Abstract
Adulteration of meat products is a serious problem in the modern society. Consumption of falsified meat products can be hazardous to health and/or lead to violating religious dietary principles. To identify such products, rapid and simple test systems for point-of-need detection are in demand along with complex laboratory methods. This study presents the first double lateral flow (immunochromatographic) test system, which allows simultaneous revealing two prevalent types of falsifications—undeclared addition of pork and chicken components to meat products. In the proposed test system, porcine myoglobin (MG) and chicken immunoglobulin Y (IgY) were used as specific biomarkers recognizable by antibodies. Within the optimization of the analysis, the concentrations of the immune reagents and regimes of their application on the working membrane were selected, which provided minimal limits of detection (LODs) for both analytes. The developed test system enables the detection of MG and IgY with the LODs of 10 and 12 ng/mL, respectively, which accords to addition of 0.1% of the undeclared meat compounds. The applicability of the test system to control the composition of raw meat mixtures and cooked food products was confirmed. The developed approach can be considered as a promising tool for monitoring composition of meat products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adulteration of meat product seems an often case in modern food production because unscrupulous manufacturers are used to replacing declared components of foodstuffs with cheaper ones (Momtaz et al. 2023). Consequences of such falsifications are not limited to misinforming consumers. Consumption of counterfeit products may have serious negative effects for health and violate religious dietary requirements (Bansal et al. 2017; Hossain et al. 2020). In the current analytical practice, the predominant methods for species identification in meat products are hybridization techniques with recognition and subsequent amplification of specific regions of nucleic acids (Azad et al. 2023). Although these methods are characterized by high selectivity and low limits of detection (LODs), their implementation involves use of complex and expensive equipment in special laboratories by highly qualified personnel. Recent developments of autonomous systems for polymerase chain reactions or isothermal amplifications are still far from wide application (Narasimhan et al. 2023). Therefore, test systems for rapid mass control of food composition, which can be used in out-of-laboratory conditions, are in great demand along the entire chain of food production—from the receipt of raw materials to meat products retailing.
Several lateral flow test strips have been reported as tools to detect proteins specific for different meat sources. Among the latter biomarkers, troponin I (Chen and Hsieh 2002; Zvereva et al. 2020a), porcine serum albumin (Masiri et al. 2016), and porcine immunoglobulins (Kuswandi et al. 2017; Hendrickson et al. 2021a; Banerjee et al. 2023) can be mentioned. However, a priority direction in the development of lateral flow immunoassay is the transformation of monoparametric testing to multiparametric one enabling simultaneous detection of several biomarkers using one test strip (Guan et al. 2022; Wu et al. 2020). Compared to monoparametric tests, the performance of multiple tests increases while the consumption of reagents and expendable materials for their manufacturing is significantly reduced. Their application in mass screening provides increased productivity and reduces cost of the testing per one analyte (Anfossi et al. 2019). Unlike immunochromatographic multiparametric tests created and used for various tasks, to date, there are no such tests developed to reveal undeclared additives in meat products. It should be noted that the development of multiparametric test strips is not an ordinary integration of several monoparametric ones. A combination of the immunoreagents having different specificities on one test strip modifies their interactions before reaching the binding zones. The integration of many immunoreagents on one test strip leads to other problems—choosing acceptable interaction conditions and ensuring similar binding levels for all of them. Simple combination of several reagents in one test strip without adapting the interaction conditions demonstrated the deterioration of analytical parameters (Bartosh et al. 2020). Besides, the best regimes cannot be complied with for all immune interactions simultaneously, which complicates the choice of new optimal conditions (Bartosh et al. 2020). Therefore, the development of multiparametric tests needs a special selection of interaction conditions, which are likely to differ from those for monoparametric testing.
Among possible ways of meat products counterfeiting, the most common ones include the use of chicken and pork raw meat as additives substituting the declared raw materials (Alikord et al. 2018). The additional importance of pork control is explained by the strong forbiddance of pork consumption in Islam and Judaism (Zia et al. 2020). As for chicken, relatively cheap production causes its frequent use for falsification of meat products composed from more expensive kinds of meat (Yang et al. 2022).
Therefore, the present study aims at the development and characterization of a double lateral flow test system for the detection of pork and chicken meat in food products using porcine myoglobin (MG) and chicken immunoglobulin Y (IgY) as specific biomarkers. The work includes obtaining reagents for immune detection of both analytes, selection of conditions for sensitive detection of both biomarkers, determination of analytical parameters for the developed double test, and validation of its applicability for the determination of pork and chicken meat in raw and cooked meat products.
