Abstract
This study aimed to evaluate the impact of combining edible gelatin coatings with lactic acid bacteria, and bifidobacteria on the quality, shelf-life, and sensory attributes of processed cooked chicken breast during 45 days of cold storage. Physicochemical analyses, survival rate of microorganisms, microbiological quality, sensory features, tissue properties, and weight loss were evaluated. These samples maintained acceptable sensory attributes up to day 45, while control samples exhibited significant quality degradation by day 30. Key quality indicators monitored over the 45-day storage period at 4 °C showed no significant changes in water activity (p > 0.05), and although pH levels decreased, this change was not statistically significant (p > 0.05). Additionally, lipid oxidation was reduced (p < 0.05), and weight loss was minimized in the coated samples compared to the control. The viable populations of bifidobacteria and lactic acid bacteria demon-strated good survival rates after 45 days, with no significant differences in pH and water activity values be-tween treated and untreated samples (p > 0.05). The type of bacteria used in the coating did not significantly affect its performance in reducing oxidation, nor did the coating affect the crispiness of the samples.
Research Highlights
-
Gelatin coating with probiotics extends chicken breast shelf life by 45 days.
-
The quality and stability of coated chicken breast improved due to reduced oxidation.
-
Gelatin coating prevents spoilage, maintains quality and weight of meat products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chicken meat is highly perishable and provides a favorable environment for the growth of spoilage and pathogenic microorganisms. These microorganisms can cause changes in the taste, texture, and nutritional value of the meat, as well as serious health risks for consumers. Oxidative processes can also lead to meat spoilage, affecting lipids, pigments, proteins, and vitamins. This can result in the loss of essential fatty acids and vitamins, changes in color and texture, and the development of rancid odor and taste, all of which affect consumer acceptance (Pavelková et al. 2014). The main strategy used by the meat industry to control microbial spoilage and lipid oxidation is the addition of antioxidants and antimicrobials to meat and meat products. However, consumers nowadays demand more natural products, which limits the industry in the use of these compounds in food. For these reasons, in recent years, many researchers have attempted to preserve meat products by different methods, among which the use of films and coatings made from natural products seems very promising (Lashkari et al. 2020).
Edible coatings defined as a layer of polymeric materials that adhere to the surface of food and can be formulated from edible biopolymers, mainly carbohydrates. They are non-polluting products because they are composed of natural products that are degradable in the environment. The choice of the type of coating material is an important step for the proper implementation of the coating, which is done according to the type of food and the properties of the different coatings (Karwowska et al. 2021). Edible gelatin coating is considered for the preservation of meat products. The gelatin matrix acts as a barrier against water and oxygen, thus slowing down water loss and oxidation of myoglobin and lipids, and extending shelf-life. Gelatin is formed when collagen is exposed to mild heat under acidic or alkaline conditions. Gelatin contains large amounts of proline, hydroxyproline, lysine and hydroxylysine, which causes cross-molecular bonds between them. The development of edible films for preserving meat products is driven by consumer demand for high-quality products, and environmental concerns regarding the non-recovery of packaging materials (Gómez-Estaca et al. 2007).
The application of edible films in meat products favors moisture retention, reduces the rate of spoilage reactions and the contamination and growth of microorganisms, and can even serve as a vehicle for the release of active compounds that incorporate additional preservative characteristics. Furthermore, antimicrobial compounds in edible coatings are released in a controlled manner, allowing the concentration required to inhibit bacterial growth to be minimized (Garavito et al. 2020). Biopreservation is a technique for extending the shelf-life of foodstuffs which also involves the incorporation of viable microorganisms into edible coatings and films. Both lactic acid bacteria and bifidobacteria produce antimicrobial substances (acids and bactericides) that can help to protect foods, as well as having other health benefits, since many strains of these bacteria are also probiotics (Agriopoulou et al. 2020; Guimarães et al. 2018; Mehdizadeh et al. 2021; Pereira et al. 2016). Today, many strategies are being developed to limit the spoilage of meat products, with recent techniques including the use of antimicrobials or microorganisms in combination with edible films made from natural polymers. This study aligns with these advanced approaches by investigating the effect of an edible gelatin coating containing strains of lactic acid bacteria and bifidobacteria on the quality and stability characteristics of processed cooked chicken breast during cold storage. Our research contributes to the field by demonstrating how such coatings can effectively preserve meat quality and extend shelf-life, thus offering a promising solution to meat spoilage challenges.
