Abstract
In order to investigate the effects of Glomus species on some physiological characteristics of two chickpea types (Pirouz cultivar of Desi type and ILC-482 of Kabuli type) under non-stress (NS) and drought stress, an experiment was conducted using a factorial arrangement based on completely randomized design with three replications. Drought stress decreased shoot and total dry weight in plants. However inoculation of plants with mycorrhiza improved these traits. Leaf chlorophyll content was decreased, but leaf proline content and guaiacol peroxidases (EC 1.11.1.7) (POD), catalase (EC 1.11.1.6) (CAT), and ascorbate peroxidase (EC 1.11.1.11) (APX) activities were increased as a result of drought stress. Drought stress had no significant effect on soluble protein content and polyphenol oxidase (EC 1.10.3.1) (PPO) enzymatic activity in chickpea plants. In general, drought stress and especially severe drought stress increased membrane lipid peroxidation (MDA) in chickpea plants, which was more evident in non-inoculated than in inoculated plants. Inoculation of chickpea by AM significantly increased POD and PPO activities compared with non-inoculated chickpea, but had no effect on CAT activity and proline content of leaves. The reaction of chickpea cultivars to inoculation by AM species and irrigation levels were different. ILC-482 showed that antioxidant enzymes activities were more and thus less MDA compared with Pirouz cultivar. In general, the most POD and PPO activities were recorded for inoculated plants with G. etunicatum and G. versiform species, and the most APX activity was observed in plants inoculated with G. intraradices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chickpea (Cicer arietinum L.) is the third most important grain legume in the world (Singh and Ocampo 1997). Two types of chickpea are recognized, Desi (colored, small seeded, angular, and fibrous) and Kabuli (beige, large seeded, rams-head shaped with lower fiber content) (Malhotra et al. 1987). Chickpea is frequently subjected to drought stress in rain-fed agriculture of arid and semi-arid areas.
In nature, plants are frequently exposed to adverse environmental conditions that have a negative effect on plant survival, development, and productivity. Drought and salinity are considered as the most important abiotic factors limiting plant growth and yield in many areas (Kramer and Boyer 1997). Water deficit occurs when water potentials in the rhizosphere are sufficiently negative to reduce water availability to sub-optimal levels for plant growth and development (Boyer 1982). The response of plants to water stress depends on several factors such as developmental stage, severity, duration of stress, and cultivar genetics. Common plant symptoms after water deficit are stunted growth, limited CO2 diffusion to chloroplasts by stomatal closure, reduced photosynthesis rate, and accelerated leaf senescence (Beltrano et al. 1994; Beltrano et al. 1999).
Abiotic stress such as drought can affect plants through generating and accumulating reactive oxygen species (ROS) such as superoxide radical (•O2), hydrogen peroxide (H2O2), hydroxyl radical (•OH) (Halliwell & Gutteridge 1985) and singlet oxygen (•O1) (Elstner 1987), which are inevitable by-products of normal cell metabolism (Dionisio-Sese et al. 1998). While it is generally assumed that the hydroxyl radical and singlet oxygen are so reactive that their production must be minimized (Jakob & Heber 1996), •O2 and H2O2 are synthesized at very high rates even under optimal conditions. The chief toxicity of •O2 and H2O2 is thought to reside in their ability to initiate cascade reactions that result in the production of the hydroxyl radicals. These radicals (and their derivatives) are among the most reactive species, capable of reacting indiscriminately to cause oxidative damage to membranes lipid (Halliwell & Gutteridge 1989; Bowler et al. 1992), photosynthetic pigments, proteins, and DNA (Sun et al. 2002). Chloroplasts, mitochondria, and peroxisomes of plant cells are important intracellular generators of ROS, which can be responsible for the occurrence of oxidative damages under abiotic stress (Francisco et al. 2002; Diego et al. 2003). Plants can respond to osmotic stress at morphological, anatomical, and cellular levels with modifications that allow the plant to avoid the stress or to increase its tolerance (Bray 1997).
The induction of ROS-scavenging enzymes, such as SOD, POD, APX, CAT (Mitter 2002) and other compounds such as carotenoids (Burke and Mahan 1991), ascorbic acid, thiols, soluble protein, and GSH (Malecka et al. 2001; Sinha et al. 2005) is the most common mechanism for detoxifying ROS synthesized during stress responses. Removing of the highly toxic H2O2 is essential for the cell to avoid inhibition of enzymes such as those controlling the Calvin cycle in the chloroplast (Creissen et al. 1994), while the H2O2 produced can be scavenged by catalase (EC 1.11.1.6) and a variety of peroxidases (EC 1.11.1.7). Catalase, which is only present in peroxisomes, dismutates H2O2 into water and molecular oxygen, whereas POD decomposes H2O2 through oxidation of co-substrates such as phenolic compounds and/or antioxidants (Bowler et al. 1994).
