Abstract
“Mallín” (plural mallines) is a particular kind of wetland occurring in Patagonian steppe and forests. In Northwest Patagonia, mallines are humid meadows with high net primary production. It was previously found that a mallín soil in the steppe devoid of actinorhizal plants had a higher Frankia nodulation capacity in Ochetophila trinervis (sin. Discaria trinervis) than other soils in the region. Under the hypothesis that mallín wetland meadows are reservoir of infective Frankia, we studied the Frankia nodulation capacity in O. trinervis of 12 mallín and their neighbouring steppe soils, by using plant bioassays. A qualitative plant bioassay showed that infective Frankia was present in most soils. The number of nodules per plant in seedlings inoculated with mallín soils was negatively correlated with soil water content while the opposite was true for plants inoculated with soils from neighbouring steppe. A quantitative bioassay was performed with eight representative soils, selected according to the number of nodules per plant produced in the qualitative assay and to the presence or not of different actinorhizal plants at the sites. Frankia nodulation units per cm3 of soil (NU) in mallín soils were higher than those in steppe. Water and organic matter content of soils were correlated with the higher nodulation capacity of mallines, which may account for the saprotrophic growth of Frankia in soils. The symbiosis was effective in plants inoculated with all soil samples. These results suggest that Northwestern Patagonian mallín wetland meadows are reservoirs of infective and effective Frankia propagules in O. trinervis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wetlands hold a high biodiversity that is associated with change over time through ecological succession and zonation both within and at the boundary of the ecosystem (Maltby 2009). A particular kind of wetland is “mallín” (plural mallines) from Patagonia, which is located either in steppe or forest environments. Northwestern Patagonian mallines can be characterized as wet meadows, with a dense cover mainly dominated by plants belonging to families Juncaceae, Cyperaceae and Poaceae, with soil containing a high percentage of organic matter, low oxygen amount and high CO2 concentration, and always associated with either surface-water or ground-water discharge. All these characteristics contribute to high net primary production. In the steppe, mallines present strongly fluctuating water regimes that can cause permanent or temporary flooding (in general, only in summer the peripheral areas of mallines may remain dry) and are heavily grazed by cattle and sheep (Raffaele 1996, 1999).
Many attributes of mallines have been well characterized, including: soils hydrology, flora, fauna, palynology, the size and composition of their seed banks and their response to environmental conditions (del Valle 1998; Boelcke et al. 1985; Perotti et al. 2005; Markgraf 1981, 1983, 1984; Raffaele 1996), however, little is known about their microbial communities.
It is known that they contain a high abundance of mycorrhizal propagules (Fontenla 2000), and diverse Actinomycetes strains, belonging to Micromonospora, Streptomyces, Actinoplanes and the Nocardioforme group (Cardoso and Vobis, unpublished results), and to the genus Frankia (Chaia et al. 2006). Frankia comprises a diverse group of strains that mainly include those with N2-fixing ability that form root nodules in symbiosis with different non-leguminous woody dicotyledonous plants, but also non-N2-fixing strains (Van Dijk and Sluimer-Stolk 1990). Polyphasic studies of this genus are increasing understanding about strain diversity and specific Frankia populations in the environment (Hahn 2008).
Infective Frankia on Ochetophila trinervis (sin. Discaria trinervis) from soils along a vegetation gradient in Northwestern Patagonia are independent of the presence of host plants (Chaia et al. 2006), as it was found in other regions (Zitzer and Dawson 1992). Nevertheless, the nodulation capacity of those soils was found to be associated to soil water content, either under a seasonal or a geographical range. The positive correlation between soil water content and the nodulation capacity of soils collected in different seasons, either stored wet or air-dried, revealed that soil water was a main factor regulating soilborne Frankia nodulation ability (Chaia et al. 2007). Furthermore, it was found that steppe soils associated with wet conditions had higher Frankia nodulation capacity than other soils from the steppe and the forest region. Particularly, a mallín soil in the steppe that had about three-fold higher nodulation units than a gallery forest along a temporary stream in the same area (Chaia et al. 2006), lead us to hypothesise that due to their singular soil conditions, such as temporary flooding and a higher organic carbon than the neighbouring sites in the steppe, mallines are reservoir of infective Frankia. We predicted that mallines that are with or without actinorhizal plants and with higher organic matter content should have a higher nodulation capacity in O. trinervis than the neighbouring sites in the steppe. The objective of this study was to analyse the Frankia nodulation capacity of mallines and their neighboring steppe sites in relation to different soil properties. In addition, as it is known that wetland soils may have nodule-forming units in Alnus glutinosa of non-N2-fixing strains (ineffective Frankia) (Wolters et al. 1997; Van Dijk et al. 1988), we aimed to establish if infective Frankia strains in O. trinervis were also effective.