Materials and methods
Chicken meat preparation
The cooked processed chicken breast was prepared by the Andre Meat Products Company (Andre, Iran). Figure 1 depicts the initial process of deboning fresh chicken by hand, separating the breast meat from the carcass, weighing it, and transporting it to a processing room. The chicken breast samples were then inserted into an injector (Filogrind, Model 360, GRONA-Spain), where a processing solution containing salt, nitrite, phosphate, stabilizer, starch, and spices was injected into the machine through needles. Subsequently, the injected chicken breast samples were placed in a cart and introduced into the Tumbler machine (Tumbling Ruhle, Germany) along with the specified solution. The Tumbler machine operated at approximately 3 °C under vacuum (75%) and with the device rotating at 25 rpm for 2.5 h. The samples were then moved to a cart and refrigerated overnight. Following this, they were transferred to the smoking chamber for 90 min of drying and 20 min of smoking, and then to the cooking chamber where they were cooked at a core temperature of 75 °C for 20 min, spending approximately 90 min in the cooking chamber at 80 °C. Subsequently, the samples were cooled to room temperature and placed under vacuum (Henkelman, Holland) for packaging.
Bacterial strains
Lactiplantibacillus plantarum 1058 (ATCC8014) and Bifidobacterium bifidum 1644 (DSM20456) strains were purchased in a lyophilized form from the Iranian Scientific and Industrial Research Organization (Iran, Tehran).
Preparation of microbial cells
The microorganisms were reactivated and pre-cultures of Lacp. plantarum 1058 and B. bifidum 1644 were prepared in De Man–Rogosa–Sharpe (MRS) broth medium (Merck, Germany) with 0.05 g /100 g L-cysteine hydrochloride (MMRS) and incubated until the exponential phase was reached under appropriate anaerobic conditions using Gazpak system at 28 °C and 37 °C, respectively. The grown cells were harvested by centrifugation at 1500×g for 15 min at 4 °C and resuspended in a sterile 0.9% (w/v) NaCl (Merck, Germany) solution for subsequent incorporation into the coatings.
Formulation of the coatings
The coating solutions, one for each strain, were prepared by dissolving 3 g (w/v) of gelatin powder (Sigma-Aldrich, United States) in 100 ml of distilled water and adding glycerol to the solution at a concentration of 25%, as a plasticizer. To obtain a suitable mixture, the solution was mixed at 45 °C for 10 min. The solution was then sterilized (by heating at 80 °C for 10 min) and cooled at room temperature. Afterward, the strains were added separately to the coating solutions to reach a final concentration of 108 CFU/ml (Ebrahimi et al. 2018).
Coating of cooked chicken breast
The samples were prepared under three conditions: 1; Control sample: cooked, processed chicken breast (without applying any coating), 2; Coating with Lacp. plantarum strain, 3; Coating with B. bifidum strain. Chicken breast samples from conditions 2 and 3 were immersed for 2 min in each of the previously described coating solutions. The excess coating solution in the treated samples was then drained for 30 s and then vacuum packed. Packages were stored at 4 °C for 45 days and at each sampling point (1, 15, 30 and 45) were analyzed for microbiological analysis, viability of the incorporated bacteria, physicochemical analysis, color, texture and sensory evaluation.
Microbiological analysis of cooked chicken breast
First, the outer layer of the cooked processed chicken breast pack was disinfected with 70% ethanol and then the coating was cut with a sterile knife. Microbial contamination was counted according to Iranian National Standard No. 5753. Samples of 10 g meat product were weighed aseptically on sampling days (0, 1, 15, 30, and 45 days) and homogenized with 90 mL of 0.1% sterile peptone water in Stomacher for 1 min at room temperature. For each sample, a sequential decimal dilution in 0.1% peptone solution was prepared and 1 mL or 0.1 mL of the samples at the appropriate dilution was prepared in three replicates and the following microbial groups were detected. Total mesophilic aerobic bacteria were counted on PCA (Merck, Germany) at 37 °C for 24 h (according to National Standard No. 5272). Coliforms were counted after incubation in Violet Red Blood Agar (Merck, Germany) at 37 °C for 24 h according to Iranian Standard No. 437. Salmonella spp. counts were performed according to Iranian Standard No. 1810. Staphylococcus aureus was counted using Baird-Parker Agar culture medium (Merck, Germany) according to Iranian Standard No. 8606-1. Molds and yeasts were counted according to Iranian Standard No. 997. Clostridium perfringens was counted according to Standard No. 2197. Escherichia coli was also counted according to Iranian Standard No. 2946 (Iran 2016).
Evaluation of the viability of microorganisms incorporated in coatings
The viability of microorganisms incorporated in the cooked chicken breast was evaluated during 45 days of storage. Lacp. plantarum was counted in MRS medium incubated under anaerobic conditions (AnaeroGen sachet) at 28 °C for 48 h; Bifidobacterium bifidum was counted in MRS supplemented with filter-sterilized 0.05% (w/v) L-cysteine·HCl incubated at 37 °C for 48 h under anaerobic conditions (Anaerogen sachet).