Plants also produce large amounts of amino acids such as proline for increasing resistance to drought. Proline is an important amino acid in plant under drought stress that prevents oxidation (Girousse et al. 1996). In addition to the intrinsic protective systems of plants against stress, plants grow in association with a number of soil microorganisms that can alleviate the stress symptoms (Smith & Read 1997). It is well documented that Arbuscular mycorrhiza fungal symbiosis can improve plant water relations or enhance drought resistance of host plants, thus protecting host plants against detrimental effects caused by drought stress (Augé 2001). AM have fundamental effects on host plant biochemistry and physiology (Koide & Schreiner 1992; Smith & Gianinazzi-Pearson 1988). Ruiz-Lozano (2003) reported that AM symbiosis might increase the drought resistance of higher plants through promoting antioxidant enzymes. Although antioxidants in bean (Lambais et al. 2003), red clover (Palma et al. 1993) and some shrub species (Alguacil et al. 2003) inoculated with AM have been investigated, the differences in antioxidant enzymes due to drought and AM have not been reported in some crops. It is well documented that AM symbiosis causes the increase of antioxidant enzymes activities of host plants. In a study, SOD activity was higher in the roots of mycorrhizal Lactuca sativa plants than those of non-AM plants (Ruiz-Lozano et al., 1996). Wu et al. (2007) revealed that inoculation with five Glomus species usually increased the SOD, G-POD and CAT activities in leaves of Citrus tangerine. Moreover, they also showed that Glomus species had different responses to SOD, G-POD and CAT. For example, G. diaphanum colonization significantly increased SOD activity, G. versiform colonization G-POD activity, and G. mosseae colonization CAT activity. Alguacil et al. (2003) also reported that inoculation with G. claroideum increased APX and GR activities in R. sphaerocarpa. Wu et al. (2006) showed that the activities of CAT and APX were significantly higher (by 15% and by 59%) in AM than in non-AM seedlings under water stress conditions. Caravaca et al. (2005) reported that the mycorrhizal inoculation treatments caused a decrease in POX activity in shoots of M. communis under both water regimes and in SOD activity in shoots of stressed and non-stressed P. angustifolia plants. A similar response was observed in mycorrhizal soybean plants that showed lower APX values relative to the corresponding non-mycorrhizal plants (Porcel et al., 2003). Study results of ZhongQun et al. (2007) showed that AM symbiosis really influenced antioxidant enzymes in different degree. However, the relationship between AM and antioxidants is poorly understood.
We wanted to determine whether the presence of AM would influence the antioxidant enzymes activities of chickpea cultivars in this study. We hypothesize that under drought conditions AM fungal colonization of chickpea assists in the antioxidant enzyme activity such as SOD, CAT and POD, which contribute to drought tolerance of the host plant. To test this, we have examined changes of antioxidant enzyme activity in AM and non-AM chickpea plants of Desi and Kabuli cultivars under drought stress and non-drought stress.
2 Materials and methods
2.1 Plant material and treatments
The experiments were conducted in the Greenhouse of the Kurdistan University from late April to early August 2009. Some soil physical and chemical properties have been presented in Table 1. The soil samples air-dried, crushed to pass through a 2-mm sieve, and mixed with sand (2:1 ratio). The certified seeds of chickpea cultivars were obtained from Agricultural Research Center of Kermanshah, Iran. These seeds were surface-sterilized with 0.1% HgCl2 solution for 5 min and washed thoroughly five times with distilled water. The experiments were carried out using a factorial arrangement based on completely randomized design with three replications. The factors were two chickpea cultivars (Pirouz cultivar of Desi type and ILC-482 of Kabuli type), three irrigation treatments including non-stress (NS), moderate drought stress (MDS) and severe drought stress (SDS) and inoculation of plants using AM species, Glomus versiforme (Karsten and Berch), Glomus intraradices (Schenk and Smith), Glomus etunicatum (Becker and Gerdman), non-mycorrhizal (non-AM) plants were considered as control. The mycorrhizal fungi obtained from University of Tabriz, Iran. The AM inoculums was consisted of a mixture of rhizospheric soil from trap cultures (Zea mays sp.) containing spores, hyphae, and mycorrhizal root fragments. The dosage inoculated was 10 g of inoculums per 1 kg soil, each pot containing approximately 790 spores. Chickpea roots were tested for mycorrhizal colonization at 30 days after sowing. When the plants were established (30 days after sowing), treatments of irrigation were applied. Duration of irrigation treatments was 55 days after plants establishment. Light duration was about 13 h, but it was reduced gradually. Temperature and relative air humidity were 27 ± 3οC and 60 ± 5% respectively.
Thirty chickpea seeds were sown in each 20 L pot filled with 20 kg of inoculated or non-inoculated soil in April 2009. After emergence, seedlings were thinned and 10 plants were kept in each pot. Irrigation treatments were: control (NS)– plants were watered daily to maintain Ψs no lower than –0.03 MPa; moderate drought stress (MDS)– plants were stressed by withholding water until a Ψs of ca. –0.6 MPa was reached; severe drought stress (SDS) plants were stressed by withholding water until a Ψs of ca. -1.2 MPa was achieved. The soil water potentials were measured with Dew Point Potentiameter (WP4) (Decagon Devices, Inc., Pullman, Washington, USA) at the sampled days and were maintained until harvest. Water content of pots was measured daily during the entire experiment with Time Domain Reflectometry TDR Method (Whalley 1993). Time domain reflectometry is a widely applied technique for the nondestructive measurement of soil water content due to its flexibility, accuracy, and the possibility for automated measurement of several probes simultaneously (Robinson et al. 2008). The pots were kept under natural photoperiod.
2.2 Mycorrhizal colonization
Two plants from each pot were sampled randomly at 50 and 70 days after sowing. Their roots were washed, cut into about 1 cm long pieces and mixed thoroughly. Root samples were then cleared with 10% (w/v) KOH and stained with 0.05% (w/v) trypan blue in lactoglycerol (Phillips and Hayman 1970). Arbuscular mycorrhizal (AM) colonisation was estimated using a modified line intersect method (McGonigle et al., 1990), where a minimum of 100 line intersections per root sample, replicated three times, were scored for the presence of AM structures. These observations were made using the light microscopy to rate the degree of root infection by AMF in two plants per replicate (six plants per treatment). The percentage of AM infection was calculated from the following equation:
2.3 Plant growth parameters
Plant height for five plants in each pot was measured. Shoots and roots were cut and dried in an oven at 70°C for 48 h and then were weighed (total, root and shoot).
2.4 Chlorophyll and carotenoid content
For chlorophyll determination, the fifth fully expanded leaves were detached from the plants after the drought treatment. Prior to extraction, fresh leaf samples were cleaned with deionized water to remove any surface contamination. Chlorophyll content was determined using the methods proposed by Harbone (1984). 500 mg of the leaf tissues was homogenized in 80% acetone at 4°C. The supernatant was taken to determine the photosynthetic pigments using the following formula:
Where, A663 and A646 are the absorbance values read at 663 and 646 nm wavelengths respectively.