2 Material and methods
2.1 Study area
The study was conducted in the ecotone steppe near S. C. Bariloche city, provinces of Rio Negro and Neuquén, Argentina (Table 1). The study area was located in the transitional zone between the xeric forest and the steppe, covering an area along 100 Km distance, with vegetation dominated by bunchgrasses (Stipa spp, Acaena spp and Festuca pallescens) as well as low shrubs such as Mulinum spinosum and Discaria spp., and also by species of Poaceae, Juncaceae and Cyperaceae in the mallines (Boelcke et al. 1985). In this region climate is temperate cool, with strong westerly winds coming from the Pacific Ocean, with maximum speeds between September to January. Mean annual temperatures are about 6–8°C, and precipitation is mainly concentrated in winter, ranging eastward from 1000 to 500 mm yr−1 (Paruelo et al. 1998). Soils are Mollisols (del Valle 1998).
2.2 Sampling and laboratory analysis
Sampling was performed in 12 sites in May 2006. Each site comprised a mallín and the neighboring steppe at a distance between 30 and 200 m. Vegetation was characterized for every site (Table 1). In every mallín, ten soil sub-samples (about 50 g each) at 0 to 20 cm depth were aseptically collected, placed in sterile plastic bags and combined. The same procedure was done to collect the soil samples for the neighboring steppe. The samples were immediately transported to the laboratory and divided in two parts. One part, to be used in the qualitative plant bioassay, was stored moist at 4°C for 2 week. The other part, for further use in the quantitative plant bioassay, and the chemical analysis, was exposed to the air for 2–5 day and stored at room temperature (Chaia et al. 2007). The following soil properties were determined: pH in water (Vásquez 2005), electrical conductivity (Blakemore et al. 1987) and organic matter (Nelson and Sommers 1996). The water content of the soil samples was determined by drying at 106°C for 48 h.
2.3 Plant bioassays
A qualitative bioassay was performed to determine the presence of infective Frankia propagules in soils (Experiment 1). Seeds of Ochetophila trinervis collected in Pampa de Huenuleo (Bariloche, February 2005) and dry-stored at −20°C, were scarified and stratified (Chaia et al. 2006). Ten seeds were sown in each 70 cm3 pot, filled with a mixture (2:2:1 v/v) sterile sand-vermiculite, as substrate, and each soil sample. After 4 weeks the seedlings were thinned to four similarly sized plants in each pot. The assay had six replicates per treatment. Three additional pots per treatment were also inoculated with Frankia strain BCU110501 (Chaia 1998) as positive control and other 20 pots with only substrate and seedlings served as negative control. At the beginning of the assay pots were watered with Evans solution diluted to one tenth of full strength and with 0.71 mM N as NH4NO3 (Huss-Danell 1978). Then, seedlings were monthly fertilised with the same solution but with N 0.071 mM and watered when necessary. Pots were arranged in a complete randomised design (Montgomery 2005).
Later, a quantitative plant bioassay (Experiment 2) to establish Frankia nodulation units per cm3 of soil (NU) was performed by using the most probable number (MPN) (Woomer 1994) and the nodulation capacity (NC) (Van Dijk 1984). For this experiment the soils of four of the sites (from mallín and the corresponding neighboring steppe) were selected according to the number of nodules per plant produced in Experiment 1, and to the presence or not of different actinorhizal plants in the sites. Each soil sample, stored air-dried for 4 months, was crushed and then sieved (2 mm mesh). Six successive 5-fold dilutions (5−1 to 5−6) were prepared directly diluting and thoroughly mixing each soil sample (on a dry weight basis) with a sterile sand and vermiculite mixture (1:1 v/v). A 30 cm3 sample, from each respective soil dilution, was placed in a sterile glass tube and watered with the Evans solution with 0.71 mM N. O. trinervis seedlings at the cotyledon stage, previously germinated on sterile moist vermiculite, were transferred to each tube. There were five replicates for each dilution, and 20 non-inoculated tubes with seedlings were used as contamination controls.
