point dried, mounted on aluminium stubs, coated with gold-palladium.
2.3 Sequencing and identification of fungi
We amplified the intergenic transcribed spacer (ITS) of fungal nuclear ribosomal DNA from sporocarps and mycorrhizae for molecular barcoding. DNA was extracted with the cetyltrimethyl ammonium bromide (CTAB) method. Primer sets ITS4B+ITS1 and ITS4+ITS1F (Gardes and Bruns 1993) were used. PCR reactions were carried out in 25 μl volumes containing 1.0 μl DNA template, 0.5 μl Taq DNA polymerase, 2.0 μl of each dNTP, 2.5 μl reaction buffer, 0.5 μl each primer, 18.0 μl deionised distilled H2O. Amplifications were performed on a thermal cycler (Applied Biosystems Veriti, Gene Company Limited) with preliminary denaturation at 94 °C for 3 min, 35 amplification cycles (94 °C for 50 s, 50 °C for 45 s, 72 °C for 1 min), and a final extension at 72 °C for 7 min. PCR products were purified with a UNIQ-10 column PCR products purification kit (Sangon, China). The sequencing reaction was performed from both strands with the amplification primers on an ABI 3700 automated sequencer (Perkin Elmer). The sequences were edited and submitted to BLAST research against the NCBI nucleotide databases (http://www.ncbi.nlm.nih.gov/) for a first generic attribution and screening PCR chimeras.
2.4 Alignments and phylogenetic analysis
Because BLAST matches were rarely satisfying (sometime less than 97 % of similarity with the closest result), a special effort for refining the identification was made on the most frequent taxa represented among root tips. Russula, Lactarius, Laccaria and Pholiota sequences were submitted to phylogenetic analyses. For each sequence, the 100 most similar BLAST results were downloaded from GenBank and UNITE databases, in addition to other sequences found with taxonomy browsers as representative taxa within the genus (for Laccaria, Pholiota, Russula and Lactarius) or the family (Strophariaceae for Pholiota). Usually, UNITE sequences are more reliably identified at species level, but mainly concerns European taxa. After preliminary Maximum Likelihood (ML) analysis of each of the four investigated clades, a second selection was made for the phylogenetically closest accessions, with elimination of redundant sequences. ML (not shown) and Bayesian analyses were obtained on this restricted set and, since they showed identical topologies, but with some better support of basal branches in Bayesian analyses, only the later are reported here. For all tree constructions, sequences were aligned under Clustal W (Higgins et al. 1994) and carefully refined manually on the editor in Mega 4.0 (Tamura et al. 2007). Bayesian analyses were performed under Mr Bayes v3.1 (Ronquist and Huelsenbeck 2003), using four Metropolis coupled Markov chain Monte Carlo, with one in every hundred trees sampled. The first 5000 trees were excluded from our analyses. For the Bayesian analyses, potential scale reduction factors were reasonably close to 1.0 for all parameters. Bayesian Posterior Probabilities of each node were obtained with 50 % majority rules with all compatible partitions. Bayesian 50 % majority rule consensus trees are provided here.
3 Results
3.1 Morphological analysis
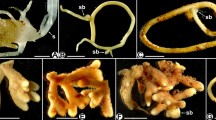
Clusters of roots were observed in the four species and were characteristic for Monotropoideae (Fig. 1a–d). All roots displayed a developed and continuous hyphal mantle (Fig. 1e and g). Typical Hartig nets occurred between cortical root cells (Fig. 1f and h). Hyphal pegs were sporadically observed in cortical cells from the four species (data not shown). The mantle surfaces displayed gloeocystidia, i.e. flask-shaped cystidia with an apical knob, on all investigated M. uniflora (n = 4) and M. humile (n = 4) mycorrhizae (Figs. 1e, g and 2b, c). In M. humile, they were accompanied by longer, spiny and sometimes branched cystidia (acanthophyses; Figs. 1g and 2c). The description of cystidia and gloeocystidia is characteristic of Russula from the sections Heterophyllidia and Foetentinae respectively. In contrast, simple hyphae covered the mantle of H. monotropa (n = 6) and M. sciaphilum (n = 6), and no superficial cystidia were observed (Fig. 2a and d).