Materials and methods
Reagents
MG and monoclonal antibodies (Mab) against MG of clone 7C3 (Mab7C3) were purchased from HyTest (Moscow, Russia). Mab against MG of clone A6 (MabA6) were obtained from the Russian Research Center of Molecular Diagnostics and Therapy (Moscow, Russia). Chicken IgY, rabbit anti-chicken immunoglobulins (RACI), goat anti-mouse immunoglobulins (GAMI), goat anti-rabbit immunoglobulins (GARI), and the conjugate of RACI with horseradish peroxidase (RACI–HRP) were obtained from Imtek (Moscow, Russia). GAMI–HRP and GARI–HRP were from Jackson ImmunoResearch (Cambridgeshire, UK).
Chloroauric acid, bovine serum albumin (BSA), streptavidin–HRP conjugate (STR-HRP), sodium citrate, sodium azide, sucrose, dimethyl sulfoxide (DMSO), and Triton X-100 were obtained from Sigma Chemicals (St. Louis, MO, USA). D-biotin-N-hydroxysuccinimide ester was from ICN Biomedicals (Irvine, CA, USA). One-component HRP substrate solution based on 3,3′,5,5′-tetramethylbenzidine was from Immunotech (Moscow, Russia). All other solvents and chemicals of analytical quality were obtained from Khimmed (Moscow, Russia).
Enzyme-linked immunosorbent assay (ELISA) was performed in Costar 9018 96-well transparent polystyrene microplates from Corning Costar (Corning, NY, USA). For the production of lateral flow test strips, CNPC-SS12 nitrocellulose working membrane (with a pore size of 15 μm) GFB-R4 separation pad, and AP045 adsorption pad (all from Advanced Mrodevices, Ambala Cantt, India) were applied.
Biotinylation of antibodies
Antibodies were preliminarily dialyzed against 50 mM K-phosphate buffer with 0.1 M NaCl, pH 7.4 (PBS) in Amicon Ultra centrifugal filters containing regenerated cellulose (Merck Millipore, Carrigtwohill, Ireland) by centrifugation at 10,000 g for 15 min. Covalent binding of biotin to antibodies was carried out at a molar ratio of 10:1 following Bayer and Wilchek (1990). A solution of D-biotin-N-hydroxysuccinimide ester in DMSO (3.1 mg/mL) was added to the antibody solution. The mixture was incubated at room temperature for 2 h and dialyzed against PBS as described above.
ELISA of biomarkers
The ELISA of MG was implemented in the sandwich format as described (Zvereva et al. 2020b). MabA6 or Mab7C3 were immobilized in microplate wells (2 μg/mL in PBS) and incubated overnight at 4 °C. Every step of the ELISA was followed by fourfold washing of the microplate with PBS containing 0.05% Triton X-100 (PBST). Solutions of MG (100 µL, 5 µg/mL–0.1 ng/mL in PBST) were added to the wells and incubated for 1 h at 37 °C. After that, 100 µL of biotinylated Mab (2 µg/mL, in PBST) and STR–HRP (1:5,000 dilution, in PBST) were successively added and incubated in the same regime.
Chicken IgY detection was carried out as described by Hendrickson et al. (2021b). For this, RACI (2 μg/mL in PBS) were absorbed in the microplate wells by overnight incubation at 4 °C. Solutions of IgY (100 μL, 30 μg/mL–0.5 ng/mL in PBST) and RACI–HRP (100 μL, diluted 1:5,000 in PBST) were successively added and incubated by the same way and with the same washing procedures as in the MG ELISA.
Finally, for both ELISAs, the same procedure of HRP activity detection was implemented. For this, HRP substrate solution (100 μL) was added to the wells and incubated for 15 min at room temperature. Then, the enzymatic reaction was stopped by the addition of 1 M H2SO4 (50 μL per well). The optical density (OD) was measured at 450 nm with a Zenyth 3100 microplate photometer (Anthos Labtec Instruments, Wals, Austria).
Preparation and characterization of gold nanoparticles
Gold nanoparticles (GNPs) with an average diameter of 25 nm were obtained by citrate reduction of HAuCl4 to Au0 as described by Frens (1973). The GNP preparation was characterized by transmission electron microscopy (TEM) using a CX-100 microscope (Jeol, Japan) as reported by Zvereva et al. 2020a.