Plants were cultivated in a growth chamber for 9 (experiment 1) and 10 (experiment 2) weeks, with 16 h photoperiod provided by metal halogen lamps (Philips HPI-T 400 W and Philips SON-T Plus 400) (photosynthetically active radiation was ca. 318 μM m−2 s−1). Average minimum and maximum temperatures were 22 and 27°C, respectively, while average relative humidity was 44%.
The plant growth and the nodulation was recorded in every plant of Experiment 1 and in plants inoculated with the 5−1 soil dilution of Experiment 2. Actinorhizal nodules were fixed in 4% glutaraldheyde. Lobes of 3 nodules per plant were excised in slices by hand, stained with cotton blue and were examined under an Olympus light microscope to determine the presence of Frankia vesicles (Wolters et al. 1997).
2.4 Data analysis
In order to organize all the sites from the whole study area according to soil properties they were classified by performing Exploratory Analysis of Clustering (Everitt 1980). Soils properties of mallín and neighboring steppe sites were compared by Wilcoxon Signed Rank test.
Data from Experiment 1 were calculated as average per plant within each pot. One-way ANOVA was used to compare the effect of mallín and neighboring steppe soils inocula on plant growth (Experiment 1). Mann–Whitney U-Test was used to compare growth of soil inoculated plants with non-inoculated controls, and also to compare Frankia NU in mallín and neighboring steppe soils.
Confidence intervals were applied to compare Frankia NU (Woomer 1994). Spearman’s correlations were performed between soils properties and the number of nodules per plant, or Frankia NU, and between Frankia NU estimated by MPN and NC (Montgomery 2005).
All tests were performed with the statistical program R.
3 Results
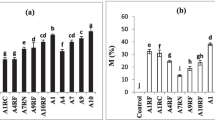
Mallín and neighboring steppe soils were clearly distinct. Mallín soils were much more variable and the steppe soils were much more uniform. Mallín soils clustered in two main groups. Mallín soils with the highest water content and lowest soil pH clustered together. The other cluster included two sub-clusters, with one of them having the lowest water content (Fig. 1). Mallín soils had significantly higher electrical conductivity, organic matter and relative water content than those from the neighboring steppe (T = −3.240, −3.120; and −3.926, p < 0.05). pH was similar in mallín and neighboring steppe soils, and varied between acid to slightly basic (Table 1). Vegetation also was different in each environment. Juncus balticus, Poa pratensis and Carex gayana were mostly present in mallines meanwhile in the neighboring steppe more commonly occurring species were Senecio sp, Stipa sp and Acaena splendens (Table 1).
Homogeneous soil groups of samples collected in mallines (Northwest Patagonian wetland meadows) and neighbour steppe sites, classified by performing Exploratory Analysis of Clustering (Everitt 1980), according to water and organic matter content, electrical conductivity and pH
The qualitative assay (Experiment 1) showed that infective Frankia in O. trinervis was present in 96% of the soils. Only the soils from Mallín 10 had no Frankia nodulation capacity, but due to the lack of nodulation also in the positive control of this site, the absence of infective Frankia is not conclusive. Negative controls had no nodules and all other positive controls were nodulated. Plants inoculated with mallín and neighboring steppe soils had similar shoot dry weight (F22 = 1.081, p = 0.310), shoot height (F22 = 0.253, p = 0.620), root length (F22 = 0.001, p = 0.980) and root/shoot (dry mass) (F22 = 0.003, p = 0.956). Plants inoculated with mallín soils had a lower root dry weight than those inoculated with neighboring steppe soils (F22 = 4.758, p = 0.040). Although not significant, the mean number of nodules per plant was slightly higher in plants inoculated with mallín soils (F22 = 1.885, p = 0.184). The plants inoculated with soils (from mallín and neighboring steppe) had significantly higher shoot dry mass (U = 20.000, p = 0.000) and lower root/shoot (dry mass) than the non-inoculated plants (U = 21.000, p = 0.000), which suggested the symbiosis was effective and aided plant growth and development (Supplementary Material S1). Number of nodules per plant inoculated with mallín soil were positively correlated with soil pH (ρ12 = 0.628, p = 0.029) but negatively correlated with soil water content (ρ12 = −0.666, p = 0.018). The number of nodules per plant inoculated with steppe soil, was positively correlated with soil water content (ρ12 = 0.637, p = 0.026). No correlation between the number of nodules per plant and the soils’ organic matter or the soils’ conductivity was found (Fig. 2).