Mycorrhizal morphology in four MH Monotropoideae. a Partial view of mycorrhizal root cluster of H. monotropa; (b) Partial view of mycorrhizal root cluster of M. uniflora; (c) Partial view of mycorrhizal root cluster of M. humile ; (d) Partial view of mycorrhizal root cluster of M. sciaphilum; (e) A root tip of M. uniflora mycorrhizae showing the thick mantle on its surface by light microscopy (transverse section); (f) Transverse section of M. uniflora mycorrhizae showing the mantle and the Hartig net by light microscopy; (g) Paradermal section of M. humile mycorrhizae showing the mantle surface by light microscopy, with numerous cystidia of two types: flask-shaped surmounted by a knob and tubular; (h) Transverse freezing microtome section of M. sciaphilum mycorrhizae showing the Hartig net
Scanning electron microscopy of the mantle surface in four species of Monotropoideae. a H. monotropa mycorrhizae; (b) M. uniflora mycorrhizae, with numerous flask-shaped cystidia surmounted by a knob; (c) M. humile mycorrhizae, with numerous cystidia of two types: flask-shaped surmounted by a knob and tubular; (d) M. sciaphilum mycorrhizae
3.2 Molecular identification of mycorrhizal fungi
In all, 56 fungal ITS sequences were obtained from 74 sampled monotropoid mycorrhizae (Table 1), due to samples failing to amplify correctly. Identical data were obtained with the two primer sets ITS4B+ITS1F or ITS4+ITS1, confirming the absence of Ascomycota.
The population of Monotropa uniflora from Qiongzhusi provided 18 sequences from 10 independent individuals (Table 1). One dominant species (14 sequences) is closely related to Russula illota Romagn. (Fig. 3a); the other fungi were related to R. crustosa Peck (n = 2; Fig. 3ba Tricholoma species and a saprotrophic species related to Pholiota multicingulata (Fig. S2). Thus, M. uniflora displayed a strong preference for the Russulales.
Phylogenetic positions of Russula taxa found in this study (in bold) based on ITS sequences and a Bayesian analysis. Subsections are precised according to Beenken (2004), and values indicate Bayesian Posterior Probabilities. a Phylogenetic reconstruction of Russula sect. Heterophyllae with R. cyanoxantha (sect. Indolentinae) as outgroup (Miller and Buyck 2002). b Phylogenetic reconstruction of Russula sect. Ingratae, subsect. Foetentinae, with the monophyletic clade subsect. Pectinatinae as outgroup (Buyck et al. 2008)
The population of Hypopitys monotropa from Qiongzhusi provided 15 sequences from 8 independent individuals (Table 1). Five Russulales included three identical sequences of the unknown Lactarius already found on M. uniflora (Fig. 4; see also Fig. 3b); four sequences originated from two distinct Cortinarius species; and the quite diverse range of species also included two Laccaria (Fig. S3), a Tricholoma, and a Tomentellopsis species. Additionally, 3 detected sequences were attributed to saprotrophic genera, namely a Mycena and the Pholiota species related to Pholiota multicingulata already found on M. uniflora (n = 2, Fig. S2). The last taxon was likely a contaminant (Sporidiobolales sp.).
Phylogenetic reconstruction of Lactarius subgenera Lactarius and Russularia, with taxa found in this study (in bold), based on ITS sequences and a Bayesian analysis. The tree was mid-point rooted and values indicate Bayesian Posterior Probabilities. Infrageneric nomenclature follows Buyck et al. (2008) modified after Buyck et al. (2010) and Verkeben et al. (2012)
The population of Monotropastrum sciaphilum from Qiongzhusi provided 11 sequences obtained from 6 individuals (Table 1). Sequences were dominated by Russulales, and included three Russula species and two Lactarius species (among which the unknown Lactarius already found above, n = 2; Fig. 4). Other sequences belonged to a Laccaria aff. L. murina (Fig. S3; ectomycorrhizal), and to the saprotrophic Pholiota found on the both the previous plant species (n = 3; Fig. S2).
