Abstract
This study emphasizes the potential of biomass-derived nanoparticles such as nanocellulose (NC), nanohemicellulose (NHC), and nanolignin (NL) as reinforcements in chitosan (C) films to produce a higher barrier active packaging film. The incorporation of NC, NHC, and NL (1.5%) significantly improves the mechanical, water, and UV barrier properties of the chitosan film (P < 0.0001). Additionally, NHC and NL reinforcement significantly enhance antioxidant and antimicrobial activity. The physicochemical, sensory, and microbiological properties of fresh meat packed in chitosan films with 1.5% nanoparticles, as well as a commercial LDPE film, were assessed when stored at 4 °C for up to 18 days. C-NHC and C-NL packaging films preserved the quality of meat until the 18th day, whereas the meat packed in the LDPE film spoiled entirely on the sixth day. In conclusion, chitosan films with biomass-derived nanoparticles could be an excellent packaging material for highly perishable food, such as fresh meat, with an extended shelf life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the Food and Agriculture Organization (FAO), globally, one-third (around 1.3 billion tonnes) of food produced for human consumption is lost or wasted every year. On the one hand, there is an excess of food production and food waste all over the world, while on the other hand, the hunger crisis is worsening (Karwowska et al. 2021). FAO reports that nearly 690 million people suffer from hunger, which accounts for around 8.9% of the total world population. The growing food waste has detrimental effects on the ecology, climate, water, and land resources worldwide (Conrad et al. 2018). Therefore, minimizing the loss or wastage of produced food is an excellent way to address these global issues, as it can provide food for those in need.
The loss or wastage of food depends not only on the quantity of production but also on the nature of the food (Karwowska et al. 2021). This primarily applies to highly perishable foods such as dairy, meats, fruits, and vegetables, which are more prone to spoilage due to various intrinsic and extrinsic factors. Among these, meat is the third most consumed food after rice and wheat (Kearney 2010). Globally, annual meat consumption has reached approximately 340 million metric tons. Spoilage of fresh meat occurs rapidly due to microbial growth and oxidation processes (Dave and Ghaly 2011) and has a shelf life of less than one day at ambient temperature and 2 to 3 days under refrigeration (4 °C) (Lambert et al. 1991). Generally, freezing inhibits microbial growth but does not kill it. Therefore, when meat is exposed to temperatures above freezing (> 0°F), microbes can quickly multiply and cause spoilage. For these reasons, approximately 20% of meat and meat products produced for consumption are wasted annually. The largest share of loss occurs after manufacturing and before reaching the hands of the consumer for consumption, primarily due to inappropriate preservation methods.
One of the best ways to prevent this loss is to protect meat from self-degradation with adequate active packaging (Nassu et al. 2010; Gil and Rudy 2023). Currently, petroleum-based plastic packaging with good mechanical and barrier properties is in use (Yadav and Chiu 2019). However, while these packaging materials protect the meat from the external environment, they fail to prevent self-degradation. Moreover, these packaging materials are predominantly non-degradable plastics, leading to the accumulation of a vast amount of solid waste and posing a severe threat to the environment (Ncube et al. 2020). Consequently, researchers are working on developing alternative bio-based packaging materials from sustainable bioresources (Shaikh et al. 2021). Many researchers have developed bio-based packaging films from starch, cellulose, guar gum, and proteins (Orsuwan et al. 2016; Yadav and Chiu 2019; Palanichamy et al. 2022). However, most of these biopolymers lack mechanical and barrier properties compared to current packaging materials. Some researchers have attempted to enhance the mechanical and barrier properties of biofilms by incorporating nanoparticles (Ashfaq et al. 2022). Nonetheless, these films have protected the food from the external environment but lack active release of antioxidant and antimicrobial compounds to prevent food self-degradation. Recent researchers have addressed this problem by adding certain additives such as rosemary oil, white cabbage extract, green tea extract, and clove oil (Rubab et al. 2020; Siripatrawan and Harte 2010; Souza et al. 2019). However, developing a packaging material with improved barrier properties and enhanced antioxidant and antimicrobial activity opens up greater opportunities for extending the shelf life of meat. This can be achieved by selecting constituent materials (base polymer and reinforcement) with built-in antioxidant and antimicrobial activity, in addition to their mechanical and barrier properties.