Total carotenoid content was calculated using the following formula:
Ca and Cb are the content of chlorophylls a and b in mg ml−1 (Lichtenthaler & Wellburn 1983).
2.5 Enzyme assays
Leaf samples were collected in an ice bucket and brought to the laboratory. Leaves were then washed with distilled water and surface moisture was wiped out. Leaf samples (0.5 g) were homogenized in ice cold 0.1 M phosphate buffer (pH 7.5) containing 0.5 mM EDTA with pre-chilled pestle and mortar. The homogenate was transferred to centrifuge tubes and was centrifuged at 4°C in Beckman refrigerated centrifuge at 15000 rpm for 15 min. The supernatant was transferred to 30 mL tubes and referred to enzyme extract.
Protein content in the enzyme extracts was determined according to Bradford (1976) using Bovine Serum Albumin V as a standard.
Catalase (CAT) (EC 1.11.1.6) activity was measured according to Beer and Sizer (1952), with minor modifications. The reaction mixture (1.5 mL) consisted of 100 mmol L−1 phosphate buffer (pH 7.0), 0.1 mmol L−1 EDTA, 20 mmol L−1 H2O2 and 20 μL enzyme extract. The reaction was started by addition of the extract. The decrease of H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient (36 mol L−1 cm) and the results expressed as CAT units per min and mg of protein.
Peroxidase (POD) (EC 1.11.1.7) activity was estimated according to Hemeda and Klein (1990). The reaction mixture contained 25 mmol L−1 phosphate buffer (pH 7.0), 0.05% guaiacol, 10 mmol L−1 H2O2 and enzyme. Activity was determined by the increase in absorbance at 470 nm due to guaiacol oxidation (E = 26.6 mM−1 cm−1).
Polyphenol oxidase (PPO) (EC 1.10.3.1) activity was assayed by the method of Kumar and Khan (1982). Assay mixture for PPO contained 2 mL of 0.1 M phosphate buffer (pH 6.0), 1 mL of 0.1 Mcatechol and 0.5 mL of enzyme extract. This was incubated for 5 min at 25°C, after which the reaction was stopped by adding 1 mL of 2.5 N H2SO4. The absorbance of the purpurogallin formed was read at 495 nm. To the blank 2.5 N H2SO4 was added of the zero time of the same assay mixture. PPO activity is expressed in U/mg protein (U = change in 0.1 absorbance min−1 mg−1protein).
Ascorbate peroxidase (APX) (EC 1.11.1.11) activity was determined according to Nakano and Asada (1981). The reaction mixture contained 50 mM potassium phosphate (pH 7.0), 0.2 mM EDTA, 0.5 mM ascorbic acid, 2% H2O2, and 0.1 mL enzyme extract in a final volume of 3 mL. The decrease in absorbance at 290 nm for 1 min was recorded and the amount of ascorbate oxidized was calculated using extinction coefficient (ε = 2.8 mM−1 APX was defined as 1 mmol mL−1 per min at 25°C cm−1). One unit of ascorbate oxidized as 1 mmol mL−1 ascorbate oxidized per min at 25°C.
2.6 Proline content
Free proline content in the leaves was determined in accordance with the method of Bates et al. (1973). Leaf samples (0.5 g) were homogenized in 5 ml of sulphosalycylic acid (3%) using mortar and pestle. About 2 mL of the extract was taken in test tube and 2 mL of glacial acetic acid and 2 mL of ninhydrin reagent were added to it. The reaction mixture was boiled in water bath at 100°C for 30 min. After cooling the reaction mixture, 6 mL of toluene was added and then transferred to a separating funnel. After thorough mixing, the chromophore containing toluene was separated and absorbance read at 520 nm in spectrophotometer against toluene blank. Content of proline was estimated by referring to a standard curve of proline.
2.7 Determination of lipid peroxidation rate
Oxidative damage to leaf lipids, resulting from drought stress, was estimated by the content of total 2-thiobarbituric acid reactive substances (TBARS) expressed as equivalents of malondialdehyde (MDA). TBARS content was estimated using Cakmak and Horst (1991) method with some modifications. Fresh leaf samples (0.2 g) were ground in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA), at 4°C. Following the centrifugation at 12000 × g for 5 min, an aliquot of 1 ml from the supernatant was added to 4 ml of 0.5% (w/v) thiobarbituric acid (TBA) in 20% (w/v) TCA. Samples were heated at 90°C for 30 min. Thereafter, the reaction was stopped in ice bath. Centrifugation was performed at 10000 × g for 5 min, and absorbance of the supernatant was recorded at 532 nm using a spectrophotometer (Model Camspec M330 UV/Vis) and corrected for non-specific turbidity through subtracting the absorbance at 600 nm. The following formula was applied to calculate MDA content using its absorption coefficient (ε) and expressed as nmol MDA g−1 fresh mass:
Where, ε is the specific extinction coefficient (=155 mM cm−1), V is the volume of crushing medium, W is the fresh weight of leaf, A600 is the absorbance at 600 nm wavelength and A532 is the absorbance at 532 nm wavelength.
2.8 Statistical analysis
Analysis of variance was performed using the SAS software (Ver. 9.1). Means were compared using the Duncan test at the 5% probability level.
3 Results
3.1 AM colonization
AM colonization rates are shown in Fig. 1. Microscopic assessment confirmed that plants of the non-inoculated treatment were not colonized by AM. Percentages of mycorrhizal colonization varied among plants inoculated with three Glomus species. The plants inoculated with mycorrhiza species had colonization percentages ranging from 40% to 83.5% in the roots of non- stressed and drought stressed plants, respectively. As it is evident in Fig. 1, the colonization rate declined with increasing the severity of drought stress. Under NS condition, plants inoculated with G. etunicatum and G. versiform had significantly more colonization rate compared with those inoculated with G. intraradices. However, there were no significant differences in colonization rates of mycorrhiza species under MDS and SDS conditions (Fig. 2).