The population of Monotropastrum humile from Zhanyi provided 12 sequences obtained from 8 independent individuals of M. humile at Zhanyi. Seven sequences corresponded to Russula aff. vesca Fr. (Fig. 3b), and two corresponded to the unknown Lactarius already found above (Fig. 4). The two other fungi were close to ectomycorrhizal Phellodon, and saprotrophic Gymnopilus. Thus M. humile, exactly like M. sciaphilum, displayed a preference for the Russulales.
The investigated MH plant species shared up to 3 fungal taxa as associates (Fig. 5). Four fungal taxa were shared by at least two of the investigated MH plant species (Table 1). Two fungal species were present on three MH plant species, namely a species related to Pholiota multicingulata (Fig. S2) and the unknown Lactarius sp. 1 (Fig. 4); the later was the only associated fungal taxa shared between the Qiongzhusi and Zhanyi sites (Fig. 5). Moreover, with the exception of the species related to Pholiota multicingulata, all shared taxa were ectomycorrhizal.
3.3 Molecular identification of sporocarps
A total of 28 fungal sporocarps were collected around MH plants (20 at Qiongzhusi and 8 at Zhanyi; Table 2), and identified to genus or family level (data not shown). DNA was successfully extracted and sequenced from all sporocarps (Table 2). Blast analyses confirmed the preliminary morphological taxonomic assignation (Table 2) with the only exception being a Cortinarius-like fungus that provided a Mycogone-like ITS sequence, probably due to a parasitic association (JQ396520; Table 2). Amanitaceae were absent on the plants roots, but were the most frequent above ground sporocarps (46 %; chi2 with Yates correction: 27.31; P < 0.0001). Russulales that dominated belowground (66 %) were less frequently encountered above ground (21 %; chi2 with Yates correction: 14.89; P < 0.0001). The dominance of ectomycorrhizal fungi was similar in both communities (86 and 89 % respectively; chi2 with Yates correction: 0.14; P > 0.05). The same trends were found when we analyzed separately the data from the Qiongzhusi area (not shown).
Several sporocarps proved to have the same phylogenetic position to the below-ground mycorrhizal fungi of the investigated Monotropoideae (Table 2). Lactarius sporocarps #12 and #13 from Qiongzhusi, and #24 from Zhanyi clustered with the unidentified Lactarius taxon found on MH plants from these two sites, i.e. H. monotropa, M. humile and M. sciaphilum (up to 2 bp difference only; Fig. 4). The Laccaria-related sporocarp #15 from Qiongzhusi had only 1 bp difference with a M. sciaphilum mycorrhizal fungus (Fig. S3) from the same site. Given they are below the 97 % similarity threshold usually relevant to delineate biological species (Hughes et al. 2009), we estimated that these two Lactarius cluster were from the same species. Thus, the percentage of similarity between below-ground mycorrhizal fungi and above-ground sporocarps, was 0 at Zhani versus 25 % (4 out of 20; two species) at Qiongzhusi.
4 Discussion
The structural examination of M. uniflora, H. monotropa, M. humile and M. sciaphilum mycorrhizae shows typical monotropoid features, i.e., a mantle, a Hartig net and fungal pegs. It matches the features reported previously in MH Monotropoideae, especially Monotropa and Hypopitys spp. (Duddridge and Read 1982; Duddridge 1985; Dexheimer and Gérard 1993; Snetselaar and Whitney 1990; Matsuda and Yamada 2003). In particular, the presence of cystidia (gloeocystidia and acanthophyses) covering mycorrhizae is congruent with reports for M. uniflora associated with Russula spp. (Martin 1986; Young et al. 2002; Yamada et al. 2008). Morphology of Russula ectomycorrhizae has been detailed by Beenken (2004) from many European tree species: the presence of both gloeocystidia and acanthophyses is described on ectomycorrhizae of Russula from the subsection Heterophyllinae (section Heterophyllae), to which the dominant symbiont of M. humile belongs (Fig. 3b); and only gloeocystidia are present on ectomycorrhizae of the subsection Foetentinae (section Ingratae) where the dominant symbiont of M. uniflora is placed (Fig. 3a). Thus there was a remarkable match between mantle surface ornamentations and molecular identifications. It is noteworthy that investigated root sections from individual plants revealed corresponding molecular identification (data not shown). Strikingly, despite an association with a MH plant, mycelial characters of the mycorrhizae do not differ from those of the “usual” ectomycorrhizal hosts, i.e. Fagaceae or Pinaceae, observed by Beenken (2004).