Physicochemical analyses
Chicken meat samples were analyzed for moisture according to Iranian National Standard No. 745, protein (INS No. 924), lipid (INS No. 742) and ash (INS No. 744). The pH was analyzed as described by Mancini et al. (2017). The oxidation (Thiobarbituric acid reactive substances) of the samples was analysed as described by Fazlara et al. 2017.
The water activity was measured using an Aw meter (Novasina AG, Switzerland) according to the manufacturer’s instructions. For weight loss assessment, one sample per coating condition was separated on the first day and weighed by the balance with an accuracy of 0.001 g during the storage period. The relative weight loss (ΔW) was calculated as follows:
Where w0 is the initial weight and w is the final weight at each sampling point (Ben Slima et al. 2017). Three readings of each sample were assessed. All these parameters were analyzed for 45 days of storage at 4 °C.
Color features
The color of the processed and cooked chicken breast was evaluated by Konica Minolta CR-400 (Japan) on the outer surface of each sample in triplicate at three randomly selected points. The colorimetry was performed at room temperature and reported as L*, a*, and b* parameters. L* indicates the brightness of the sample and its range varies from 0 (pure black) to 100 (pure white). Parameter a* indicates the redness of the sample and its range varies from − 60 (pure green) to + 60 (pure red). Parameter b* indicates the yellowness of the sample and its range varies from − 60 (pure blue) to + 60 (pure yellow). From the measured values, the total color difference between the control sample and each of the treatment samples (ΔE*) are calculated according to Eq. (1): (Mancini et al. 2017).
Textural features
The tissue characteristics of the treated and control meat products during 45 days of storage at 4° C were obtained by analysing the tissue properties in a tissue analyzer (Brookfield, CT3 Texture Analyzer, USA) equipped with a 25 kg load cell and a 25 mm diameter acrylic probe at room temperature. The sample was cut into 20-mm-long cylinders and compressed to approximately half its original height in two consecutive cycles at a constant speed of 1 mm/s with a waiting time of 5 s. From the force deformation curves, the parameters of the tissue profile were calculated: hardness (the maximum force required to cause deformation), cohesivity (strength of the internal bonds forming the body of the product), springiness (the amount of return of the sample to its original shape after compression), adhesiveness and chewiness (Mancini et al. 2017).
Sensory evaluation
Samples of treated and control chicken breasts were analysed from a sensory point of view using a hedonic test (liking or pleasure) on a 5-point scale according to the characteristics of tissue, taste, color, juiciness, and general acceptance of samples. Samples with a Kor three-digit code (using a random number table) were provided to the evaluators in a randomized complete block design (30 untrained evaluators aged 20–35 years, of both sexes equally). The only selection criterion for the group of evaluators was liking and consumption of this type of meat product. Samples were placed separately in disposable plastic dishes and given to the evaluators along with a special questionnaire. Warm samples (38 °C) were given to the evaluators, who were asked to use mineral water and unsalted crackers to rinse their mouths between each sample. To avoid the negative impact of external factors on the results of sensory evaluation, the evaluation panel was asked to abstain from eating, chewing gum, and smoking for at least one hour before the evaluation process. The sensory evaluation was performed after the microbial safety test of the samples, which confirmed that the samples could be consumed (Fernandes et al. 2016).
Statistical analysis
Data collection for physicochemical, microbial, and colorimetric tests was performed by sampling and experimentally using laboratory equipment. Sensory testing of cooked processed chicken breast samples was also performed in a consumer-oriented manner, using a questionnaire and subsequent statistical analysis. The results obtained in the experiments are expressed as the mean ± standard deviation (SD) of the three-replicate measurements. Colony-forming units (CFUs) are converted to their logarithmic values before statistical analysis. Experimental data were compared by one-way ANOVA. Statistical analysis of the results was performed with SPSS software version 27. A significance level of p ≤ 0.05 was considered for all data comparisons. For the statistical analysis of the sensory evaluation, the non-parametric Kruskal-Wallis method was used to compare treatments at a specific storage time and the non –parametric Friedman method to compare a treatment during storage time.
Results and discussion
Microbiological analysis of cooked chicken breast
The findings indicated that there was no growth of mesophilic aerobic bacteria, Coliforms, Salmonella spp., Staphylococcus aureus, Clostridium perfringens, and Molds and Yeasts on the media.