One such biopolymer that has recently gained attention is chitosan, a polysaccharide extracted from the outer shells of crustaceans such as crabs, lobsters, and shrimp. Chitosan exhibits intrinsic antimicrobial and antioxidant activity (Barbosa et al. 2011; Singh et al. 2021). However, there is a need to enhance these properties in order to extend the shelf life of highly perishable foods such as meat and dairy products. Furthermore, there is room for improvement in the mechanical and barrier properties of chitosan (Souza et al. 2020). Some researchers have improved the mechanical and barrier properties of chitosan biopolymer by reinforcing it with nanocellulose (NC) (Yadav et al. 2020). However, such reinforcement did not significantly influence the antimicrobial and antioxidant activity. Our recent research reports that other biomass-derived nanoparticles, such as nanohemicellulose (NHC) and nanolignin (NL), exhibit similar mechanical and barrier properties to cellulose nanoparticles (Jacob Rani and Venkatachalam 2022). Additionally, hemicellulose and lignin have intrinsic antimicrobial and antioxidant activity. Therefore, it is expected that reinforcing chitosan with such biomass-derived polymers will enhance the activity of the packaging material with improved mechanical and barrier properties.
Hence, our focus was on developing a high-barrier active packaging solution to extend the shelf life of fresh meat. We achieved this by utilizing chitosan biopolymer and biomass-derived nanoparticles. This study specifically investigated the individual effects of all three biomass-derived nanoparticles as reinforcements, aiming to enhance the mechanical, barrier, antimicrobial, and antioxidant properties of chitosan films.
Materials and methods
Materials
Chitosan with minimum degree of acetylation 90% was procured from SRL Pvt. Ltd. Glacial acetic acid, DPPH assay, methanol, nutrient agar, plate count agar (PCA), DRBC agar, peptone water were purchased from Sisco Research Laboratories Pvt. Ltd., India.
Biomass-derived nanoparticles such as nanocellulose (NC), nanohemicellulose (NHC) and nanolignin (NL) (purity > 95% and particle size 50 to 100 nm) were produced from Prosopis juliflora wood as described in our previous article (Jacob Rani and Venkatachalam 2022).
Preparation of films
The chitosan solution was prepared by dissolving 1 g of chitosan in 50 ml of 2% acetic acid solution. On the other hand, nanoparticles (0, 5, 10, 15 and 20 mg) were suspended in 50 ml of distilled water. These suspensions were added to the chitosan solution to attain 0, 0.5, 1, 1.5 and 2% w/w concentration of reinforcement and stirred for 4 h at 800 rpm in magnetic stirrer. Then this film forming solution were poured into glass petri dish with 15 mm diameter and dried at 60 °C for 24 h until the solvent was completely evaporated. The resultant films were conditioned in a humidity control chamber (Sub Zero Pvt. Ltd., India) at 25 °C and 50% relative humidity (RH) for 48 h prior to testing. The obtained films with 0, 0.5, 1, 1.5, 2 w/w% of NC, NHC and NL were coded as C, C-0.5NC, C-1NC, C-1.5NC, C-2NC, C-0.5NHC, C-1NHC, C-1.5NHC, C-2NHC, C-0.5NL, C-1NL, C-1.5NL, C-2L respectively. The thickness of the film was measured using a dial type thickness gauge (Mitutoyo 2109s-10, Japan) as an average of 10 readings.
Mechanical characterization
Mechanical Properties such as tensile strength (TS), elongation percentage at break (EB) and ultimate modulus (UM) were measured as per ASTM D882 standard using a Universal tensile testing machine (International Equipment’s, Mumbai). Each sample (150 × 25 mm) was mounted between the grips and tested with a crosshead speed of 50 mm/min. Measurements represented an average of three replications. The obtained results were subjected to statistical analysis using the ordinary One-way ANOVA test with GraphPad Prism 9.1.2 software.
Analysis of water barrier properties
Water solubility (WS)
Film water solubility was measured in accordance with a previously described method (Orsuwan et al. 2016). Samples were cutted into 20 × 20 mm2 and dried at 40 °C for 24 h to find the initial dry weight (Wi). Then, the film samples were soaked in 20 mL distilled water with mild shaking for 24 h, removed and dried at 40 °C for 24 h to find the undissolved final dry weight (Wf). The water solubility (%) was determined using the Eq. 1.