Russulales are dominant in frequency on M. uniflora (88 %). Russula and some Lactarius spp. were found to associate with M. uniflora in North America, Eurasia and Japan (Martin 1986; Cullings et al. 1996; Bidartondo and Bruns 2001, 2005; Young et al. 2002; Yang and Pfister 2006). Though different Russulales were found at various locations in these previous studies, our results provide further evidence for a rather specific association in China and throughout the Northern Hemisphere. The two species of Monotropastrum showed a preference for Russulales that accounted for 83 % of M. humile symbionts and 56 % of M. sciaphilum symbionts. Bidartondo and Bruns (2001), Yokoyama et al. (2005) and Yamada et al. (2008) already demonstrated that Russula and Lactarius associated with M. humile. One study of M. humile in Japan revealed that among the 50 taxa found, 49 belonged to Russulales, while the last one belonged to the Thelephoraceae (Matsuda et al. 2011). M. sciaphilum, an endemic to China, has been recommended as an addition to the Red List of the International Union for Conservation of Nature and Natural Resources (Min et al. 2011). The present study provides the first analysis of the mycorrhizal associations in this rare plant. It revealed the presence of additional symbionts, including ectomycorrhizal Laccaria spp. Thus, our data support the view that the Monotropeae clade formed by M. uniflora+Monotropastrum spp. also has a preference for Russulales (Smith and Read 2008), although M. humile var. glaberrimum may be an exception to this (Tsukaya et al. 2008). Our investigation of surrounding sporocarps, dominated by Amanitaceae, suggests that the preferences mentioned above do not simply reflect the locally available fungal community, but constitutes partner choices filtering this community. The Amanitaceae are, however, often infrequent on roots compared to their abundance in fruiting communities (Gardes and Bruns 1996).
Contrary to the three previous species, H. monotropa, which occupies a quite different phylogenetic position within Monotropoideae (Bidartondo and Bruns 2001, 2002; Tsukaya et al. 2008), displayed unexpected results. This species has long been reported to associate specifically with the genus Tricholoma in North America, Eurasia and Japan (Martin 1985; Cullings 1996; Bidartondo and Bruns 2002, 2002; unpubl. data from M.-A. Selosse & M. Sauve from six European H. monotropa populations). Our results show a non-specific association with various ectomycorrhizal fungi, including three Russulales, and a single Tricholoma. In addition, Thelephoraceae and Cortinarius, and some putatively saprotrophic fungi were also found, among which only the Thelephoraceae have been reported previously from MH Monotropeae (in M. humile sensu lato; Yokoyama et al. 2005; Matsuda et al. 2011). The fact that some MH plants are not specific has been reported in the related MH Pyrola aphylla (Pyroleae; Hynson and Bruns 2009) and in several MH orchids (Roy et al. 2009; Martos et al. 2009). Our result should be considered under the fact that H. monotropa is a complex circumboreal species. Diversity in America has arisen following survival on multiple glacial refugia (Beatty and Provan 2011) and several color morphs exist that display substantial genetic differentiation (Klooster and Culley 2010). Over the Northern Hemisphere, it was recognized that the clades of associated Tricholoma tended to vary from one continent to another (Bidartondo and Bruns 2001; M.-A. Selosse & M. Sauve, unpubl. data). Thus, the investigated Chinese population may be a mix of representatives from different subspecies (each of which may exhibit some specificity), or, more likely, belong to a different Hypopitys species. Because of our limited sampling effort, additional sampling is required from Asia as well as further phylogenetic analyses within H. monotropa sensu lato worldwide before firm conclusion can be made.