Survival of bacteria inoculated in the coating during storage
At the start of experimentation, both Lacp. plantarum and B. bifidum were present at a concentration of about 7.5 log cfu/g (Table 1). During storage, the survival rate of bacteria in the coated chicken breast slightly decreased, reaching a value of about 6.0 log cfu/g in both samples after 45 days of storage (p < 0.001). The high survival of bacteria used is relevant since they have the potential to be used as biopreservation, due to the ability to produce antimicrobial substances as lactic acid or for the competition of nutrients. These results are quite consistent with those of another study in which the survival of Lactobacillus sacchi incorporated into sodium caseinate films was almost unchanged when stored at 4 and 25 °C for 30 days (Gialamas et al. 2010). Pereira et al. 2018; also found that the survival of B. animalis Bb-12 and L. casei 01 was high in the coating of sliced ham with whey protein isolate (WPI) stored at 4 °C. One of the most recent approaches to improve the survival of functional bacteria is their immobilization in edible films (Fernandes et al. 2016).
Results of aw, pH, TBA (Thiobarbituric acid reactive substances) and weight loss of samples during storage and proximate composition of chicken breast samples
The proximate composition of chicken breast was reported in Table 2.
The pH, water activity and lipid oxidation in coated and uncoated samples during 45 days of refrigeration were investigated (Fig. 2). For water activity (Fig. 2A), the results showed that there were no significant differences (p > 0.05) in water activity in all treatments. The results of pH analysis (Fig. 2B) showed that in all samples the pH slightly decreased during storage (p < 0.001). After 45 days, no difference in pH values was detected between uncoated and coated chicken breast, as also highlighted by Pereira et al. 2018. On the contrary, Pavli et al. (2020) showed that the use of edible films affected the pH value and a rapid decrease in pH value was observed at all temperatures.
During storage, lipid oxidation (Fig. 2C) was initially stable in treatments with gelatin coatings containing Bifidobacterium bifidum and Lactobacillus plantarum. However, lipid oxidation during storage subsequently decreased, particularly in samples treated with the gelatin and bacteria coatings. This pattern may be attributed to the initial oxidative reactions followed by the stabilization of the antioxidative properties of the gelatin coatings. This coating was effective up to the 30th day of storage (p < 0.001). In general, samples with gelatin and bacteria coating performed better than the control. The type of bacteria used did not affect the performance of the coating in reducing oxidation. The susceptibility of meat to fat oxidation and increased TBA depends on various factors, such as the species of animal, type of muscle, shelf-life, methods of packing and addition of antioxidants. Although free radicals are known to aggravate fat oxidation in meat, the amount of fat and fatty acid composition are also important for the oxidation of meat fat during storage (Hassanzadeh et al. 2012). The study of Taghizadeh and Rezaei (2012) showed that initial products of lipid oxidation (peroxide) increased significantly over time until day 10, indicating fat oxidation, while from day 10 onwards the rate decreased, which can be due to the decomposition of hydroperoxides into oxidation by-products. The peroxide content of the coated fillets was significantly lower than that of the uncoated fillets only at day 15, probably due to gelatin coating preventing oxygen from coming into contact with the product surface. In general, weight loss (Fig. 2D) occurred in all samples during shelf-life. However, this weight loss was less in coated samples, which shows a positive effect of the coating in preventing product weight loss. In general, the lowest weight loss occurred in treatments 1 and 2 at day 1 (9.74 ± 0.64) and the highest rate occurred in the control at day 45 (29.1 ± 0.5). It was shown in other studies that during the storage of ham with edible whey protein coating, the coating prevented weight loss (Pereira et al. 2018).
Results of sensory evaluation
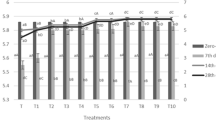
The results of sensory evaluation are shown in Fig. 3. The overall acceptance of samples with edible gelatin coating containing B. bifidum decreased during storage. The lowest acceptance rate in treatment 2 and control (2.5 ± 1.106) was at day 15 and the highest rate was in control at day 45 (3.9 ± 0.845), with no significant differences (p > 0.05) between them at day 45. The hydration of product decreased during the storage period in treatment 1. But in control sample and treatment 2, there is an increase, so the highest rate was in control at day 30 (3.87 ± 0.973). The crispiness of the treatment samples increased during storage. But in treatment 2, the crispiness has decreased slightly. Crispiness has also increased in control samples. It can be concluded that the coating did not have a significant effect on crispiness of the samples. In general, the lowest crispiness was in control and treatment 2 at day 15 (2.3 ± 1.081) and the highest in control was at day 45 (3.87 ± 0.571). In terms of texture, the control sample increased during storage. In case of treatment 1, the highest amount was reported at day 30. Tissue specificity increased in treatment 2 during storage. The lowest rate was in treatment 2 and control on day 15 (3.07 ± 1.202) and the highest rate was in treatment 1 on day 30 (3.87 ± 1.008). In terms of product taste, the results show that in all samples, the taste has improved with increasing shelf life by 30 days. The lowest rate was in treatment 2 and control at day 15 (2.6 ± 1.221) and the highest rate was in the control at day 30 (3.93 ± 1.172). Bulut and Candoğan (2022) developed 3D printed functional chicken meat-based snack to optimization of process parameters and gelatin level. The results showed the sample with 1.79% gelatin addition received significantly higher scores for all attributes (P < 0.05), with ratings ranging from 8.0 to 8.2 (indicating a preference of “like very much”). The improved scores for the gelatin-added samples can be attributed to factors such as gelatinization, enhanced printability, and increased viscosity.