Preparation of antibody–GNP conjugates
The antibody–GNP conjugates were prepared as described by Zvereva et al (2020b), and Hendrickson et al. (2021b). Firstly, Mab7C3, MabA6, and RACI were dialyzed against 10 mM Tris–HCl buffer, pH 9.0. The pH of the GNP solution was adjusted to 8.5–9.0 by 0.1 M K2CO3. For the conjugation, the antibodies were added to the GNP solution with concentrations of 20 µg/mL for Mab7C3, 8 µg/mL—for MabA6, and 10 µg/mL—for RACI. The mixtures were incubated for 45 min with stirring at room temperature. After that, a solution of 10% BSA was added to its final content of 0.25%, and the mixtures were stirred for another 15 min. The obtained conjugates were precipitated by centrifugation at 12,800 g for 15 min at 4 °C and resuspended in 10 mM Tris–HCl buffer, pH 8.5, containing 1.0% BSA, 1.0% sucrose, and 0.05% sodium azide. The conjugates were stored at 4 °C.
Preparation of immunochromatographic test strips
The reagents listed below were applied on the working membranes using an Iso-Flow dispenser (Imagene Technology, Hanover, NH, USA) with a loading of 0.1 µL per 1 mm of the membrane. All solutions were prepared in PBS.
Individual test strips for chicken IgY detection. The test zone (TZ) was formed using RACI (0.75, 1.0, or 2.0 mg/mL). The control zone (CZ) was formed using GARI (0.5 mg/mL).
Individual test strips for MG detection. The TZ was formed using MAb7C3 or MabA6 (0.75, 1.0, or 2.0 mg/mL). The CZ was formed using GAMI (0.5 mg/mL).
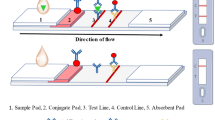
Double test strip. The TZ for chicken IgY detection was formed using RACI (0.75 mg/mL), and the TZ zone for MG detection was formed using MAb7C3 (2.0 mg/mL). The CZ was formed using a mixture of GARI and GAMI solutions with concentrations of 0.35 mg/mL for every protein.
Working membranes with applied immunoreagents were dried for at least 20 h at room temperature, composed with separation and adsorption pads, and cut into test strips (with 3.0 mm width) using an automatic guillotine cutter (KinBio, Shanghai, China).
Sample pretreatment
Raw meat (chicken, turkey, pork, beef, goat, rabbit, and horse) as well as vegetarian and turkey meat sausages were purchased from local supermarkets. Beef and pork-based cooked sausages with a confirmed composition were produced and kindly provided by the V.M. Gorbatov Federal Research Center for Food Systems of the Russian Academy of Sciences (Moscow, Russia). Meat mixtures were obtained in our laboratory from minced raw meat. To prepare heat-treated meat mixtures, they were incubated in vacuum bags for 45 min at 80 °C. After that, the mixtures were cooled to 35 °C by spraying the bags with water for 30 min. The prepared mixtures were stored at 4 °C before the analysis.
Meat samples preparation was carried out as proposed by Hendrickson et al. (2021a). Meat samples were minced by a household homogenizer. Then, to 250 mg of every homogenate, 5 mL of the extraction buffer (PBS containing 0.1% Triton X-100 and 0.5 M KCl) was added and incubated with stirring for 10 min at room temperature. Then, the samples were sonicated for 10 min and centrifuged for 10 min at 5,000 g. The resulting supernatants were analyzed by the test strips.
Lateral flow immunosensing
Individual assays. The tested sample (100 µL), RACI–GNP (OD520 = 6, 0.5 µL), or MabA6–GNP conjugate (OD520 = 17.5, 0.5 µL) were mixed and incubated with test strips for 15 min.
Double assays. The tested sample (100 µL), RACI–GNP (OD520 = 6, 0.5 µL), and MabA6–GNP (OD520 = 17.5, 0.5 µL) were mixed and incubated with test strips for 15 min.
After the analysis, test strips were scanned using the CanoScanLiDE 90 scanner (Canon, Tokyo, Japan). The obtained images were processed with TotalLab software (TotalLab, Newcastle upon Tyne, UK). The total level of coloration intensity in the binding zones was used to confirm the presence of target analytes in the samples and to estimate their content.
Processing of the assay results
Average and RSD values for the measured responses (OD450 for ELISAs or intensity of the TZ coloration for lateral flow tests) were determined based on triplicate measurements. The concentration dependences of the responses were approximated using the Origin software (Origin Lab, Northampton, MA, USA).