The extreme specificity of the investigated Monotropeae is also challenged for other investigated MH species by two further findings. First, for each species, up to three discovered fungal taxa were shared with another species, e.g. a Lactarius-related sequence was found in all species except M. uniflora. In particular, the finding of a R. aff. illota sequence on a M. sciaphilum root is very unexpected since this is the symbiont dominating on M. uniflora. All pairs of plant species, but M. uniflora and M. humilis, shared at least one fungal taxon (since the different plant species were not barcoded at the same time in the lab, we eliminate the possibility of a cross-contamination). This is an unexpected situation since, whenever MH Monotropoideae have been found in sympatry up to now, they did not share fungal partners (Cullings et al. 1996; Bidartondo and Bruns 2001; Smith and Read 2008). However, a recent study on the phylogenetically related MH Pterospora andromedea (Pterosporeae) revealed a mycorrhizal fungus that usually associates with another MH Pterosporeae, Sarcodes sanguinea (Dowie et al. 2011). This suggests that overlaps in fungal partners sometime happen. These may simply reflect cross-colonization, but one might also speculate that MH plants might not form a guild where each species avoids competition for fungal partners.
A second observation that more directly challenges the concept of specificity is that although the Russulales dominate in all investigated MH species, non-Russulales taxa also occur. We note that the occasional finding of Thelephoraceae reported in Japanese M. humile reported above (Matsuda et al. 2011) may also be considered as a deviation from strict specificity. These fungi belonged to taxa that are ectomycorrhizal or, more unexpectedly, saprotrophic (such as the Pholiota aff. multicingulata found on all Qionzhusi plant species). They might be derived from soil contaminations, but this possibility is unlikely since no Ascomycota (among which many common soil fungal saprotrophs are placed), were found on the plant roots, even when using the universal ITS1+ITS4 primer combination. However, direct observation of these symbionts on roots would be required to ensure their mycorrhizal status. Some saprotrophic Basidiomycota, like Mycena spp., are sometimes isolated from orchid roots (Martos et al. 2009; see review in Selosse and Roy 2009), while others can interact with living roots, like Hypholoma spp. (Vasiliauskas et al. 2007). However, Pholiota spp. usually do not grow in healthy living tissues. Hynson & Bruns also reported saprotrophic fungi from the MH Pyrola aphylla (see Table S1 in Hynson and Bruns 2009), and some MH orchids are even supported by saprotrophic fungi (Martos et al. 2009; Selosse et al. 2010). To summarize, we ignore the exact interaction with MH hosts of the fungi that do not belong to dominant taxa, and especially of the saprotrophs found here. We cannot conclude to a mycorrhizal infection, or a superficial growth or an endophytic or pathogenic colonisation. Until further analysis is conducted, i.e. investigations of other populations and more physiological experiments such as germination trials, we see these fungi only as potentially challenging the concept of strict specificity, and the concept of a strict association to ectomycorrhizal fungi.
5 Conclusions
Our data provide evidence from the investigated Chinese MH Monotropoideae of a preference for Russulales. Subtle differences in targeted clades, when compared with the fungal symbionts of these plants in other regions, fit well the idea of a geographic mosaic for symbioses in MH plants. The question of strict specificity is challenged by the discovery of fungal sharing between MH species and the marginal presence of diverse non-Russulales taxa on root clusters, which deserve further morphological corroborations. However, a preferential association is obvious when comparing root fungi to the available fungal community. It is beyond doubt that our limited sampling effort makes these results preliminary. The existence of unexpectedly low specificity H. monotropa clades in China, as well as the diversity of saprotrophic fungi in MH roots and possible overlaps in fungi between MH species, suggests a more opportunistic recruitment of local fungi by MH plants than usually considered. We recommend warmly reporting all marginal taxa found on roots of MH plants in future works (Selosse et al. 2010). However, whatever the geographical area considered, a similar selection of ectomycorrhizal lineages of Basidiomycota operates on the MH Monotropoideae side, whose mechanisms deserve further studies. We now hope to undertake studies on a larger geographic scale, to provide a general picture of MH associations in Monotropoideae over China and the Northern Hemisphere.