Regarding the appearance of treatments, it was reported that in treatment 2, appearance improved during the maintenance period. The least improvement in appearance was in treatment 1 at day 45 (2.47 ± 0.819) and the highest improvement was in control at day 30 (3.7 ± 0.988). In treatment 2, quality of appearance was better than other treatments. It can be said that the difference in sensory characteristics at days 1 and 15 of test was not significant. A study by Brannan reported that the use of grape seed extract (GSE) in chicken breast alone does not cause any change in sensory score or color of samples, but rather reduces unpleasant taste and odor in chicken, which is not consistent with the present study in terms of taste changes (Brannan 2009). In a research conducted by Kakaei and Shahbazi, the effect of chitosan-gelatin film containing ethanolic extract of grape seed (in amounts of 1 and 2%) and essential oil of Ziziphora clinopodioides (in amounts of 1 and 2%) separately and in combination increased the shelf life of minced fish fillets at refrigeration temperature (4 °C) over 11 days was examined. Samples with chitosan-gelatin film containing 2% Ziziphora clinopodioides essential oil and 1% ethanolic extract of grape seed as well as 2% essential oil of Ziziphora clinopodioides and 2% ethanolic extract of grape seed showed the best sensory properties (Kakaei and Shahbazi 2016). In the study of Pavli et al. a slight difference was observed between the control and probiotic cases, mainly in the properties of redness, odor and acidic taste. The acidic taste was slightly more pronounced in samples with microorganisms, but was not considered unpleasant. Features specified are significantly affected by temperature (Pavli et al. 2020). Taghizadeh and Rezaei 2012 also stated in a study that both treatments decreased in quality during the maintenance period. At the end of the storage period, uncoated fillets were not significantly different from gelatin-coated fillets. Also, texture, odor, color and general acceptance of control samples as well as gelatin-coated fillets at day 10 were unacceptable (Brannan 2009).
Results of textural properties
The results of the texture property analysis (Fig. 4) showed that the hardness of the products increased during shelf-life. No significant difference (p > 0.05) was found between the treatments at days 1, 15 and 30. After 45 days, the lowest hardness value was related to the sample coated with Treatment 2. Khoshnoudi-Nia and Sedaghat (2019) conducted a study to assess how active edible coating and temperature impact the quality of roasted pistachio nuts during storage. The results indicated that the gelatin-coated samples had significantly higher instrumental hardness compared to the other samples at both 20 and 35 °C. Additionally, the instrumental hardness of the control samples increased over time, whereas the gelatin-coated samples showed a decreasing trend. In the study of Rahnemoon et al., chicken samples stored in alginate coating had a significantly higher hardness value than control samples (Rahnemoon et al. 2018). The hardness of meat texture increased as the amount of water in the meat decreased. Mbaga et al. showed that the texture of chicken meat softened after 10 days of storage at 4 °C (Mbaga et al. 2014). The highest adhesion values were recorded at 1 day of storage for coated samples. Thereafter, the adhesion rate decreased. On the contrary, some studies found that adhesion increased in all samples during storage (Rahnemoon et al. 2018). This could depend on the adhesive and glazing properties of the different foods. Cohesiveness was approximately the same during the shelf-life of all samples. Chewiness was significantly different between coated and uncoated (control) samples after 30 days of storage. According to the results, springiness was almost the same during shelf-life of all samples.
Results of texture properties of chicken breast samples during storage at 4 °C. A-H shows the textural properties of processed chicken breast (coated and uncoated) for their hardness at cycle 1, hardness work at cycle 1, adhesivess, hardness cycle 2, hardness work cycle 2, cohesivess, springiness and chewiness respectively
Results of color characteristics of samples
Color indicators were examined in this study (Fig. 5). Color characteristics are an important parameter that affects the consumers’ assessment of product quality. The L-index remained more or less stable during storage. The control sample showed a slight reduction compared to the coated samples that showed no difference. The lowest L value was recorded in the control sample at day 30 (68.2 ± 0.1). The addition of bacterial cells to the films can affect the passage of light, probably due to increased light scattering (Kanmani and Lim 2013). This finding is similar to that reported by Soukoulis et al. (2014) that absorption of light by the films can be due to a reduction of undesirable chemical reactions such as lipid oxidation, which leads to a loss of nutritional value.