Root colonization percent in chickpea subjected to different irrigation treatments [non-stress (NS), moderate drought stress (MDS) and severe drought stress (SDS)] and AM species application levels [non-inoculated (control) and inoculated chickpea by Glomus species (G. versiforme, G. intraradices and G. etunicatum)]. The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
3.2 Plant growth parameters
Drought stress decreased plant height in chickpea plants (Fig. 3). Also, drought stress decreased shoot and total dry weight in non-inoculated chickpea plants, especially under SDS condition (Table 2). Plant colonization with AM considerably increased plant height, total dry weight, root and shoot dry weights of chickpea, especially under the severe drought stress. Mycorrhiza species had no significant effect on plant height, total dry weight, root and shoot dry weights under any of the irrigation treatments (Table 2). However, plant colonization with AM considerably improved these traits under all irrigation treatments, particularly under NS and SDS conditions. There was no significant difference between inoculated and non- inoculated plants in height under MDS condition (Table 2).
Plant height in chickpea subjected to different irrigation treatments [non-stress (NS), moderate drought stress (MDS) and severe drought stress (SDS)]. The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
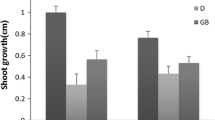
In both Pirouz and ILC482 cultivars, plant height and root dry weight were not significantly differed among mycorrhiza species. Although, in Pirouz cultivar, plants inoculated with G. versiform significantly had more root dry weight in comparison with G. intraradices. However, ILC-482 inoculated with different AM species had the highest plant height and root dry weight (Table 3). ILC-482 cultivar significantly had more total dry weight than Pirouz cultivar (Fig. 4).
3.3 Chlorophyll and carotenoid content
Table 4 shows that drought stress partly decreased chlorophyll a and total chlorophyll content in chickpea plants. However, there was no significant difference between plants under moderate and severe stress. Chlorophyll a content in chickpea leaf was also affected by inoculation with different species of AM (Table 4). In general, chickpea plants inoculated with AM produced significantly more chlorophyll a in comparison with non-inoculated plants. Although different species of AM had similar effect on chlorophyll a under SDS and MDS, the plants inoculated with G. versiform noticiably produced the highest chlorophyll a under NS condition, followed by inoculated plants with G. etunicatum and G. intraradices, respectively (Table 4). Two cultivars of Pirouz and ILC-482 showed similar reaction to non-inoculation and inoculation with G. etunicatum and G. versiform in leaf chlorophyll a content. However, inoculation by G. intraradices had more positive effect on chlorophyll a content of ILC-482, compared to that of Pirouz (Fig. 5).
Chlorophyll a (Chl a) content in non-inoculated (control) and inoculated chickpea by Glomus species (G. versiforme, G. intraradices and G. etunicatum). The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
Table 4 indicates that under SDS and MDS conditions, different AM species had similar effect on T chl production in chickpea plant, but under NS condition, the plants inoculated with G. versiform AM species produced the highest T chl amount. In general, the highest and the lowest T chl content were recorded for plants inoculated with G. versiform under NS conditions and non-inoculated plants in SDS, respectively. Total chlorophyll content of inoculated and non-inoculated plants under SDS was statistically similar to those under MDS condition (Table 4). In Pirouz cultivar, the highest T chl production was related to plants inoculated with G. versiform. There was no significant difference in total chlorophyll content of these plants and those inoculated with G. etunicatum. In this cultivar, non-inoculated plants had significantly less T ch production than those inoculated with G. etunicatum and G. versiform species. However, there was no significant difference between non-inoculated and inoculated plants with G. intraradices in Pirouz cultivar. In ILC-482, there was noticeable difference between non-inoculated plants and those inoculated with AM species. The non-inoculated plants had the least T chl content. T chl content of non-inoculated plants and those inoculated with G. etunicatum and G. versiform species was similar for both cultivars. Whereas inoculating two cultivars with G. intraradices mycorrhiza species resulted in more T chl production in ILC-482 (Fig. 6). Drought stress and plant inoculation had no significant effects on carotenoid content of chickpea leaves (P ˃ 0.05).
Total chlorpphyll (T chl) content in non-inoculated (control) and inoculated chickpea by Glomus species (G. versiforme, G. intraradices and G. etunicatum). The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
3.4 Anti-oxidative enzymes activities
The effects of different irrigation levels partly were different on POD activity in two chickpea cultivars. In Pirouz cultivar, plants under drought stress conditions (MDS and SDS) had significantly more POD activity, compared with non-stressed plants. There was no noticeable difference in POD activity between plants under MDS and SDS conditions (Table 5).
In ILC-482, the highest POD activity was observed under SDS condition. In this cultivar, plants under MDS showed more POD activity, compared with NS plants, but this difference was not statistically significant. ILC-482 had significantly more POD activity in comparison with Pirouz cultivar under SDS condition. However, there was no significant difference in POD activity of these two cultivars under other irrigation treatments (Table 5).
Figure 7 clearly shows that POD activity of chickpea was affected by inoculation with AM, so that the plants inoculated with G. etunicatum had the most, but non-inoculated plants had the least POD activity. Peroxidase activity did not significantly differ between plants inoculated with G. etunicatum and G. versiform species and between G. versiform and G. intraradices species. There was no noticeable difference between non-inoculated plants and those inoculated with G. intraradices.
Polyphenol oxidase (PPO) and peroxides (POD) enzymes activities in non-inoculated (control) and inoculated chickpea by Glomus species (G. versiforme, G. intraradices and G. etunicatum). The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
Drought stress had no effect on PPO activity in chickpea plants. The plants lacking AM had significantly low PPO activity, compared with plants having AM. Although the highest activity of this enzyme was observed in plants inoculated with G. versiform, it had no significant difference with plants inoculated by G. etunicatum or G. intraradices (Fig. 7).