Moisture absorption (MA)
Moisture absorption was measured as described by Noshirvani et al. (2016). Samples sectioned into 20 × 20 mm2 were dried and weighed for initial weight (W0). After that, they were placed in a humidity control chamber at 25 °C and 98% RH. The samples were weighed at desired interval until the equilibrium state (constant weight) was reached. MA of the film was determined using the initial weight (W0) and the equilibrium weight (We) (Eq. 2).
Water absorbency (WA)
WA was estimated in terms of percentage swelling ratio as described by Yadav and Chiu (2019). Samples were sectioned into 20 × 20 mm, dried and pre-weighed (Wd). Each sample was soaked in a 20 mL distilled water for 24 h at room temperature. The swollen samples were removed, wiped the surface water droplets using a tissue paper and then dried and weighed (Ws). WA was calculated using the Eq. 3.
Water vapour permeability (WVP)
The WVP of the film was analyzed using ASTM (1996) E96 method. The samples were placed on top of a glass permeation cell containing anhydrous CaCl2 maintaining a relative humidity (RH) of 0% and weighed. Then, it was placed in a humidity control chamber at 25 °C and 75% RH. Weight gain was measured for 8 h at an interval of 1 h. WVP was determined using the Eq. 4.
where Δw represents change in weight in specific interval of time (Δt), A represents the area of the film covered the permeation cell (m2), d represents the thickness (m), S represents the saturation vapor pressure of water (Pa) at 25 °C, R1 represents RH in the humidity Control Chamber and R2 represents RH in the permeation cell. WVP was mentioned as an average of three replications.
Water contact angle
The water contact angle of the films was measured using a Drop Shape Analysis System (Data physics instrument, Germany). The deionized water was dropped on the surface of the film. Then the image was captured and analyzed using Data physics SCA 20 software to obtain the contact angle. Measurements were performed in triplicate, and their average values were taken.
UV barrier and opacity of the films
Absorbance and transmittance of the film samples were measured at different wavelength 300 nm, 400 nm, 500 nm and 600 nm using a UV/Vis spectrophotometer (LMSP-UV1200, Labman Scientific Instruments, India). Opacity was calculated using the Eq. 5.
Anti-oxidant and anti-microbial studies
The anti-oxidant activity of the film samples was determined using DPPH free radical scavenging assay as described in Siripatrawan and Harte (2010). In brief, 3 ml of film extract solution were mixed with 1 ml of 1 mM methanolic solution of DPPH and incubated in dark for 30 min. The absorbance was measured at 517 nm. The DPPH scavenging effect was determined using the Eq. 6.
The antimicrobial activities of the films were tested against a gram-negative bacteria (E. coli), a gram-positive bacteria (Bacillus subtillis), a yeast (Candida albicans) and a mould (Aspergillus niger) by disc diffusion method. The films were cut into a disc of 10 mm and placed on a nutrient agar plate inoculated with corresponding microorganisms. The plates were incubated for 24 h to test the antibacterial activity and 48 h for antifungal analysis. The diameter of the inhibitory zone surrounding the film disc was measured.
Biodegradability test
The soil burial degradation test was performed to assess the biodegradability of the film samples. The film samples were sectioned into 100 × 20 mm2, dried and pre-weighed (M0). The samples were buried in the soil for degradation and weighed (M1) at regular interval for 28 days. Weight loss (WL) was determined using the Eq. 7.
Fresh meat packaging and shelf life studies
Freshly cut goat meat (mutton) samples with a postmortem period of less than 1 h were collected from the slaughterhouse (Saidapet, Chennai, India). Samples were cleaned and packed in the commercial LDPE film and the prepared chitosan-based nanocomposites. The shelf life of the packed meat was assessed by testing the physicochemical and microbiological properties of the packed samples refrigerated under 4 °C on the 3rd, 6th, 10th and 15th day.
Physico-chemical characterization
The pH of the meat sample was measured on homogenate using AOAC, 1995 method as described by Chandra Mohan et al. 2017. A 10 g of meat sample was homogenized in 100 ml of distilled water. PH was measured at ambient temperature using a digital pH meter with a glass electrode (LI 120, Elico Ltd., India). Titratable acidity (TA) of the samples was analyzed by titrating the homogenized meat against 0.1N NaOH solution, and the results were expressed as % (w/w) of acetic acid equivalent.