Relationship between the properties of soils (a, pH, b, water content, c, organic matter, d, electrical conductivity) from mallines (Northwest Patagonian wetland meadows) and neighboring steppe sites and the nodulation of Ochetophila trinervis seedlings, 9 weeks after soil inoculation treatment (Experiment 1)
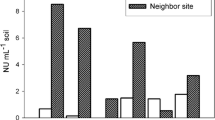
More specific enumeration of the nodulation capacity performed in eight representative soils (Experiment 2) (see Table 2), selected according to the number of nodules per plant produced in the first bioassay, and to the presence or not of different actinorhizal plants in the sites (Table 1) (Supplementary Material S1), showed that Frankia NU in mallín soils was higher than those in steppe soils (U = 0.000, p = 0.029). Frankia NU were positively correlated with soil water content (ρ8 = 0.778, p = 0.023), and soil organic matter (ρ8 = 0.755, p = 0.031) (Fig. 3). The Frankia NU estimated through MPN and NC were positively correlated (ρ8 = 0.934, p = 0.001). Plants inoculated with the first dilution series of soils (from mallín and neighbour steppe) had a higher plant dry mass (U = 119.000, p = 0.001) and lower root shoot ratio (length) (U = 20.000, p = 0.001) than the non-inoculated plants (Table 2).
Relationship between the properties of soils (a, organic matter, b, water content) from mallines (Northwest Patagonian wetland meadows) and neighboring steppe sites and the nodulation capacity of Ochetophila trinervis-infective Frankia (Experiment 2). NU data were log10 transformed to distinguish NU values in the range 0.1 to 28 (see Table 2)
Examined plants from both experiments had vesicles and had no sporangia inside nodules.
4 Discussion and conclusions
We found that most soils from mallín and from neighboring steppe nodulated O. trinervis plants and that mallín soils, including those devoid of actinorhizal plants, had higher Frankia NU than those from the steppe. It is rather common that soils near actinorhizal hosts have greater nodulation capacity than surrounding soils (Zimpfer et al. 1999; Jeong and Myrold 2001), including soils in primary successional volcanic sites (Seeds and Bishop 2009), although Frankia also occurs in sites lacking hosts (Zitzer et al. 1996; Gtari et al. 2007), and may be abundant in the rhizosphere of some non-actinorhizal plants like Betula (Smolander 1990), Rubus (Markham and Chanway 1996), or Alphitonia (Gauthier et al. 2000). But, what factors would support a higher abundance of Frankia NU in Northwest Patagonian mallín soils, including those devoid of host plants, than in neighbour steppe?
We suggest that the higher Frankia abundance in mallines than in neighbour steppe is a consequence of Frankia dispersal and saprophytic growth in the sites. Patagonian wetlands are located in valley bottoms in the steppe. The strong winds and the precipitation flows might contribute to dispersal of soil microorganisms and accumulation in those places as it was proposed for mycorrhizal propagules (Fontenla 2000). Frankia might be transported in this way by both mechanisms (water and wind). An indication that wind could transport Frankia in the environment was the presence of “Elaeagnus-infective” Frankia on particulates removed from air filters of a greenhouse that had been in use for 12 months. It was suggested that since it is a subsurface soil microorganism, its dispersal could happen via rafting on soil particles or by mechanical disturbance to soil profile resulting in propagules liberation (Paschke 1993). It was shown that water may be a dispersal agent for short distances from the bulk soil to the rhizoplane (Paschke and Dawson 1993), or for long distances in riparian environments (Zitzer and Dawson 1992; Huss-Danell et al. 1997). Water movement may account the horizontal spread of Frankia in peat, outside irrigated plots where nodulated alders were introduced (Arveby and Huss Danell 1988). Occasional flooding from some nearby river or stream could account nodulation in Casuarina (Bond 1976). The assumption of water dispersal in the northwest Patagonian region is supported by the occurrence of infective Frankia in lake sediments at long distances from the shores (Chaia et al. 2005).