CAT activity was significantly affected by irrigation treatments in both cultivars. The activity amount of this enzyme was increased with increasing the severity of drought stress. CAT activity under SDS condition was enhanced by 37% and 71.9% in Pirouz and 69.4% and 82.6% in ILC-482, compared with that under MDS and NS conditions, respectively. ILC-482 had significantly more CAT activity than Pirouz under SDS condition. However, there was no significant difference in CAT activity of two chickpea cultivars under other irrigation treatments (Table 5).
The results revealed (Table 5) that APX activity was somewhat affected by different irrigation levels in both Pirouz and ILC-482 cultivars. Drought stress increased the activity of this enzyme in Pirouz. In this cultivar, the highest APX activity was observed in plants under drought stress (MDS and SDS). In ILC-482, although plants under SDS condition had more APX activity, there was no significant difference between plants under SDS and NS conditions. Furthermore, there was no significant difference in APX activity of plants under MDS and NS conditions. Although Pirouz had comparatively more APX activity under all irrigation treatments, significant superiority of Pirouz over ILC-482 was observed under MDS condition (Table 5).
APX activity of Pirouz and ILC-482 cultivars was differently affected by application and non-application of different AM species. In Pirouz, the highest activity of APX was recorded in plants inoculated with G. etunicatum. Difference between inoculated chickpeas with G. etunicatum and G. intraradices was not significant. In this cultivar, there was no noticeable difference in APX activity between plants inoculated with G. versiform and non-inoculated plants and also between plants inoculated with G. intraradices and non-inoculated plants. In ILC-482, the highest APX activity was observed in plants inoculated with G. intraradices. In this cultivar, there was no noticeable difference between plants inoculated with G. intraradices and G. versiform. Chickpea plants inoculated with G. etunicatum was significantly superior in APX activity, compared with non-inoculated chickpeas (Fig. 8).
Ascorbate peroxidase (APX) activity in non-inoculated (control) and inoculated chickpea by Glomus species (G. versiforme, G. intraradices and G. etunicatum). The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
3.5 Protein content
Drought stress had no effect on soluble protein content of chickpea plants. Under NS condition, inoculated plants significantly produced more soluble protein, compared with non-inoculated plants. There was no noticeable difference in protein content of AM pants under NS condition. Protein content of non-inoculated and inoculated plants was not significantly differed under MDS and SDS conditions. In other words, inoculating chickpea plants with AM did not significantly increase leaf soluble protein content under SDS and MDS conditions (Table 6).
3.6 Proline content
Figure 9 shows that proline content was partly increased with increasing drought stress severity, so that the least and the highest proline accumulation was observed in well-irrigated plants (15.42 mg g-1 DW) and plants under SDS (15.82 mg g-1 DW), respectively. There was no significant difference in proline content of plants under MDS and SDS. Proline accumulation in chickpea was not affected by plant inoculation with AM.
Proline content in chickpea leaves subjected to different irrigation treatments [non-stress (NS), moderate drought stress (MDS) and severe drought stress (SDS)]. The results are means (n = 3) ± SE. Means with the same letter for each stage are not significantly different (Duncan’s Multiple Range test; P ≤ 0.05)
3.7 Lipid peroxidation (MDA)
Membrane lipid peroxidation (MDA) was affected by irrigation treatments (Table 6). MDA was increased with increasing the severity of drought stress in non-inoculated chickpea plants. There was no significant difference in lipid peroxidation between AM chickpea plants under SDS and MDS conditions (Table 6). Inoculation and non-inoculation of chickpea plants with different AM species significantly affected MDA. Moreover, it was observed that under NS conditions, non-inoculated plants had fewer MDA, compared with plants inoculated with AM. Although inoculated plants had less MDA under MDS condition, there was no significant difference between them. The chickpea plants inoculated with G. etunicatum had the highest membrane lipid peroxidation in this irrigation level. However, there was no significant difference in lipid peroxidation of non-inoculated and inoculated plants under SDS condition (Table 6). There was no significant difference in lipid peroxidation of inoculated plants under NS and SDS conditions (Table 6). MDA was significantly more in Pirouz than in ILC-482 (Fig. 10).
4 Discussion
The lowest colonization was observed in plants under severe drought stress. Al-Karaki et al. (2004) also showed that mycorrhizal colonization was higher in well-watered wheat plants colonized with AM fungi species than drought- stressed wheat plants. The lower level of colonization under drought stress could be attributed to the reduced carbon availability from the host plant (Kehri and Chandra 1990), because the plant ability to absorb water and nutrients and consequently for photosynthesis decreases under drought stress.
Percentage of mycorrhizal colonization varied among plants colonized with three Glomus species under NS condition, as plants inoculated with G. etunicatum and G. versiform had significantly more colonization rate than those inoculated with G. intraradices. The inter and intraspecific differences in the effectiveness of AM fungi for root colonization and plant growth are well known (Harley and Smith 1983). However, there was no significant difference among mycorrhiza species under MDS and SDS conditions (Fig. 1). In general, two cultivars of Pirouz and ILC-482 showed similar reaction to colonization by mycorrhiza species (Fig. 2).