To determine the peroxide value (PV), 5 g of meat sample was homogenized with 30 mL of acetic acid-chloroform solution and add 0.5 mL of saturated KI. Slightly heat the above mixture and add 30 mL of distilled water, then titrate it against 0.1 N sodium thiosulphate solution. Peroxide value was calculated using the Eq. (8)
where S represents sample titration value, B represents blank titration value and N represents normality of sodium thiosulfate.
Sensory properties
A panel consisting of 10 experts, comprising 5 men and 5 women aged between 30 and 50, was involved in assessing the sensory properties of the product. The evaluation focused on attributes such as color, odor, and muscle elasticity. To evaluate color and odor, a 10-point Hedonic Scale system was utilized, where a score of 10 represented excellent quality, 8–9 indicated good quality, 6–7 denoted fair quality, and scores of 5 or less indicated that the product was unmarketable. Muscle elasticity was assessed using a 3-point scale, with a score of 3 indicating a quick return to the original state, 2 representing a slow return, and 1 indicating a failure to return to the original state.
Microbiological growth
Homogenize a 10-g meat sample with 90 mL of sterile peptone water and spread 0.1 mL of this homogenized sample over the agar plates. Total plate count (TPC) was determined using PCA plates with incubation at 37 °C for 48 h. Coliform was determined using violet red bile lactose agar plate with incubation at 30–37 °C for 24 h. Yeast and mold growth were determined using DRBC agar plates with incubation at 25 °C for 5 days. The culture plates were observed visually for typical colony counts and results were expressed as log CFU g−1 (Rubab et al. 2020).
Statistical analysis
The results obtained for mechanical and water barrier properties were subjected to statistical analysis using the ordinary One-way ANOVA test with GraphPad Prism 9.1.2 software. This statistical test enables the comparison of effects among three different nanoparticles while also evaluating the influence of their concentration on these properties.
Results and discussion
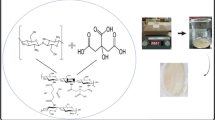
NC, NHC and NL produced from Prosopis juliflora were added as a reinforcing agent in the chitosan solution at different concentrations and the films were prepared using the casting method (Fig. 1). The chitosan and nanoparticles exhibit a stronger interaction with each other and form a 3D network structure. The prepared films were thin, translucent and appeared in light golden color. The thickness of the films were measured using a thickness gauge and was found to be 60 ± 5 μm.
Mechanical properties
Mechanical properties are essential for all packaging materials for their mobility, durability and correctness while forming a packaging unit. Reinforcement of different nanoparticles in the chitosan films showed different trends toward its mechanical behaviour (Fig. 2).
Adding 1% of NC shows a significant increase in TS, whereas its EB was moderately increased. Further increase in NC had a saturated effect in TS with a drastic reduction in EB. Likewise, NL reinforcement shows a threefold increase in TS at 1.5% with significantly low EB. This increased tensile strength was due to strong interaction between chitosan and the nanoparticles, which also decreases the motion of the interface between reinforcement and base polymer, leading to the reduction of EB (Yadav et al. 2020). However, the addition of NHC significantly improved the elongation property of the film rather than TS and formed more flexible films. This decreased tensile strength is attributed to the replacement of strong interactive bonds in the chitosan by weak interactive bonds of NHC and also shows the plasticizing effect of hemicellulose (Zhang et al. 2020). Stiffness (UM) of NC and NL reinforced film was increased with an increase in concentration, while in contrast, NHC reinforced film had a low ultimate modulus. The films with different properties can be potentially used for specific applications. The films with higher tensile strength, such as 1% NC, and 1.5% NL with low elongations, can be used as food carrying pouches (Louis et al. 2022), whereas the flexible films with higher elongation and low stress (1.5% NHC) could be used as a wrapper for the food materials.