Concerning the a-index, at the beginning of the trial, the coated samples had the highest value compared to the control sample. During storage, the control sample showed an increase in the level of the a-index, while samples 1 and 2 showed the opposite trend. Martins et al. (2012) reported that the moisture content of the films may change the reflection of light on film surface (a-index values decrease), leading to a reduction in red color of the films. Regarding the b-index, the control sample initially had the highest value compared to the coated samples. During storage, the latter showed an increase in value, although it was still lower than the control sample. No significant difference (p > 0.05) was found between any of the treatments on any of the test days. In general, the lowest value of this index was observed in treatments 1 and 2 at day 1 (1.19 ± 0.1). The results of ΔE study, which indicates the difference in color between the control and each of the treatments, showed that in all samples this difference decreased during storage. Campaniello et al. (2020) evaluated the development of apple-based carriers for B. animalis subsp. lactis DSM 10,140. They showed that the probiotic remained viable during storage at 4 and 8 °C, but it led to an increase in browning index and color deterioration. However, applying a coating (alginate- or gelatin-based) mitigated this effect. They found that the most significant impact on color (largest increase in Δa) was observed in pieces coated with alginate and stored at 8 °C.
In another study (Pereira et al. 2018), it was reported that the presence of an edible film (whey concentrate) with Lactobacillus casei-01 or Bifidobacterium animalis Bb-12 had no significant effect on the color of chopped ham (*L, *a, *b and ΔE). Color and light transfer properties are very important in making edible films, as they directly affect the appearance and acceptance of packaged or coated food products. It should be noted that processing chicken breast products involve processing operations that result in other side streams such as feathers and bones that are often discarded. Most of the feathers end up in landfills without further use, and were associated with a risk of soil and water pollution (Prasanthi et al. 2016). By prolonging the shelf life of processed chicken breast will reduce the quantity of wasted feathers as well. The utilization of feathers will encourage the zero-waste concept and help to raise the economic aspects since it is possible to use feathers for the hydrolysis process in the production of products such as hydrolysate or aspartic acid for commercial gain (Solcova et al. 2021).
Concluding remarks
The study demonstrates that gelatin-based edible coatings containing Lactobacillus plantarum and Bifidobacterium bifidum are effective in preserving the quality and extending the shelf life of refrigerated chicken breast. The conclusions that can be drawn from the study are as follows:
-
No growth of mesophilic aerobic bacteria, coliforms, Salmonella spp., Staphylococcus aureus, Clostridium perfringens, and molds and yeasts was detected in the coated samples, indicating effective microbial control.
-
The incorporated probiotics (L. plantarum and B. bifidum) maintained high survival rates, decreasing only slightly from 7.5 log cfu/g to about 6.0 log cfu/g over 45 days, suggesting their potential use as biopreservatives.
-
Water activity remained stable with no significant differences among treatments. The pH values slightly decreased over the storage period but showed no significant difference between coated and uncoated samples after 45 days.
-
The gelatin coatings with bacteria significantly reduced lipid oxidation, particularly up to 30 days of storage, helping to preserve meat quality by stabilizing antioxidative properties.
-
Coated samples experienced less weight loss compared to the control, demonstrating the coating’s effectiveness in moisture retention during storage.
-
Sensory evaluation showed that overall acceptance of coated samples decreased slightly over time but was not significantly different from the control by the end of the storage period.
-
The hardness of the meat increased during storage for all samples, with the lowest increase observed in the samples coated with Treatment 2, indicating better texture preservation.
-
The L-index remained stable while the a-index and b-index values varied, suggesting the coatings influenced light scattering and helped maintain color stability of the meat.