Results and discussion
Obtaining and characterization of reagents for lateral flow immunosensing
The analytical capabilities of the immunoreagents were characterized in the sandwich ELISA. To perform the ELISA of MG and find the best combination of capture and detection antibodies, both Mab7C3 and MabA6 were biotinylated. It was shown that all variants of antibody combination as immobilized and biotinylated compounds provided specific detection of porcine MG. The minimal LOD of 9 ng/mL was achieved in the case of Mab7C3 immobilization (capture antibodies) and biotinylated MabA6 interacting with the STR–HRP conjugate (Fig. 1S a). The use of RACI immobilized in the microplate wells and the RACI–HRP conjugate in the sandwich ELISA allowed the revealing chicken IgY with the LOD of 2.5 ng/mL conjugate (Fig. 1S b). Therefore, the demonstrated high sensitivity of biomarker detection ensured using the considered antibodies for the development of lateral flow test systems.
As a colored marker and a carrier for antibodies, GNPs were synthesized and characterized by TEM. According to the obtained images, the average diameter of GNPs was 24.9 ± 4.6 nm with an ellipticity of 1.2 ± 0.1 (in total, 49 nanoparticles were processed) (Fig. 2S). Antibody concentrations for conjugation with GNPs were selected as described in our previous studies (Hendrickson et al. 2021b; Zvereva et al. 2020b): 20 µg/mL—for Mab7C3, 8 µg/mL—for MabA6, and 10 µg/mL—for RACI.
The dependence of the coloration intensity of TZ1 (grey columns) and TZ2 (black columns) on the amount of the MabA6–GNP conjugate during the analysis of MG; 2.5 (1), 0.5 (2), and 0.25 µL of MabA6–GNP (3) are added to the tested sample. The TZ coloration is characterized in the absence of the analytes (columns 1.0, 2.0, and 3.0) and at their concentration of 1,000 ng/mL (columns 1.1000, 2.1000, and 3.1000)
Obtaining and characterization of individual lateral flow test strips for chicken IgY
The works started with the production and characterization of individual lateral flow assays for both biomarkers to develop test systems for comparison with double lateral flow tests, where analytical parameters were expected to retain after the integration of reactants with different specificities. The development included the selection of the reactants’ concentrations and conditions of their interactions to reach the minimal LODs and high coloration of the formed zones on the test strips. Two types of nitrocellulose working membranes were tested for the application of immunoreagents: the CNPC-SS12 (Advanced Microdevices, Ambala Cantt, India) and the UniSart CN95 (Sartorius, Goettingen, Germany) membranes. As a result, the CNPC-SS12 working membrane was chosen for further experiments because of the formation of more distinct and uniformly colored zones during immunochromatography. To form the TZ and the CZ, RACI and GARI were applied to the working membrane, respectively. To initiate the assay, the tested sample was mixed with the RACI–GNP conjugate, and the test strip was immersed in this mixture. In the presence of the analyte in the sample, specific complexes IgY—RACI–GNP were formed, which moved with the liquid along the test strip and bound to RACI forming a colored band in the TZ. The excess of labeled antibodies moved further, bound in the CZ forming the second colored band there or transferred to the adsorption pad of the test strip.
The best analytical characteristics of the individual test strip for IgY detection were achieved by immobilization of RACI in the TZ at a concentration of 1 mg/mL and the addition of 0.5 µL of RACI–GNP conjugate (OD520 = 6) to the sample. The LOD of IgY in the individual test strips was 12.8 ng/mL; the assay duration was 15 min conjugate (Fig. 3S a).
Obtaining and characterization of individual lateral flow test strips for MG
To develop individual lateral flow test strips for MG, two options of reagent combination were first approbated. As capture/labeled antibodies, MabA6/Mab7C3–GNP and MabA6/MabA6–GNP were used. The coloration of the TZ was observed after the analysis in both variants. However, upon the MabA6/Mab7C3–GNP combination, non-specific coloration at the zero point (no MG in the sample) was demonstrated, whereas for the MabA6/MabA6–GNP combination, the coloration intensity of TZ was weak even for high (> 500 ng/mL) concentrations of MG. Upon immobilization of Mab7C3 in the TZ (2 mg/mL) and the use of the MabA6–GNP conjugate (0.5 µL, OD520 = 17.5), the coloration intensity was very high. As a result, the LOD of MG reached 6.8 ng/mL conjugate (Fig. 3S b). GAMI was applied to the CZ at a concentration of 0.5 mg/mL to reach comparable signals of both binding zones for high (> 500 ng/mL) concentrations of MG in the sample.