Water barrier properties
Water barrier properties plays a vital role in the quality of food packaging films while exposed to external environment and also to maintain the quality and shelf-life of the food products (Yadav and Chiu 2019). A decreased water solubility of the film is one of the desirable property for most food packaging because humid food such as meat and fresh cut fruits will disintegrate the hydrophilic materials. Addition of NC and NL had significantly decreased the water solubility (P < 0.0001), whereas the solubility slightly decreased by the incorporation of NHC (Fig. 3a). This decrease in solubility may be due to the 3D network structure formed between the chitosan and nanoparticles, which hinder the movement of soluble polymers to the water (Yadav et al. 2020). However, hemicellulose was more likely soluble in water when comparable to other nanoparticles was the reason behind the higher water solubility observed in NHC reinforced films. In similar way, moisture absorption and water absorbency of the NC and NL reinforced film had decreased significantly, whereas the same was increased in film incorporated with NHC (Fig. 3b and c). The better interaction of the chitosan and NC by hydrogen bonding decreased the chances to absorb the moisture and water molecules. Likewise, phenolic groups in lignin also fit into the chitosan with strong covalent or hydrogen bonding, leads to decreased affinity towards water (Siripatrawan and Harte 2010). In case of NHC, some strong bonds present in the chitosan was replaced by the weak hydrophilic bonds in the hemicellulose and leads to increased water interaction with the film (Zhang et al. 2020).
Hindering the passage of water is one of the major objective of the packaging film to protect the food. Hence, the WVP of the film should be as low as possible. Addition of nanoparticles had reduced the WVP of the chitosan films significantly (Fig. 3d). The physical barrier developed in the film due to the structural interaction and good dispersion of nanoparticles into the matrix (Yadav and Chiu 2019). As the percentage of nanoparticles increased to 1.5%, the WVP decreased significantly (P < 0.0001) and the lowest value (0.164 × 10−11 gm−1 s−1 Pa−1) was observed in the film loaded with 1.5% NL. However, further addition of nanoparticles doesn’t make any effective change in WVP.
Contact angle is the measure of surface affinity of film towards water. Herein, contact angle of NC and NL reinforced film had improved significantly (Fig. 3e). But NHC incorporated film had no significant changes. Higher contact angle of 98.6° was observed in the film with 2% NC. The increase in hydrophobicity was due to the structural interaction between the Chitosan and nanoparticles by the formation of hydrogen bonds and beneficial improved the water resistance of the films (Wardhono et al. 2019). Overall, chitosan films with nanoparticle reinforcement had better water barrier properties when comparing with other biopolymer based films such as starch, guar gum etc. (Romero Figueroa 2018; Louis et al. 2022; Palanichamy et al. 2022). These improved water barrier properties could increase the shelf life of food in packaging applications.
UV barrier and light transmittance of the films
Clear visibility of the packed food is essential to evaluate the quality of food by the consumer visually. Hence, transparency becomes a necessary property for the packaging film. However, higher transmittance towards visible light is more pronounced for the appearance of the food. At the same time, the packaging material must restrict the passage of UV rays to preserve the quality of the food because exposure to UV radiation will oxidize the foods and degrade them quickly (Orsuwan et al. 2016). Hence, the transmittance of the films in different wavelengths such as 300 nm (UV-B radiation), 400 nm (UV-A radiation), 500 nm and 600 nm (visible light radiation) were measured (Fig. 3a). The transmittance of plain chitosan film for visible light (500 to 600 nm) was around 60 to 70%, which was reduced by incorporating nanoparticles (0 to 2%). This slight decrease did not affect the film's visual transparency because the nanoparticles were evenly distributed throughout the film without any visible aggregations. Moreover, the reinforcement of nanoparticles significantly reduced the UV transmittance. All the films reinforced with nanoparticles had high resistance toward UV-B radiation, whereas films with 1% and 1.5% reinforcement had a relatively high resistance than other films towards UV-A rays. The results indicate that the prepared films will potentially avoid the oxidative rancidity caused by UV rays and protect food quality with extended shelf life. Hence, it could be a preferable packing material for high lipid content foods such as meat, creams, cheese, and butter.
The results indicate that the incorporation of biomass-derived nanoparticles significantly enhanced the mechanical and barrier properties of the film. In all, by comparing those properties of the film, 1.5% nanoparticle reinforced film was selected for further studies.