Shoot and total dry weight of chickpea plants was decreased under drought stress. However, inoculation of plants with AM significantly increased plant height, total dry weight, root and shoot dry weights, particularly under SDS condition (Table 2). Improvement of growth parameters in plants inoculated with mycorrhiza species under drought stress and non- stress conditions can be related to ability of mycorrhiza in increasing the water absorption capacity of plant by increasing root hydraulic conductivity, the absorptive surface area of the root system, and access to small soil pores (Augé 2001). As in our study inoculation of plants with mycorrhiza increased root dry weight, leading to improvements in other plant growth parameters (Table 2). These results are matching with Al-Karaki et al. (2004). They stated that biomass was higher in AM inoculated than non- inoculated wheat plants. Results revealed that inoculation of ILC-482 cultivar by each of the AM species had significantly more positive effect on increasing root dry weight than Pirouz cultivar, under SDS (data no shown). Therefore, superiority of ILC-482 cultivar in plant height may probably be due to greater root growth and improving water and nutrient uptake.
A decrease in leaf chlorophyll content (a and T chl) of chickpea under drought stress (Table 4) would be a typical symptom of oxidative stress (Seel et al. 1992; Moran et al. 1994). Drought stress increases the formation of reactive oxygen species (ROS) that damage plants through oxidizing photosynthetic pigments (Smirnoff 1993; Alscher et al. 1997; Yordanov et al. 2000). Chlorophyll a content of leaves increased because of plant inoculation with AM (Table 4). This is possibly due to the fact that under drought stress, mycorrhizal plants can absorb more water than non-mycorrhizal ones. Also mycorrhizae, by improving nutrition (McArthur and Knowles 1993), can enhance chlorophyll content (Rachel et al. 1992). These results are in agreement with those previously found by Mathur and Vyas (2000). They detected that Arbuscular mycorrhizal root colonization increased chlorophyll synthesis. Furthermore, Beltrano and Ronco (2007) reported that either moderately or severely stressed mycorrhizal plants had significantly higher chlorophyll content compared with non-AM plants.
Proline content of leaves was not statically different between chickpea cultivars and between plants inoculated and non-inoculated, however drought stress increased significantly proline accumulation in chickpea leaves (Fig. 9). Osmotic adjustment has been reported in chickpea under water deficit conditions (Gunes et al. 2008). Increasing proline content of leaves with decreasing available water means that an efficient mechanism for osmotic regulation, stabilizing sub-cellular structures, and cellular adaptation to water stress was provided (Valentovic et al. 2006; Gunes et al. 2008). Proline also serves as a sink for energy to regulate redox potentials, as a hydroxyl radical scavenger, as a solute that protects macromolecules against denaturation and as a means of reducing acidity in the cell (Kishor et al. 1995; Smirnoff 1993).
The highest soluble protein content of leaves was observed in plants inoculated with AM under NS conditions and in plants non-inoculated under MDS conditions. Under SDS conditions, there was no significant difference between plants inoculated and non-inoculated (Table 6). This result support that of Wu et al. (2006), who detected that in spite of AM roots had significantly higher soluble protein than non- AM roots grown under well-irrigated conditions, no significance was found in AM and non-AM roots under water stress conditions.
Drought stress (MDS and SDS) increased POD activity in Pirouz cultivar. However, in ILC-482 cultivar, only severe drought stress increased POD activity. Drought stress increased CAT activity in both cultivars (Table 5). Many of the degenerative reactions associated with several environmental stresses including water deficit result in the production of reactive oxygen species (ROS) in plants, causing additional oxidative stress (Ruiz-Lozano 2003). Plants can protect themselves by synthesizing antioxidants (carotenoids, ascorbate, and glutathione) and increase anti-oxidative enzymes (POD, CAT, and APX) (Egert & Tevini 2002). CAT, which is present only in peroxisomes, transmutes H2O2 into water and molecular oxygen, whereas POD decomposes H2O2 through oxidation of co-substrates such as phenolic compounds and/or antioxidants (Bowler et al. 1994). The activities of CAT and POD were significantly more in ILC-482 than in Pirouz cultivar under SDS condition (Table 5). Hence, less oxidative damage (lipid peroxidation) in ILC-482 (Kabuli type) may be resulted from the greater CAT and POD activities. These observations are different from previous reports on genotypes of other plant species that have different water stress sensitivity, where CAT activity was observed to be greater in stress-tolerant types than their counterparts (Sairam et al., 2004). Our observations agree with the proposal by Bartels (2001) that both the prevention of oxidative stress and the elimination of reactive oxygen species are the most effective approaches used by plants to gain tolerance against several abiotic stresses, including drought. In Pirouz cultivar, drought stress increased APX activity, but had no significant effect on this enzyme in ILC_482 cultivar. In general, APX , which catalyses the conversion of H2O2 to water using ASC as substrate, was found to be significantly higher in Desi than in Kabuli types which is in agreement with those previously found by Nayyar et al. (2006).
In most cases, inoculation of chickpea by AM increased POD, PPO and APX activities significantly. However it had no effect on CAT activity (Figs. 7 and 8). Most of the POD activity was recorded for inoculated plants with G. etunicatum (Fig. 7). The highest APX activity was observed in chickpeas inoculated with G. intraradices (Fig. 8). In fact, AM colonization seemed partly affect the antioxidant production. Several antioxidant enzymes have been studied under similar stress conditions and it was found that different responses in mycorrhizal plants have frequently been observed (Ruiz-Lozano et al. 1996). Ruiz-Lozano (2003) reported that AM symbiosis might increase the drought resistance of higher plants through promoting antioxidant enzymes. Wu and Zou (2009) detected that the mechanism might be that AM themselves possesses several special genes encoding for antioxidant enzymes. Therefore, expression patterns can regulate the activities of antioxidant enzymes independently regardless of the mycorrhizal colonized levels.
Reaction of chickpea cultivars to inoculation by AM species and irrigation levels were different. This result is in agreement with those of Hetrick et al. (1996), who investigated the responses of wheat to AM and drought stress and reported significant differences in responses to AM among wheat cultivars.