Development of double lateral flow test strips
The design of the double lateral flow test strips for the simultaneous determination of IgY and MG in a sandwich format of immunochromatography is shown in Fig. 1. The working membrane of the double test strip contained two TZ (TZ1 and TZ2 with the reagents of different specificities) and one CZ. Accordingly, the development of the double assay included the choice of the TZ arrangement and the concentrations of the capture and labeled antibodies. Because the CZ was formed by a mixture of secondary antibodies of different specificities, the amount of each antibody could be decreased in comparison with monoparametric tests. Thus, GAMI and GARI in the concentration of 0.35 mg/mL each were applied to form the CZ. It was demonstrated that the TZ coloration in the individual test of IgY was higher than that in the test system of MG at the same concentrations of both analytes. Therefore, we decided to arrange binding zones as follows: TZ1 (MG detection; Mab7C3) → TZ2 (IgY detection; RACI) → CZ. Upon such zone location, less dispersion of reagents during their movement along the membrane and more efficient binding MG were achieved. To converge the marker binding in the two TZ, the RACI concentration in TZ2 was slightly reduced compared to the individual IgY tests. Finally, Mab7C3 (2 mg/mL) and RACI (0.75 mg/mL) were applied to the test strip.
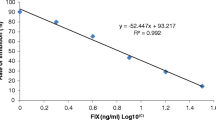
In the next step, the optimal concentrations of labeled Mab were chosen. The optimality criterion was high (and similar) coloration intensities for TZ1 and TZ2 at the same concentrations of both analytes. Figure 2 shows the dependence of the coloration intensity of TZ1 upon the change of MabA6–GNPs content in the reaction medium. TZ coloration was assessed in two variants of the analysis—upon zero and high (1,000 ng/mL) concentration of MG. To do this, analyte-free or IgY and MG-containing samples (50 µL) were mixed with the RACI–GNP conjugate (0.5 µL, OD520 = 6) and the MabA6–GNP conjugate (0.25–2.5 µL, OD520 = 17.5). It was shown that the optimal composition of the double test strip, which ensures the high intensity of TZ coloration and the absence of nonspecific coloration at the zero point, includes Mab7C3 (2 mg/mL) and RACI (0.75 mg/mL) immobilized in the TZ1 and TZ2, respectively, and RACI–GNP (0.5 µL, OD520 = 6) and MabA6–GNP (0.5 µL, OD520 = 17.5) added to tested sample (100 µL). The resulting calibration curves of IgY and MG are presented in Fig. 3. The LODs of IgY and MG for the double immunosensing were 12 ng/mL and 10 ng/mL, respectively. According to the literature data, both biomarkers are contained in relatively high concentrations in muscle tissues of the corresponding species. Thus, the average MG content is estimated as 1 mg per 1 g of porcine meat (Liu et al. 2021) while 1 g of chicken muscles contains 0.2 mg of IgY (Hendrickson et al. 2022). Therefore, the demonstrated detection sensitivity for both biomarkers gives reason for the application of the developed double test system to analyze real raw meat mixtures and cooked meat products.
Analysis of meat samples from different species using the developed double test system
To confirm the specificity of the developed test system, the meat of different animals and birds was tested. For this, extracts of pork, chicken, turkey, beef, lamb, horse, and rabbit meat were prepared and analyzed. The obtained data demonstrate that coloration of the TZ1 (MG detection) appeared only after the analysis of pork extract (column 1 in Fig. 4a), and the coloration in TZ2 (IgY detection) appeared for chicken and turkey meat extracts (columns 2, 3 in Fig. 4b). These results confirmed the high specificity of the developed test system and its suitability to control the undeclared additives.
Analysis of raw meat mixtures of different compositions
Mixtures of various compositions were prepared from minced meat of beef as the main component with the addition of pork and chicken (10, 5, and 1%). The results of the analysis of meat mixtures by the double test system are presented in Fig. 5a–c. The reliable coloration of TZ1 and TZ2 was detected for all mixtures with pork and chicken meat, respectively. The coloration of each TZ appeared independently of the signal in another TZ. Therefore, the developed test system confirmed its efficiency for qualitative («yes–no») control of counterfeit additives in raw meat mixtures. It should be noted that the regulations accepted for the meat industry consider only the presence of undeclared sources in the amount of at least 1% or more (and even 5% or more) as critical manifestations of falsifications committed by manufacturers (Feng et al. 2023). Such attitude is associated with a decrease in economic reasons for small-scale falsifying additives. However, evaluation of the analytical method’s capability to detect undeclared additives with higher sensitivity is also required because small variations in the composition may also lead to health risks and violation of religious dietary requirements. It was demonstrated that the TZ coloration intensity is a concentration-dependent parameter and the results of its quantitative assessment satisfy linear approximations for both TZ1 and TZ2. The calculated LODs for the developed double test system indicate the possibility to reveal at least 0.1% of pork and chicken meat added to raw materials. The determined sensitivity is comparable (in terms of w/w content of undeclared meat) with that reported in the earlier proposed immune and other receptor-based techniques for individual control of porcine and chicken meat: 0.063% (Hendrickson et al. 2021b), 0.1% (Cheubong et al. 2021), 0.25% (Nhari et al. 2018), 0.5% (Chen and Hsieh 2020), and 1% (Ofori and Hsieh 2017).