Antioxidant activity of the films
The antioxidant activity of plain chitosan and 1.5% nanoparticle-reinforced films was assessed in terms of DPPH scavenging activity (Fig. 3b). The plain chitosan film exhibited 60.9 ± 0.8% scavenging activity on DPPH. The free amino group in chitosan reacts with DPPH, forming stable radicals (Siripatrawan and Harte 2010). The addition of nanoparticles enhanced the antioxidant activity of the films, with the degree of enhancement varying among different nanoparticles. Notably, NHC and NL demonstrated greater free radical scavenging activity due to the presence of phenolic structures, as reported (Crouvisier-Urion et al. 2016; Wu et al. 2019). In similar way, the antioxidant activity of films reinforced with NHC and NL exceeded that of NC films. The addition of NL, in particular, had a significant impact, increasing the antioxidant activity to an impressive 78.7 ± 0.4%. This achievement surpasses the results of recent research involving chitosan films reinforced with N and P-doped carbon dots for meat packaging, which reported a maximum activity of 76.6 ± 0.9% (Khan et al. 2023). This heightened antioxidant activity will help mitigate the oxidative degradation of food materials packaged with it. Therefore, this material could be the preferred choice for packaging high-lipid foods like meat.
Antimicrobial activity of the films
The antibacterial activity of the chitosan films incorporated with nanoparticles against gram-positive and gram-negative bacteria was compared with plain chitosan film and paper disc containing antibacterial drug azithromycin (30 µg/mL) (Fig. 4a–c). The plain chitosan film showed antibacterial activity on the contact surface underneath the films with negligible inhibition zone for both gram-negative and gram-positive bacteria. Though chitosan have intrinsic antimicrobial activity, it is possible to have negligible inhibition when it is in the form of insoluble films. However, chitosan incorporated with NC show little inhibition against gram negative whereas for gram positive bacteria it shows only on the contact surface area. The incorporation of NHC and NL have exhibited antibacterial activity in contact surface with significant inhibition zone against gram negative bacteria and comparatively lesser zone in gram positive bacteria. The antimicrobial action depends on the chemical composition of the materials and their biochemical actions involved at the bacterial cell wall. When comparing with antibacterial drug, NHC reinforced films has 75% activity against E. coli and around 47% activity against B. subtilis, whereas NL reinforced film has 85% activity against E. coli and around 50% activity against B. subtilis. This difference in activity towards gram-positive and gram-negative bacterial was due to the structural difference in its cell wall. The thicker peptidoglycan layers present in gram-positive bacteria need more activity to destroy it than the thin peptidoglycan cell wall of gram-negative bacteria.
The antifungal activity of the chitosan films incorporated with nanoparticles against a yeast (Candida albicans) and a mold (Aspergillus niger) and compared with the antifungal drug Clotrimazole (30 µg/mL) (Fig. 4d–f). The Candida albicans has grown even in the contact surface area of the plain chitosan film, whereas NC and NHC incorporated films also have growth in some spots of contact area. Only NL reinforced film shows significant antifungal activity against Candida albicans on the contact area surrounded with little inhibition zone. The activity against a mold (Aspergillus niger) also show the similar trend for plain chitosan film. NC and NHC reinforced film has growth in some spot of its contact surface area, whereas no mold growth was observed on the surface of NL reinforced chitosan films. These results clearly demonstrated the higher antimicrobial activity of lignin due to its phenolic structures than the other biomass components. Over all, NC, NHC and NL reinforced films are likely to be used to pack highly perishable foods such as meat.
Biodegradability of the films
The weight loss percentage of the film samples during the soil burial test is shown in Fig. 3c. Samples were collected every five days until they completely degraded. Around 75 to 85% of films were degraded until the 20th day. After the 20th day, samples were highly disintegrated, which made it difficult to collect them. Chitosan, cellulose, hemicellulose and lignin are natural biopolymers found to degrade within 30 days. The fully biodegradable capability of the prepared films, coupled with their improved mechanical and barrier properties, could make this an excellent substitute for traditional non-biodegradable packaging materials.
Shelf-life assessment of packed meat
Fresh meat is a highly perishable food, which could be affected by various intrinsic and extrinsic factors such as microbial growth and the oxidation of unsaturated lipids. Fresh meat's shelf life is much less even under refrigerator conditions (Pirsa and Shamusi 2019). Fresh goat meats packed in commercial film (LDPE) and chitosan nanocomposites reinforced with NC, NHC and NL (Fig. 5a–f) were stored under refrigerated conditions (4 °C) and were analyzed every three days until 18th day.