The different soil properties measured that correlated with higher nodulation capacity in mallín soils (i.e. soil water) may stimulate the saprophytic growth of Frankia (Mirza et al. 2007, 2009). Therefore some soil factors would favour Frankia growth in soils. Water content affected soil nodulation capacity (Van Dijk 1984; Righetti et al. 1986; Dawson et al. 1989; McCray Batzli et al. 2004). Frankia NU decreased when soil samples were air-dried, which suggested that soil moisture is a relevant factor that affect the nodulation capacity of Patagonian soils (Chaia et al. 2007). Interestingly, two opposite trends seemed to occur in Patagonian soils considering soil water contents, where a range of soil water values favoured the nodulation capacity (neighbour steppe) but values below and above that range contributed to decreasing nodule numbers per plant (see Fig. 2). A similar situation was previously found for O. trinervis (Chaia et al. 2006) and for other rhamnaceous species occurring in a dry environment such as the Californian chaparral (Pratt et al. 1997) where increasing soil moisture favoured nodulation capacity, but if this increments were up to flooding levels, again nodulation capacity decreased. This trend was also found for Myrica and Alnus infective Frankia in sand dune successional communities, with soil moisture levels up to 50% (McCray Batzli et al. 2004). Soil nodulation capacity for Frankia strains infective in Alnus spp. declined according the decrease of soil water content, supporting the hypothesis that soil populations of these strains were dependent on water saturation of the soil (Wolters 1998; Van Dijk 1984).
Soil pH is other of the soil properties that was found to affect Frankia infective populations in soils. Generally positive correlations with nodulation capacity of soils have been found for pH in a range of acidic values (Smolander and Sundman 1987; Myrold and Huss-Danell 1994; Martin et al. 2003). We found a strong positive correlation between the number of nodules per plant inoculated with mallín soils and pH, that was in a range from acidic to slightly alkaline (5.1–7.9), but no correlation was found between pH and the number of nodules per plant inoculated with steppe soils, or with the NU of selected soils from mallín and steppe, which had a smaller range (6.0–7.6) and near neutral pH values.
The positive association between NU and organic matter (Fig. 3) might be related to an enhancement of saprophytic growth (Burleigh and Dawson 1994). Organic matter, from leaf litter that was amended to soils lacking hosts and inoculated with Frankia strains, favoured Frankia infectivity again suggesting saprophytic growth (Nickel et al. 2001). Regarding soils populations in both kinds of environments, mallín and steppe, it would be interesting in further research to investigate if they belong to the same group of strains considering that Frankia populations in soils may be determined by soil environment. Nodular Frankia strains of Myrica gale genetically characterized by PCR-RFLP of the 16S–23S intergenic spacer were found to occur in two groups according to soil pH and organic matter characteristics of the sites. One group was associated with peaty acidic and organic soils and the other group was associated with flooded soils, neutral pH values and moderate level of carbon (Huguet et al. 2004). It is possible that there is one main factor driving Frankia infectivity in soil like was suggested by Myrold and Huss-Danell (1994) who found a negative correlation between total soil C and Frankia NU and proposed that C rather than pH, was regulating the size of the infective Frankia populations.
In this study comprising soils with different moisture levels up to flooding (Table 1), nodules lacking vesicles, presumptively ineffective, were not found. Different explanations may be suggested for this result. Perhaps these ineffective strains simply do not exist in the studied soils. But if present, these strains might be not compatible with O. trinervis or the host plant could have developed resistance towards infection (van Dijk and Sluimer-Stolk 1990), another possibility could be that these strains are infective and might co-exist in nodules with effective ones, as found in Ceanothus caeruleus (Rhamnaceae) nodules (Ramirez-Saad et al. 1988), and therefore were not detected.