Although, drought stress especially SDS increased MDA (a final product of the peroxidation of unsaturated fatty acids in phospholipids) in inoculated and non-inoculated chickpea plants, but increase of MDA was more in non-inoculated than in inoculated plants (Table 6). Results indicate that there was no significant difference in MDA between plants inoculated with AM under SDS and NS conditions (Table 6). In fact, AM colonization seemed partly adjusted effect of drought stress on MDA. But it was found that in non-inoculated plants, MDA was noticeably increased under SDS conditions compared with NS conditions. One possible explanation for this result is that plant inoculation by AM, increases antioxidant enzyme activity. Our findings are in agreement with those of Zhong Qun et al. (2007). Beltrano and Ronco (2007) demonstrated that the normal status of cell membranes is restored more efficiently in mycorrhizal plants than in non-mycorrhizal plants. Water stress can induce ROS, because of lipid peroxidation and production of MDA (Lacan & Baccou 1998). Therefore, the oxidation of membrane lipids is a reliable indication of uncontrolled free radical production, and hence, of oxidative stress (Noctor & Foyer 1998). ILC-482 had less MDA than Pirouz (Fig. 10). These observations are different from previous findings by Nayyar et al. (2006). They proportionately showed a markedly higher content of MDA in Kabuli type. Consequently, ILC-482 had more root dry weight and POD and CAT activities under SDS conditions, although Pirouz had more APX activity under MDS conditions (Table 5). Thus, increasing antioxidant enzymes activity (POD and CAT) and improving water absorption probably decreases the amount of membrane lipid peroxidation. Plants inoculated with G. intraradices showed the least antioxidant enzymatic activities, although had more APX activity than other AM species (Fig. 7). Hence, they had more MDA compared with plants inoculated with other AM species under SDS conditions, although there was no significant difference between them (Table 6). It appears that the POD and CAT have a greater impact in reducing the effects of severe drought stress in ILC-482 cultivar compared with Pirouz cultivar.
5 Conclusion
It can be concluded that drought stress decreases plant growth parameters and chlorophyll content and increases leaf proline content in chickpea cultivars. There are differences in biochemical responses of Desi and Kabuli types of chickpea to water stress and symbiosis with some of AM species. In present study, ILC-482 cultivar significantly had more plant height and root dry weight and also had less Membrane lipid peroxidation than Pirouz cultivar. On the other hand, it was appeared that POD and CAT have a greater impact in reducing the effect of severe drought stress in ILC-482 cultivar compared with Pirouz cultivar. Therefore, induced POD and CAT in AM symbiosis may be an important mechanism to improve water deficit resistance in this cultivar.
Abbreviations
- AM:
-
Arbuscular mycorrhiza fungal
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- MDA:
-
malondialdehyde
- MDS:
-
moderate drought stress
- NS:
-
non-stress
- POD:
-
peroxidase
- PPO:
-
polyphenol oxidase
- SDS:
-
severe drought stress
References
Alguacil MM, Hernandez JA, Caravaea F, Portillo B, Roldan A (2003) Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiol Plant 118:562–570
Al-Karaki GGN, McMichael B, ZAK J (2004) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14:263–269
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233
Augé RM (2001) Water relations, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Bartels D (2001) Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance. Trends Plant Sci 6:284–286
Bates LS, Waldran RP, Teare ID (1973) Raipid determination of free proline for water studies. Plant Soil 39:205–208
Beer RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Beltrano J, Carbone A, Montaldi ER, Guiamet JJ (1994) Ethylene as promoter of wheat grain maturation and ear senescence. Plant Growth Regul 15:107–112
Beltrano J, Ronco M, Montaldi ER (1999) Drought stress syndrome in wheat is proved by ethylene evolution and reversed by rewatering, aminoethoxyvinylglycine, or sodium benzoate. J Plant Growth Regul 18:59–64
Beltrano J, Ronco MG (2007) Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus claroideum: Effect on growth and cell membrane stability. Braz J Plant Physiol 20(1):29–37
Bowler C, Van Camp W, Van Montagu M, Inzé D (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13:199–218
Bowler C, Van Montagu M, Inzé D (1992) Superoxide dismutase and stress tolerance. Ann Rev Plant Physiol Plant Mol Biol 43:83–116
Boyer JS (1982) Plant productivity and environment. Sci 218:443–448
Bradford MN (1976) A rapid and sensitive method for the quantiation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2:48–54
Burke JJ, Mahan JR (1991) Environmental regulation of cellular protection systems. In: Gausman HW (ed) Plant Biochemical Regulators. Marcel Dekker, Inc, New York, pp 47–58
Cakmak I, Horst J (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Caravaca F, Alguacil MM, Hernandes JA, Roldan A (2005) Involvement of antioxidant enzyme and nitrate reductase activities during water stress and recovery of mycorrhizal Myrtus communis and Phillyrea angustifolia plants. Plant Sci 169:191–197
Creissen GP, Edwards AE, Mullineaux PM (1994) Glutathione reductase and ascorbate peroxidase. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 343–364
Diego AM, Oliva MA, Carlos AM, Cambraia J (2003) Photosyntesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Dionisio-Sese, Maribel L, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Egert M, Tevini M (2002) Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ Exp Bot 48:43–49
Elstner EF (1987) Metabolism of activated oxygen species. In: Davies DD (ed) The Biochemistry of Plants.vol. II, Biochemistry of Metabolism. Academic, San Diego, CA, pp 252–315
Francisco JP, Daniel V, Nilo M (2002) Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry 60:573–580
Girousse C, Bournoville R, Bonnemain JL (1996) Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol 111:109–113
Gunes A, Inal A, Adak MS, Bagci EG, Cicek N, Eraslan F (2008) Effect of drought stress implemented at pre- or post- anthesis stage some physiological as screening criteria in chickpea cultivars. Russian J Plant Physiol 55(1):59–67