References
Beatty GE, Provan J (2011) Phylogeographic analysis of North American populations of the parasitic herbaceous plant Monotropa hypopitys L. reveals a complex history of range expansion from multiple late glacial refugia. J Biogeogr 38:1585–1599
Beenken L (2004) Les ectomycorhizes du genre Russula. Bull Soc Mycol Fr 120:293–333
Bidartondo MI, Bruns TD (2001) Extreme specificity in epiparasitic Monotropoideae (Ericaceae): widespread phylogenetic and geographical structure. Mol Ecol 10:2285–2295
Bidartondo MI, Bruns TD (2002) Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Mol Ecol 11:557–569
Bidartondo MI, Bruns TD (2005) On the origins of extreme mycorrhizal specificity in the Monotropoideae (Ericaceae): performance trade-offs during seed germination and seedling development. Mol Ecol 14:1549–1560
Björkman E (1960) Monotropa hypopithys L.—an epiparasite on tree roots. Plant Physiol 13:308–327
Bridge PD, Schlitt T, Cannon PF, Buddie AG, Baker M, Borman AM (2008) Domain II hairpin structure in ITS1 sequence as an aid in differentiating recently evolved animal and plant pathogenic fungi. Mycopathologia 166:1–16
Buyck B, Hofstetter V, Eberhardt U, Verbeken A, Kauff F (2008) Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Divers 28:15–40
Buyck B, Hofstetter V, Verbeken A, Walleyn R (2010) Prop. 1919: To conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Mycotaxon 111:504–508
Cullings K, Szaro T, Bruns T (1996) Evolution of extreme specialization within a lineage of ectomycorrhizal epiparasites. Nature 379:63–66
Dexheimer J, Gérard J (1993) Application de quelques techniques cytochimiques à l’étude des interfaces des ectendomycorhizes de Monotrope (Monotropa hypopitys L.). Acta Bot Gall 140:459–472
Douhan GW, Vincenot L, Gryta H, Selosse M-A (2011) Population genetics of ectomycorrhizal fungi: from current knowledge to emerging directions. Fungal Biol 115:569–597
Dowie NJ, Hemenway JJ, Trowbridge SM, Miller SL (2011) Mycobiont overlap between two mycoheterotrophic genera of Monotropoideae (Pterospora andromedea and Sarcodes sanguinea) found in the Greater Yellowstone Ecosystem. Symbiosis 54:29–36
Duddridge JA (1985) A comparative ultrastructural analysis of the host-fungus interface in mycorrhizal and parasitic association. In: Moore D, Casselton LA, Wood DA, Frankland JC (eds) Developmental biology of higher fungi. (British Mycology Society Symposium 10) Cambridge University Press, Cambridge
Duddridge JA, Read DJ (1982) An ultrastructural analysis of the development of mycorrhizas in Monotropa hypopitys L. New Phytol 92:203–214
Fang M, Fang R, Fang RC, He M, Hu L, Yang H, Qin H, Min T, Chamberlain DC, Stevens PF, Wallace GD, Anderberg A (2005) Ericaceae. In: Wu Z, Raven P, Hong D (eds) Flora of China volume 14 (Apiaceae through Ericaceae). Science Press, Beijin, pp 242–517
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—applications to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gardes M, Bruns TD (1996) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can J Bot 74:1572–1583
Hashimoto Y, Fukukawa S, Kunishi A, Suga H, Richard F, Sauve M, Selosse M-A (2012) Mycoheterotrophic germination of Pyrola asarifolia dust seeds reveals convergences with germination in orchids. New Phytol in press
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins G, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Hughes KW, Petersen RH, Lickey EB (2009) Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species’ delimitation across basidiomycete fungi. New Phytol 182:795–798
Hynson NA, Bruns TD (2009) Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proc Roy Soc B 276:4053–4059