Our results, indicating a higher abundance of Frankia propagules in mallín soils than in neighboring steppe, supported the hypothesis that northwest Patagonian wetlands are reservoirs of Ochetophila trinervis infective Frankia.
These results provide a new argument for conservation of Patagonian wetlands. The finding of a higher Frankia abundance in the wetlands open new insights in favor of the protection of this kind of environments as sources of Frankia inocula, for further use in land reclamation with native actinorhizal plants in eroded areas in the region.
Abbreviations
- MPN:
-
Most Probable Number Method
- NC:
-
Nodulation Capacity Method
- NU:
-
Nodulation units per cm3 of soil
References
Arveby AS, Huss Danell K (1988) Presence and dispersal of infective Frankia in peat and meadow soils in Sweeden. Biol Fertil Soils 6:39–44
Blakemore LC, Searle PL, Daly BK (1987) Soluble salts, part 9. In: Department of Scientific and Industrial Research (eds) Methods for chemical analysis of soils, NZ Soil Bureau Scientific Report 80, Lower Hunt, New Zealand, pp 77–82
Boelcke O, Moore DM, Roig FA (1985) (eds) Transecta Botánica de la Patagonia Austral. Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina, Instituto de la Patagonia, Chile, Royal Society, Gran Bretaña, pp 733
Bond G (1976) The results of the IBP survey of root- nodule formation in non-leguminous angiosperms. In: Nutman PS (ed) Symbiotic nitrogen fixation in plants. Cambridge University Press, Cambridge, pp 443–474
Burleigh SH, Dawson JO (1994) Occurrence of Myrica-nodulating Frankia in Hawaiian volcanic soils. Plant Soil 164:283–289
Chaia E (1998) Isolation of an effective strain of Frankia from nodules of Discaria trinervis (Rhamnaceae). Plant Soil 205:99–102
Chaia E, Ribeiro Guevara S, Rizzo A, Arriberé M (2005) Occurrence of Discaria trinervis nodulating Frankia in dated sediments of glacial Andean lakes. Symbiosis 39:67–75
Chaia EE, Fontenla S, Vobis G, Wall LG (2006) Infectivity of soilborne Frankia and mycorrhizae in Discaria trinervis along a vegetation gradient in Patagonian soil. J Basic Microbiol 46:263–274
Chaia EE, Solans M, Vobis G, Wall LG (2007) Infectivity variation of Discaria trinervis-nodulating Frankia in Patagonian soil according to season and storage conditions. Physiol Plant 130:357–363
Dawson JO, Kowalski DG, Dart PJ (1989) Variation with soil depth, topographic position and host species in the capacity of soils from an Australian locale to nodulate Casuarina and Allocasuarina seedlings. Plant Soil 118:1–11
del Valle H (1998) Patagonian soils: a regional synthesis. Ecología Austral 8:103–123
Everitt B (1980) Cluster analysis, 2nd edn. Heinemann Educational Books, London, p 136
Fontenla SB (2000) Repercusión de las micorrizas en Patagonia, entre plantas hospedadoras y no hospedadoras. Doctoral Thesis, Universidad Nacional del Comahue. Argentina
Gauthier D, Jaffre T, Prin Y (2000) Abundance of Frankia from Gymnostoma spp. in the rhizosphere of Alphitonia neocaledonica, a non-nodulated Rhamnaceae endemic to New Caledonia. Eur J Soil Biol 36:169–175
Gtari M, Daffonchio D, Boudabous A (2007) Occurrence and diversity of Frankia in tunisian soil. Physiol Plant 130:315–470
Hahn D (2008) Polyphasic taxonomy of the genus Frankia. In: Pawloski K, Newton WE (eds) Nitrogen-fixing actinorhizal symbioses. Springer, The Netherlands, pp 25–48
Huguet V, Mergeay M, Cervantes E, Fernandez MP (2004) Diversity of Frankia strains associated to Myrica gale in Western Europe: impact of host plant (Myrica vs. Alnus) and of edaphic factors. Environ Microbiol 6:1032–1041