The current study on the effectiveness of gelatin-based edible coatings containing Lactobacillus plantarum and Bifidobacterium bifidum in preserving the quality and extending the shelf life of refrigerated chicken breast presents several limitations. Firstly, the study’s duration was limited to 45 days, which may not provide a comprehensive understanding of the long-term effects and stability of the coatings. Future research should explore longer storage periods to better evaluate the coatings’ effectiveness over time. Additionally, while the study focused on specific probiotic strains, it did not investigate the potential benefits or differences that other probiotic strains might offer. This could limit the generalizability of the findings to other potentially beneficial strains. The study also did not explore other biopolymer materials that might provide enhanced or complementary protective properties compared to gelatin. Understanding the specific mechanisms by which the coatings reduce lipid oxidation and microbial growth was not deeply analyzed, which could aid in optimizing formulation and application methods. In future research, exploring other biopolymer materials for coatings might provide improved or complementary protective properties compared to gelatin. It is also important to research the specific mechanisms by which the coatings reduce lipid oxidation and microbial growth to optimize formulation and application methods. Evaluating the nutritional impact of the coatings, particularly their potential to provide additional health benefits such as enhanced probiotic intake, is crucial. Finally, performing cost-benefit analyses will determine the economic feasibility of using these coatings in commercial meat processing and distribution.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Agriopoulou S, Stamatelopoulou E, Sachadyn-Król M, Varzakas T (2020) Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: quality and safety aspects. Microorganisms 8:952–976. https://doi.org/10.3390/microorganisms8060952
Brannan RG (2009) Effect of grape seed extract on descriptive sensory analysis of ground chicken during refrigerated storage. Meat Sci 8:589–595. https://doi.org/10.1016/j.meatsci.2008.10.014
Bulut EG, Candoğan K (2022) Development and characterization of a 3D printed functional chicken meat based snack: optimization of process parameters and gelatin level. LWT 154:112768. https://doi.org/10.1016/j.lwt.2021.112768
Campaniello D, Bevilacqua A, Speranza B, Sinigaglia M, Corbo MR (2020) Alginate-and gelatin-coated apple pieces as carriers for Bifidobacterium animalis subsp. lactis DSM 10140. Front Microbiol 11:566596. https://doi.org/10.3389/fmicb.2020.566596
Ebrahimi B, Mohammadi R, Rouhi M, Mortazavian AM, Shojaee-Aliabadi S, Koushki MR (2018) Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT 87:54–60. https://doi.org/10.1016/j.lwt.2017.08.066
Fazlara A, Pourmahdi M, Zarei M, Karimi T (2017) Effect of edible chitosan-rosemary coating on quality and shelf life of refrigerated chicken fillets. Iran Vet J 13:78–90. https://doi.org/10.22055/ivj.2017.17675.1453
Fernandes RD, Trindade MA, Lorenzo JM, Munekata PE, De Melo MP (2016) Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control 63:65–67. https://doi.org/10.1016/j.foodcont.2015.11.027
Garavito J, Moncayo-Martínez D, Castellanos DA (2020) Evaluation of antimicrobial coatings on preservation and shelf life of fresh chicken breast fillets under cold storage. Foods 9:1203–1219. https://doi.org/10.3390/foods9091203
Gialamas H, Zinoviadou KG, Biliaderis CG, Koutsoumanis KP (2010) Development of a novel bioactive packaging based on the incorporation of Lactobacillus sakei into sodium-caseinate films for controlling Listeria monocytogenes in foods. Food Res Int 43:2402–2408. https://doi.org/10.1016/j.foodres.2010.09.020
Gómez-Estaca J, Montero P, Gimenez B, Gomez-Guillen M (2007) Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus). Food Chem 105:511–520. https://doi.org/10.1016/j.foodchem.2007.04.006
Guimarães A, Abrunhosa L, Pastrana LM, Cerqueira MA (2018) Edible Films and Coatings as Carriers of Living Microorganisms: a New Strategy towards Biopreservation and healthier. Foods Compr Rev Food Sci Food Saf 17:594–614. https://doi.org/10.1111/1541-4337.12345
Hassanzadeh P, Moradi M, Vaezi N, Moosavy MH, Mahmoudi R (2012) Application of chitosan edible coating containing grape seed extract on the quality and shelf life of refrigerated chicken meat. Food Res J 21:467–482
Iran National Standards Organization (2016) https://en.inso.gov.ir/portal/home. Accessed 5 April 2024
Kakaei S, Shahbazi Y (2016) Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT 72:432–438. https://doi.org/10.1016/j.lwt.2016.05.021