Images of double test strips after testing meat mixtures (a-c) and cooked meat samples (d). a samples containing 5, 2.5, and 0.5% of porcine meat; b samples containing 5, 2.5, 0.5% of chicken meat; c samples containing 5, 2.5, and 0.5% of chicken meat and 5, 2.5, 0.5% of porcine meat; d vegetarian sausages (1), beef/pork cooked sausages (2), turkey sausages (3), heat-treated mixture of pork (90%) and chicken (10%) meat (4), heat-treated mixture of pork (60%) and chicken (40%) meat (5)
Analysis of cooked meat products
The manufacturing of semi-finished and finished meat products usually includes thermal and enzymatic processing (Dao et al. 2022). Therefore, the use of morphological methods for identifying components in such foodstuffs is limited (Abdel Hafeez et al. 2016). On the other hand, food processing is associated with the risks of changing the structure or complete destruction of antigenic determinants recognized by antibodies. That is why testing the capabilities of the developed analytical system for the detection of the target biomarkers in processed meat products is of greatest interest. To perform this, extracts of sausages with different compositions were obtained, namely, vegetarian sausages, sausages containing beef and pork, and sausages containing turkey. Besides, extracts of thermally processed meat mixtures containing pork and chicken in the 90%/10% and 60%/40% ratios were detected. The obtained results are presented in Fig. 5d. It was shown that the developed double test system enabled the detection of proteins originated from the muscle tissue of pork, chicken, and turkey in finished meat products.
Conclusion
The developed test system allows for the detection of MG and IgY as biomarkers of porcine and chicken meat in meat-based products. The assay combines qualitative and quantitative modes. The achieved LODs were 10 ng/mL and 12 ng/mL for MG and IgY, respectively. They accord to the presence of the corresponding undeclared meat compounds in the concentration of at least 0.1%, whereas the concrete cut-off could depend on meat sources. The undoubted advantage of the developed test system is the possibility of simultaneous detection of two relevant analytes, which increases testing efficiency. The selectivity of the developed assay and its applicability for raw and cooked meat analysis was confirmed. Due to rapidity and methodological simplicity, the developed test system can be proposed as an effective tool for detecting added undeclared pork and chicken meat in meat products.
Data availability
The data supporting the findings of this study are contained within the article. Initial data of instrumental measurements are available on request from the corresponding author.
Code availability
Not Applicable.