Huss-Danell K (1978) Nitrogenase activity measurements in intact plants of Alnus incana. Physiol Plant 43:372–376
Huss-Danell K, Uliassi D, Renberg I (1997) River and lake sediments as sources of infective Frankia (Alnus). Plant Soil 197:35–39
Jeong SC, Myrold DD (2001) Population size and diversity of Frankia in soils of Ceanothus velutinus and Douglas-fir stands. Soil Biol Biochem 33:931–941
Maltby E (2009) The changing wetland paradigm. In: Maltby E, Barker T (eds) Wetlands handbook. Blackwell Publishing Ltd. http://www3.interscience.wiley.com/cgibin/bookhome/122580025?CRETRY=1&SRETRY=0. Accessed 7 July 2010
Markgraf V (1981) Modern pollen dispersal in Argentina. Palynology 5:43–63
Markgraf V (1983) Late and post-glacial vegetational and paleoclimatic changes in subantartic, temperate and arid environments in Argentina. Palynology 7:43–70
Markgraf V (1984) Late Pleistocene and Holocene vegetation history of temperate Argentina: Lago Morenito, Bariloche. Dissertations Bot 72:235–254
Markham JH, Chanway CP (1996) Alnus rubra nodulation capacity of soil under five species from harvested forest sites in coastal British Columbia 178:283–286
Martin KJ, Posavatz NJ, Myrold DD (2003) Nodulation potential of red alder stands covering a wide age range. Plant Soil 254:187–192
McCray Batzli J, Zimpfer JF, Huguet V, Smyth CA, Fernandez M, Dawson JO (2004) Distribution and abundance of infective, soilborne Frankia and host symbionts Shepherdia, Alnus and Myrica in a sand dune ecosystem. Can J Bot 82:700–709
Mirza BS, Welsh A, Hahn D (2007) Saprophytic growth of inoculated Frankia sp in soil microcosms. Microbiol Ecol 62:280–289
Mirza BS, Welsh A, Hahn D (2009) Growth of Frankia strains in leaf litter-amended soil and the rhizosphere of a nonactinorhizal plant. FEMS Microbiol Ecol 70:132–141
Montgomery D (2005) Diseño y Análisis de Experimentos, 2da edición. Limusa Wiley, México, p 165
Myrold DD, Huss-Danell K (1994) Population dynamics of Alnus-infective Frankia in a forest soil with and without host trees. Soil Biol Biochem 26:533–540
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Bigham JM (ed) Methods of soil analysis, Part 3, Chemical methods, Book Series 5. ASA and SSSA, Madison, pp 961–1010
Nickel A, Pelz O, Hahn D, Saurer M, Siegwolf R, Zeyer J (2001) Effect of inoculation and leaf litter amendment on establishment of nodule-forming Frankia populations in soil. Appl Environ Microbiol 67:2603–2609
Paruelo JM, Beltrán A, Jobbágy E, Sala OE, Golluscio RA (1998) The climate of Patagonia: general patterns and controls on biotic processes. Ecología Austral 8:85–101
Paschke MW (1993) Distribution and dispersal of Frankia. PhD Thesis. University of Illinois at Urbana-Champaign
Paschke MW, Dawson JO (1993) Avian dispersal of Frankia. Can J Bot 71:1128–1131
Perotti MG, Diéguez MC, Jara FG (2005) Estado del conocimiento de humedales del norte patagónico (Argentina): Aspectos relevantes e importancia para la conservación de la biodiversidad regional. Rev Chil Hist Nat 78:723–737
Pratt SD, Konopka AS, Murry MA, Ewers FW, Davis SD (1997) Influence of soil moisture on the nodulation of post-fire seedlings of Ceanothus spp. growing in the Santa Monica mountains of Southern California. Physiol Plant 99:673–679
Raffaele E (1996) Relationship between seed and spore banks and vegetation of a mountain flood meadow (mallín) in Patagonia, Argentina. Wetlands 16:1–9
Raffaele E (1999) Mallines: Aspectos generales y problemas particulares. In: Málvarez AI (ed) Tópicos sobre Humedales Templados y Tropicales de Sudamérica. UNESCO, Montevideo, pp 27–33