Klooster MR, Culley TM (2010) Population genetic structure of the mycoheterotroph Monotropa hypopitys L. (Ericaceae) and differentiation between red and yellow color forms. Int J Plant Sci 171:167–174
Kron KA, Judd WS, Stevens PF, Crayn DM, Anderberg AA, Gadek PA, Quinn CJ, Luteyn JL (2002) Phylogenetic classification of the Ericaceae: molecular and morphological evidence. Bot Rev 68:335–424
Leake JR (1994) The biology of myco-heterotrophic (“saprophytic”) plants. New Phytol 127:171–216
Leake JR, McKendrick SL, Bidartondo M, Read DJ (2004) Symbiotic germination and development of the myco-heterotroph Monotropa hypopitys in nature and its requirement for locally distributed Tricholoma spp. New Phytol 163:405–423
Martin JF (1985) Sur la mycorhization de Monotropa hypopitys par quelques espèces du genre Tricholoma. Bull Soc Mycol France 101:249–256
Martin JF (1986) Mycorhization de Monotropa uniflora L. par des Russulaceae. Bull Soc Mycol France 102:155–159
Martos F, Dulormne M, Pailler T, Bonfante P, Faccio A, Fournel J, Dubois M-P, Selosse M-A (2009) Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol 184:668–681
Massicotte HB, Melville LH, Peterson RL (2005) Structural features of mycorrhizal associations in two members of the Monotropoideae, Monotropa uniflora and Pterospora andromedea. Mycorrhiza 15:101–110
Massicotte HB, Melville LH, Peterson RL, Tackaberry LE, Luoma DL (2010) Structural characteristics of root–fungus associations in two mycoheterotrophic species, Allotropa virgata and Pleuricospora fimbriolata (Monotropoideae), from southwest Oregon, USA. Mycorrhiza 20:391–397
Matsuda Y, Yamada A (2003) Mycorrhizal morphology of Monotropastrum humile collected from six different forests in central Japan. Mycologia 95:993–997
Matsuda Y, Okochi S, Katayama T, Yamada A, Ito S-I (2011) Mycorrhizal fungi associated with Monotropastrum humile (Ericaceae) in central Japan. Mycorrhiza 21:569–576
Miller SL, Buyck B (2002) Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol Res 106:259–276
Min S, Chang-Qin Z, Wallace GD (2011) Rediscovery of Monotropastrum sciaphilum (Andres) G. D. Wallace in China after 91 years. Aliso 29:115–118
Robertson DC, Robertson JA (1982) Ultrastructure of Pterospora andromedea Nuttall and Sarcodes sanguinea Torrey mycorrhizas. New Phytol 92:539–551
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse MA (2009) Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol 7:51
Selosse M-A, Roy M (2009) Green plants eating fungi: facts and questions about mixotrophy. Trends Plant Sci 14:64–70
Selosse M-A, Weiss M, Jany JL, Tillier A (2002) Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis and neighbouring tree ectomycorrhizae. Mol Ecol 11:1831–1844
Selosse M-A, Richard F, He X, Simard S (2006) Mycorrhizal networks: les liaisons dangereuses. Trends Ecol Evol 11:621–628
Selosse M-A, Martos F, Perry B, Maj P, Roy M, Pailler T (2010) Saprotrophic fungal symbionts in tropical achlorophyllous orchids: Finding treasures among the ‘molecular scraps’? Plant Signal Behav 5:1–5
Selosse M-A, Boullard B, Richardson D (2011) Noël Bernard (1874–1911): orchids to symbiosis in a dozen years, one century ago. Symbiosis 54:61–68
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Snetselaar KM, Whitney KD (1990) Fungal calcium oxalate in mycorrhizae of Monotropa uniflora. Can J Bot 68:533–543
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Taylor DL, Bruns TD (1997) Independent, specialized invasions of the ectomycorrhizal mutualism by two non-photosynthetic orchids. PNAS 94:4510–4515
Tsukaya H, Yokoyama J, Imaichi R, Ohba H (2008) Taxonomic status of Monotropastrum humile, with special reference to M. humile var. glaberrimum (Ericaceae, Monotropoideae). J Plant Res 121:271–278