Halliwell B, Gutteridge JMC (1985) Free radicals in biology and medicin. Clarendon, Oxford
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon, Oxford, UK
Harborne JB (1984) Phytochemical Methods. A guide to modern techniques of plant analysis. Chapman and Hall, London, p 288
Harley JL, Smith SE (1983) Mycorrhizal Symbiosis. London and New York: Academic Press
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hetrick BAD, Wilson GWT, Todd TC (1996) Mycorrhizal response in wheat cultivars: relationship to phosphorus. Can J Bot 74:19–25
Jakob B, Heber U (1996) Photoproduction and detoxification of hydroxyl radicals in chloroplast and leaves in relation to photoinactivation of pohotosystems I and II. Plant Cell Physiol 37:629–35
Kehri HK, Chandra S (1990) VAM association in urd as affected by water stress condition in soil and foliar spray. Acta Bot Ind 18:316–318
Kishor PB, Hong Z, Miao GH, Hu CA, Verma DPS (1995) Overexpression of D1-pyrroline- 5-carboxilate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–94
Koide RT, Schreiner RP (1992) Regulation of the vesicular arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol 43:557–581
Kramer PJ, Boyer JS (1997) Water relations of plants and soils. Academic, San Diego, Calif
Kumar KB, Khan PA (1982) Peroxidase and polyphenoloxidase in excisedragi (Eleusine coracana cv. PR 202) leaves during senescence. Ind J Exp Bot 20:412–416
Lacan D, Baccou JC (1998) High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 204:377–382
Lambais MR, Rios-Ruiz WE, Andrade RM (2003) Antioxidant responses in bean (Phaseolus vulgaris) roots colonized by arbuscular mycorrhizal fungi. New Phytol 160:421–428
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoides and chlorophylls ‘a’ and ‘b’ of leaf extract in different solvent. Biochem Soc Trans 11:591–592
McArthur DAJ, Knowles NR (1993) Influence of VAM fungi and nutrition on growth, development, and mineral nutrition of potato. Plant Physiol 102:771–782
Malecka A, Jarmuszkiewicz W, Tomaszewska B (2001) Antioxidative defense to lead stress in subcellular compartments of pea root cells. Acta Biochim Pol 48:687–698
Malhotra RS, Pundir RPS, Slinkard AE (1987) Genetic resources of chickpea. In: Saxena MC, Singh KB (eds) The Chickpea. CAB International Cambrian News, Aberystwyth, pp 67–81
Mathur N, Vyas A (2000) Influence of arbuscular mycorrhizae on biomass production, nutrient uptake and physiological changes in Ziziphus mauritana Lam. under water stress. J Arid Environ 45:191–195
McGonigle TP, Miller MH, Evans DG, Fairchild DL, Swan GA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Mitter R (2002) Oxidatve stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P (1994) Drought induces oxidative stress in pea plants. Planta 194:346–352
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nayyar H, Singh S, Kaur S, Kumar SD, Upadhyaya H (2006) Different sensitivity of macrocarpa and microcarpa types of chickpea (Cicer arietinum L.) to water stress:association of contrasting stress response with oxidative injury. Journal Integrative Plant Biology 48(1):1318–1329
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–79
Palma JM, Longa MA, del Rio LA, Arines J (1993) Supperoxide dismutase in vesicular arbuscular-mycorrhizal red clover plants. Physiol Plant 87:77–83
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T British Mycol Soc 55:158–161.l
Porcel R, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhiz soybean plants and their possible relationship to the process of nodule senescence. New Phytol 157:135–143
Rachel EK, Reddy SR, Reddy SM (1992) Seedling preinoculation with AM fungi on transplant survival and growth of sunflower. Proc Natl Acad Sci Ind B 62:429–432
Robinson DA, Campbell CS, Hopmans JW, Hornbuckle BK, Jones SB, Knight R, Ogden F, Selker J, Wendroth O (2008) Soil moisture measurement for ecological and hydrological watershed-scale observatories. A review Vadose Zone J 7:358–389
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress: new perspectives for molecular studies. Mycorrhiza 13:309–317
Ruiz-Lozano JM, Azcón R, Palma JM (1996) Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. New Phytol 134:327–333
Sairam RK, Rao KV, Srivastava GC (2004) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Seel WE, Hendry GAF, Lee JA (1992) The combined effect of desiccation and irradiance on mosses from xeric and hydric habitats. J Exp Bot 43:1023–1030
Singh KB, Ocampo B (1997) Exploitation of wild Cicer species for yield improvement in chickpea. Theor Appl Genet 95:418–423
Sinha S, Saxena R, Singh S (2005) Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: role of antioxidants and antioxidant enzymes. Chemosphere 58:595–604
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Smith SE, Gianinazzi-Pearson V (1988) Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu Rev Plant Physiol 39:221–244
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, San Diego, Calif
Sun W, Montagu MV, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577:1–9
Valentovic P, Luxova M, Kolarovic L, Gasparikova O (2006) Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ 4:186–191
Whalley WR (1993) Considerations on the use of time-domain reflectometry (TDR) for measuring soil water content. J Soil Sci 44:1–9
Wu QS, Zou YN (2009) Mycorrhiza has a direct effect on reactive oxygen metabolism of drought-stressed citrus. Plant Soil Environ 55(10):436–442
Wu QS, Zou YN, Xia RX (2006) Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur J Soil Biol 42:166–172
Wu QS, Zou YN, Xia RX, Wang MY (2007) Five Glomus species affect water relations of Citrus tangerine during drought stress. Bot Stud 48:147–154
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38:171–186
ZhongQun H, ChaoXing H, ZhiBin Z, ZhiRong Z, HuaiSong W (2007) Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids and Surfaces B: Biointerfaces 59(2):128–133
Acknowledgment
We are grateful to the University of Kurdistan for financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohrabi, Y., Heidari, G., Weisany, W. et al. Changes of antioxidative enzymes, lipid peroxidation and chlorophyll content in chickpea types colonized by different Glomus species under drought stress. Symbiosis 56, 5–18 (2012). https://doi.org/10.1007/s13199-012-0152-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-012-0152-8