Ramirez-Saad HC, Janse JD, Akkermans ADL (1988) Root nodules of Ceanothus caeruleus contain both the N2-fixing Frankia endophyte and a phylogenetically related Nod-/Fix- actinomycete. Can J Microbiol 44:140–148
Righetti TL, Chard CH, Backkhaus RA (1986) Soil and environmental factors related to nodulation in Cowania and Purshia. Plant Soil 91:147–160
Seeds JD, Bishop JG (2009) Low Frankia inoculation potentials in primary successional sites at Mount St. Helens, Washington, USA. Plant Soil 323:225–233
Smolander A (1990) Frankia populations in soils under different tree species with special emphasis on soils under Betula pendula. Plant Soil 121:1–10
Smolander A, Sundman V (1987) Frankia in acid soils devoid of actinorhizal plants. Physiol Plant 70:297–303
Van Dijk C (1984) Ecological aspects of spore formation in the Frankia-Alnus symbiosis. PhD Thesis. Leiden State University, The Netherlands
Van Dijk C, Sluimer-Stolk A (1990) An ineffective strain type of Frankia in the soil of natural stands of Alnus glutinosa (L.) Gaertner. Plant Soil 127:107–121
Van Dijk C, Sluimer A, Weber A (1988) Host range differentiation of spore-positive and spore-negative strain types of Frankia in stands of Alnus glutinosa and Alnus incana in Finland. Physiol Plant 72:349–358
Vásquez ME (2005) Acidez del suelo. En: Tecnologías en Análisis de suelos: Alcance a Laboratorios Agropecuarios, Marbán L, Ratto SE (eds) Primera Edición. Asociación Argentina de la Ciencia del Suelo, Buenos Aires, pp 120–126
Wolters DJ (1998) Ineffective Frankia in wet alder Soils. PhD Thesis. Wageningen Agricultural University in Wageningen, The Netherlands
Wolters DJ, Akkermans ADL, Van Dijk C (1997) Ineffective Frankia strains in the wet stands of Alnus glutinosa L. Gaertn. in The Netherlands. Soil Biol Biochem 29:1707–1712
Woomer PL (1994) Most probable number counts. In: Weaver RW, Angle JS, Bottomley PS (eds) Methods of soil analysis, part 2. Microbiological and biochemical properties. Book series 5. ASA, SSSA, Wisconsin, pp 59–79
Zimpfer JF, Kennedy GJ, Smyth CA, Hamelin J, Navarro E, Dawson JO (1999) Localization of Casuarina-infective Frankia near Casuarina cunninghamiana trees in Jamaica. Can J Bot 77:1248–1256
Zitzer SF, Dawson JO (1992) Soil properties and actinorhizal vegetation influence nodulation of Alnus glutinosa and Elaeagnus angustifolia by Frankia. Plant Soil 140:197–204
Zitzer SF, Archer SR, Boutton TW (1996) Spatial variability in the potential for symbiotic N2 fixation by woody plants in a subtropical savanna ecosystem. J Appl Ecol 33:1125–1136
Acknowledgements
We thank G Bernardi for calculations for the MPN method and to two anonymous referees that contributed to improve the quality of the paper. This work was funded by Universidad Nacional del Comahue, Agencia Nacional de Promoción Científica y Tecnológica, and CONICET Proyecto de Investigación Plurianual 5066.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Mean growth (and standard deviation) of O. trinervis seedlings inoculated with moist soils from 12 mallines (M) and 12 neighboring steppe (S) sites in Northwest Patagonia, and of non-inoculated controls (Experiment 1). Measurements were made 9 weeks after inoculation treatment (n = 6). DW, dry weight. (DOC 71 kb)
Rights and permissions
About this article
Cite this article
Cardoso, B.M., Chaia, E.E. & Raffaele, E. Are Northwestern Patagonian “mallín” wetland meadows reservoirs of Ochetophila trinervis infective Frankia? . Symbiosis 52, 11–19 (2010). https://doi.org/10.1007/s13199-010-0095-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-010-0095-x