Kanmani P, Lim ST (2013) Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem 141:1041–1049. https://doi.org/10.1016/j.foodchem.2013.03.103
Karwowska M, Łaba S, Szczepański K (2021) Food loss and waste in meat sector—why the consumption stage generates the most losses? Sustainability 13:6227–6240
Khoshnoudi-Nia S, Sedaghat N (2019) Effect of active edible coating and temperature on quality properties of roasted pistachio nuts during storage. J Food Process Preserv 43:e14121
Lashkari H, Halabinejad M, Rafati A, Namdar A (2020) Shelf life extension of veal meat by edible coating incorporated with Zataria multiflora essential oil. J Food Qual 2020(8871857). https://doi.org/10.1155/2020/8871857
Mancini S, Paci G, Fratini F, Torracca B, Nuvoloni R, Dal Bosco A, Preziuso G (2017) Improving pork burgers quality using Zingiber officinale Roscoe powder (ginger). Meat Sci 129:161–168. https://doi.org/10.1016/j.meatsci.2017.03.004
Martins JT, Cerqueira MA, Bourbon AI, Pinheiro AC, Souza BWS, Vicente AA (2012) Synergistic effects between κ-carrageenan and Locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll 29:280–289. https://doi.org/10.1016/j.foodhyd.2012.03.004
Mbaga S, Sanka Y, Katule A, Mushi D (2014) Effects of storage time on the quality of local chicken meat. Tanzan J Agric Sci 13
Mehdizadeh T, Kaboudari A, Reale A (2021) Stimulatory effect of Allium ampeloprasum L. ssp. Iranicum Wendelbo on the probiotic Bifidobacterium bifidum in Iranian white cheese. J Dairy Sci 104:10550–10557. https://doi.org/10.3168/jds.2021-20371
Pavelková A, Kačániová M, Horská E, Rovná K, Hleba L, Petrová J (2014) The effect of vacuum packaging, EDTA, oregano and thyme oils on the microbiological quality of chicken’s breast. Anaerobe 29:128–133. https://doi.org/10.1016/j.anaerobe.2013.09.002
Pavli FG, Argyri AA, Chorianopoulos NG, Nychas GJE, Tassou CC (2020) Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 118:108810. https://doi.org/10.1016/j.lwt.2019.108810
Pereira JO, Soares J, Sousa S, Madureira AR, Gomes A, Pintado M (2016) Edible films as carrier for lactic acid bacteria. LWT 73:543–550. https://doi.org/10.1016/j.lwt.2016.06.060
Pereira JO, Soares J, Monteiro MJ, Gomes A, Pintado M (2018) Impact of whey protein coating incorporated with Bifidobacterium and Lactobacillus on sliced ham properties. Meat Sci 139:125–133. https://doi.org/10.1016/j.meatsci.2018.01.016
Prasanthi N, Bhargavi S, Machiraju P (2016) Chicken feather waste: a threat to the environment. Int J Innov Res Sci Eng Technol 5:16759–16764. https://doi.org/10.15680/IJIRSET.2016.0509188
Rahnemoon P, Sarabi Jamab M, Javanmard Dakheli M, Bostan A (2018) The effect of alginate coating containing pomegranate peel extract on shelf life, texture and color characteristics of chicken breast meat. Innov Food Technol 5:583–596. https://doi.org/10.22104/jift.2018.2552.1599
Slima SB, Ktari N, Trabelsi I, Triki M, Feki-Tounsi M, Moussa H, Makni I, Herrero A, Jiménez-Colmenero F, Perez CR, Salah RB (2017) Effect of partial replacement of nitrite with a novel probiotic Lactobacillus plantarum TN8 on color, physico-chemical, texture and microbiological properties of beef sausages. LWT 86:219–226. https://doi.org/10.1016/j.lwt.2017.07.058
Solcova O, Knapek J, Wimmerova L, Vavrova K, Kralik T, Rouskova M, Hanika J (2021) Environmental aspects and economic evaluation of new green hydrolysis method for waste feather processing. Clean Technol Environ Policy 23:1863–1872. https://doi.org/10.1007/s10098-021-02072-5
Soukoulis C, Yonekura L, Gan HH, Behboudi-Jobbehdar S, Parmenter C, Fisk I (2014) Probiotic edible films as a new strategy for developing functional bakery products: the case of pan bread. Food Hydrocoll 39:231–242. https://doi.org/10.1016/j.foodhyd.2014.01.023
Taghizadeh AG, Rezaei M (2012) Effect of gelatin coatings on chemical, microbial and sensory properties of refrigerated rainbow trout fillet (Oncorhynchus mykiss). J food Sci Technol (Iran) 9:67–76
Acknowledgements
The support of the Research Vice-Chancellor of Qazvin University of Medical Sciences (IR.QUMS.REC.1398.401) is wholeheartedly appreciated.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.M., and D.R.; methodology, A.M., and H.K.M.; software, S.S., and A.A.; validation, A.M., and R.M.; formal analysis, A.A.; investigation, A.M., and P.G.; resources, A.M. and R.M.; data curation, A.M. and H.K.M.; writing—original draft preparation, A.M., H.K.M.; writing—review and editing, A.M., S.S., P.G., R.M., and D.R.; supervision, R.M.; project administration, A.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehrabi, A., Morasa, H.K., Ghajarbeygi, P. et al. Effects of gelatin coating on the preservative and sensory qualities of cooked chicken breast. J Food Sci Technol (2024). https://doi.org/10.1007/s13197-024-06074-1
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13197-024-06074-1