Tucker G (2009) Ericaceae. In: Flora of North America Editorial Committee (ed) Flora of North America, volume 8. Oxford University Press, New-York, pp 370–535
United States Department of Agriculture (1993) Forest ecosystem management: an ecological, economic, and social assessment. A report of the Forest Ecosystem Management Assessment Team. U.S. Department of Agriculture/U.S. Department of the Interior/National Oceanic and Atmospheric Administration/U.S. Environmental Protection Agency, Portland (Oregon)
Vasiliauskas R, Menkis A, Finlay RD, Stenlid J (2007) Wood-decay fungi in fine living roots of conifer seedlings. New Phytol 174:441–446
Verkeben A, Nuytinck J, Buyck B (2012) New combinations in Lactifluus. 1. L. subgenera Edules, Lactariopsis, and Russulopsis. Mycotaxon 118:447–453
Wallace GD (1987) Transfer of Eremotropa sciaphila to Monotropastrum (Ericaceae: Monotropoideae). Taxon 36:128–130
Yamada A, Kitamura D, Setoguchi M, Matsuda Y, Hashimoto Y, Matsushita N, Fukuda M (2008) Monotropastrum humile var. humile is associated with diverse ectomycorrhizal Russulaceae fungi in Japanese forests. Ecol Res 23:983–993
Yang S, Pfister DH (2006) Monotropa uniflora plants of eastern Massachusetts form mycorrhizae with a diversity of russulacean fungi. Mycologia 98:535–540
Yokoyama J, Fukuda T, Tsukaya H (2005) Molecular identification of the mycorrhizal fungi of the epiparasitic plant Monotropastrum humile var. glaberrimum (Ericaceae). J Plant Res 118:53–56
Young BW, Massicotte HB, Tackaberry LE, Baldwin QF, Egger KN (2002) Monotropa uniflora: morphological and molecular assessment of mycorrhizae retrieved from sites in the subboreal spruce biogeoclimatic zone in central British Columbia. Mycorrhiza 12:75–82
Acknowledgments
We are grateful to Wang Nian for his helpful working on essay writing, Wu Zhi-Kun for collection in the field, Walter Till and Heimo Rainer (Herbarium of Wien University), as well as Reinhard Agerer and Franck Richard for helpful discussions. We acknowledge David Richardson and two anonymous referees that made helpful and detailed corrections on earlier versions of this paper. This study was supported by the Ministry of Science and Technology of Peoples’ Republic of China (No. 2009GB2F300343) as well as the Chinese plans of New Production of Yunnan (No. 2009BB001) and of Condition Platform Construction of Yunnan (No. 2010 DH011). M.-A. S. is supported by the French Agence Nationale de la Recherche and the CNRS.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Figure S1
The four MH plants investigated in this study. (a) Monotropa uniflora; (b) Hypopitys monotropa; (c) Monotropastrum humile; (d) M. sciaphilum (a, b and d are reproduced from Shen Min et al. 2011). (PDF 181 kb)
Figure S2
Phylogenetic reconstruction of the Strophariaceae family with taxa found in this study (in bold), based on ITS sequences and a Bayesian analysis. Values indicate Bayesian Posterior Probabilities. The tree was rooted by Deconica (Psilocybe) montana as a basal group in Strophariaceae (Bridge et al. 2008). (PDF 163 kb)
Figure S3
Phylogenetic reconstruction of the genus Laccaria with taxa found in this study (in bold), based on ITS sequences and a Bayesian analysis. Values indicate Bayesian Posterior Probabilities. The tree was rooted by Laccaria glabripes, Hydnangium carneum and Porohydnangium australe following the hypothesis of basal origin of Australian lineages of this clade. (PDF 129 kb)
Rights and permissions
About this article
Cite this article
Min, S., Chang-Qin, Z., Yong-Peng, M. et al. Mycorrhizal features and fungal partners of four mycoheterotrophic Monotropoideae (Ericaceae) species from Yunnan, China. Symbiosis 57, 1–13 (2012). https://doi.org/10.1007/s13199-012-0180-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-012-0180-4