Abbreviations
- BSA:
-
Bovine serum albumin
- CZ:
-
Control zone
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- GAMI:
-
Goat anti-mouse immunoglobulins
- GARI:
-
Goat anti-rabbit immunoglobulins
- GNPs:
-
Gold nanoparticles
- IgY:
-
Chicken immunoglobulin of class Y
- LOD:
-
Limit of detection
- Mab:
-
Monoclonal antibodies
- MG:
-
Myoglobin
- PBS:
-
50 MM K-phosphate buffer with 0.1 M NaCl, pH 7.4
- PBST:
-
PBS containing 0.05% Triton X-100
- RACI:
-
Rabbit anti-chicken immunoglobulins
- RACI–HRP:
-
The conjugate of RACI with horseradish peroxidase
- TEM:
-
Transmission electron microscopy
- TZ:
-
Test zone
References
Abdel Hafeez HH, Zaki RS, Abd El-Mageed DS (2016) Applying light, histochemical and scanning histological methods for the detection of unauthorized animal and herbal content in street meat sandwich: What is in the sandwich we eat? J Food Proces Technol 7(12):2. https://doi.org/10.4172/2157-7110.1000643
Alikord M, Momtaz H, Keramat J, Kadivar M, Rad AH (2018) Species identification and animal authentication in meat products: a review. Food Measure 12:145–155. https://doi.org/10.1007/s11694-017-9625-z
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Baggiani C (2019) Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing. Biosensors 9(1):2. https://doi.org/10.3390/bios9010002
Azad MAK, Dey M, Khanam F, Biswas B, Akhter S (2023) Authentication of meat and meat products using molecular assays: a review. J Agric Food Res 12:100586. https://doi.org/10.1016/j.jafr.2023.100586
Banerjee R, Maheswarappa NB, Biswas S, Dasoju S, Barbuddhe S, Rajan VM, Patra G, Bhattacharyya D (2023) Lateral flow immunoassay-based absolute point-of-care technique for authentication of meat and commercial meat products. J Food Sci Technol 60:772–782. https://doi.org/10.1007/s13197-022-05663-2
Bansal S, Singh A, Mangal M, Mangal AK, Kumar S (2017) Food adulteration: sources, health risks, and detection methods. Critical Rev Food Sci Nutr 7(6):1174–1189. https://doi.org/10.1080/10408398.2014.967834
Bartosh AV, Sotnikov DV, Hendrickson OD, Zherdev AV, Dzantiev BB (2020) Design of multiplex lateral flow tests: a case study for simultaneous detection of three antibiotics. Biosensors 10(3):17. https://doi.org/10.3390/bios10030017
Bayer EA, Wilchek M (1990) Protein Biotinylation. Meth Enzymol 184:138–160. https://doi.org/10.1016/0076-6879(90)84268-l
Chen FC, Hsieh YHP (2002) Porcine troponin I: a thermostable species marker protein. Meat Sci 61:55–60. https://doi.org/10.1016/s0309-1740(01)00162-0
Chen FC, Hsieh YHP (2020) Detection of pork in heat-processed meat products by monoclonal antibody-based ELISA. J AOAC Int 83(1):79–85. https://doi.org/10.1093/jaoac/83.1.79
Cheubong C, Takano E, Kitayama Y, Sunayama H, Minamoto K, Takeuchi R, Furutani S, Takeuchi T (2021) Molecularly imprinted polymer nanogel-based fluorescence sensing of pork contamination in halal meat extracts. Biosens Bioelectron 172:112775. https://doi.org/10.1016/j.bios.2020.112775
Dao DTA, Hien LTM, Thinh NP, Thang N, Ha DV, Tam LN, Nguyen VD (2022) Recovery of amino acids and peptides from pig bone soup using thermal pre-treatment and enzymatic hydrolysis. J Food Process Preserv 46(7):e16700. https://doi.org/10.1111/jfpp.16700
Feng J, Lan H, Pan D (2023) Triplex-colored nucleic acid lateral flow strip and multiplex polymerase chain reaction coupled method for quantitative identification of beef, pork and chicken. J Food Comp Anal 123:105493. https://doi.org/10.1016/j.jfca.2023.105493
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys 241:20–22. https://doi.org/10.1038/physci241020a0
Guan T, Xu Z, Wang J, Liu Y, Shen X, Li X, Sun Y, Lei H (2022) Multiplex optical bioassays for food safety analysis: toward on-site detection. Compr Rev Food Sci Food Saf 21:1627–1656. https://doi.org/10.1111/1541-4337.12914
Hendrickson OD, Zvereva EA, Dzantiev BB, Zherdev AV (2021a) Sensitive lateral flow immunoassay for detection of pork additives in raw and cooked meat products. Food Chem 359:129927. https://doi.org/10.1016/j.foodchem.2021.129927
Hendrickson OD, Zvereva EA, Vostrikova NL, Chernukha IM, Dzantiev BB, Zherdev AV (2021b) Lateral flow immunoassay for sensitive detection of undeclared chicken meat in meat products. Food Chem 344:128598. https://doi.org/10.1016/j.foodchem.2020.128598
Hendrickson OD, Zvereva EA, Zherdev AV, Dzantiev BB (2022) Double qualitative immunochromatographic test for simultaneous control of chicken muscles and eggs in food. J Food Comp Anal 106:104324. https://doi.org/10.1016/j.jfca.2021.104324
Hossain MAM, Uddin SMK, Sultana S, Wahab YA, Sagadevan S, Johan MR, Ali ME (2020) Authentication of Halal and Kosher meat and meat products: analytical approaches, current progresses and future prospects. Crit Rev Food Sci Nutr 62(2):285–310. https://doi.org/10.1080/10408398.2020.1814691
Kuswandi B, Gani AA, Ahmad M (2017) Immuno strip test for detection of pork adulteration in cooked meatballs. Food Biosci 19:1–6. https://doi.org/10.1016/j.fbio.2017.05.001
Liu Q, Long Y, Zhang YF, Zhang ZY, Yang B, Chen CY, Huang LS, Su Y (2021) Phenotypic and genetic correlations of pork myoglobin content with meat colour and other traits in an eight breed-crossed heterogeneous population. Animal 15(11):100364. https://doi.org/10.1016/j.animal.2021.100364
Masiri J, Benoit L, Barrios-Lopez B, Thienes C, Meshgi M, Agapov A, Dobritsa A, Nadala C, Samadpour M (2016) Development and validation of a rapid test system for detection of pork meat and collagen residues. Meat Sci 121:397–402. https://doi.org/10.1016/j.meatsci.2016.07.006
Momtaz M, Bubli SY, Khan MS (2023) Mechanisms and health aspects of food adulteration: a comprehensive review. Foods 12(1):199. https://doi.org/10.3390/foods12010199
Narasimhan V, Kim H, Lee SH, Kang H, Siddique RH, Park H, Wang YM, Choo H, Kim Y, Kuma S (2023) Nucleic acid amplification-based technologies (NAAT)—toward accessible, autonomous, and mobile diagnostics. Adv Mat Technol. https://doi.org/10.1002/admt.202300230
Nhari RMHR, Mokhtar NFK, Hanish I, Hamid M, Rashidi MAAM, Shahidan NM (2018) Monoclonal antibody-based enzyme immunoassay for detection of porcine plasma in fish surimi. Food Addit Contam 35(5):807–817. https://doi.org/10.1080/19440049.2017.1420920
Ofori JA, Hsieh YHP (2017) Immunodetection of porcine red blood cell containing food ingredients using a porcine-hemoglobin-specific monoclonal antibody. Foods 6(11):101. https://doi.org/10.3390/foods6110101
Wu Y, Zhou Y, Leng Y, Lai W, Huang X, Xiong Y (2020) Emerging design strategies for constructing multiplex lateral flow test strip sensors. Biosens Bioelectron 157:112168. https://doi.org/10.1016/j.bios.2020.112168
Yang C, Zhong G, Zhou S, Guo Y, Pan D, Wang S, Liu Q, Xia Q, Cai Z (2022) Detection and characterization of meat adulteration in various types of meat products by using a high-efficiency multiplex polymerase chain reaction technique. Front Nutr 9:979977. https://doi.org/10.3389/fnut.2022.979977
Zia Q, Alawami M, Mokhtar NFK, Nhari RMHR, Hanish I (2020) Current analytical methods for porcine identification in meat and meat products. Food Chem 324:126664. https://doi.org/10.1016/j.foodchem.2020.126664
Zvereva EA, Popravko DS, Hendrickson OD, Vostrikova NL, Chernukha IM, Dzantiev BB, Zherdev AV (2020a) Lateral flow immunoassay to detect the addition of beef, pork, lamb, and horse muscles in raw meat mixtures and finished meat products. Foods 9(11):1662. https://doi.org/10.3390/foods9111662
Zvereva EA, Byzova NA, Hendrickson OD, Popravko DS, Belichenko KA, Dzantiev BB, Zherdev AV (2020b) Immunochromatographic detection of myoglobin as a specific biomarker of porcine muscle tissues in meat products. Appl Sci 10(21):7437. https://doi.org/10.3390/app10217437
Acknowledgements
The authors are grateful to S.M. Pridvorova (Research Center of Biotechnology) for TEM studies, and D.S. Popravko for the design of Figure 1.
Funding
This study was financially supported by the Russian Science Foundation (project No 19-16-00108).
Author information
Authors and Affiliations
Contributions
Conceptualization, EAZ, AVZ and BBD; Methodology, EAZ; Validation, EAZ; Investigation, EAZ and ODH; Resources, BBD and AVZ; Writing—Original Draft Preparation, EAZ; Writing—Review & Editing, AVZ; Visualization, AVZ; Project Administration, BBD; Funding Acquisition, AVZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The studies did not involve human participants or animals.
Consent to participate
All authors have read and agreed to the published version of the manuscript.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zvereva, E.A., Hendrickson, O.D., Dzantiev, B.B. et al. Double lateral flow immunosensing of undeclared pork and chicken components of meat products. J Food Sci Technol 61, 1148–1156 (2024). https://doi.org/10.1007/s13197-024-05944-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-024-05944-y