Sensory properties of goat meat
The panel of six people using a hedonic scoring system evaluated sensory properties such as color, odor and elasticity of the fresh and packed meats (Table S1). According to the panel, the meat packed in the C-NL and C-NHC nanocomposite had good sensory properties until 18th day. In contrast, rapid deterioration in color, odor and elasticity were observed in meat packed in a commercial film. Meat packed in the commercial film becomes unmarketable on 6th day, whereas chitosan film packed meat and C-NC packed meat were reasonably good compared with commercial packaging film. Overall, the sensory evaluation suggests that C-NL and C-NHC are the most appropriate packaging film for protecting fresh meat without much deterioration.
Physico-chemical properties
The changes in physicochemical properties were periodically assessed over the storage period, as shown in Fig. 5g–j. The pH of the meat is a crucial parameter in assessing its quality, with raw fresh meat typically having a pH of 5.5–6.2. If the pH falls below this range, the meat loses its quality for consumption. In this study, the initial pH of the meat used was 6.1, which decreased over time. However, the pH of meat packed in C-NL and C-NHC films showed no significant change until the 18th day, and the recorded values remained within the allowable limit. Similarly, the moisture content of the fresh meat (77%) also decreased over time for all packaging methods, but the changes were not significant for meat packed in C-NHC and C-NL films. The acidity of the meat increased over time for all packaging methods, but again, the changes were not significant for chitosan nanocomposites packed meat.
Due to the high lipid content in meat, it is more susceptible to oxidative degradation. Therefore, the peroxide value of the meat is essential in assessing its quality and marketability. The peroxide value of the fresh meat was initially very low (0.12 meq/kg), but it increased over the storage period. The peroxide value of meat packed in commercial film, plain chitosan film, and C-NC film increased significantly, while meat packed in C-NHC and C-NL films showed no significant changes in its peroxide value until the 18th day (< 0.5 meq/kg). The observed changes in physicochemical properties may be attributed to the production of volatile components by microbial growth and the oxidative degradation of fats and lipids (Souza et al. 2019). Therefore, meat packed in packaging with higher antioxidant and antimicrobial activity (C-NHC and C-NL) experienced fewer changes in its properties and maintained its quality throughout the shelf-life assessment.
Microbiological quality
As expected, the total plate count of meat packed in the commercial film increased over the storage period. Initially, the meat packed in the commercial film had total plate counts and coliform levels within the permissible limits until the 6th day, after which it spoiled. However, meat packed in chitosan nanocomposites with antimicrobial activity showed a decrease in microbial counts (Fig. 6). This can be attributed to the active release of antimicrobial components, which not only inhibits microbial growth but also kills the initial microbes present in the raw meat. In the following days, there was a slight increase in microbial count even in chitosan-based films, indicating a reduction in the films' activity over time. The plain chitosan film had a shelf life of 9 days, while the C-NC film maintained microbial growth within the permissible limit until the 12th day. The C-NHC films reached their maximum allowable microbial growth limit on the 18th day and lasted six more days than the C-NC films due to their antimicrobial nature. Meat packed in the film with higher antimicrobial activity (C-NL) exhibited a better shelf life compared to the other films used in this study, as it did not exceed the allowable limit until the 18th day. The growth of yeast and molds was within the permissible limit for all the packaging films; however, the growth rate was significantly lower for meat packed in C-NL film. Overall, the results suggest that the C-NL film is the most preferred packaging material for extending the shelf life of highly perishable foods such as meat, thanks to its antioxidant and antimicrobial properties.
Conclusion
The successful development of biomass-derived nanoparticle-reinforced chitosan films represents a significant advancement in active packaging technology. These films exhibit improved tensile strength, elongation properties, water and UV barrier properties, as well as enhanced antioxidant and antimicrobial activities compared to the unreinforced chitosan films. In the case of goat meat packaging, the chitosan nanocomposite films outperformed the commercial LDPE film. While the commercial film led to drastic microbial growth, the chitosan nanocomposites effectively reduced microbial counts initially and maintained meat quality throughout the storage period. Notably, the C-NL film demonstrated the longest shelf life, exceeding 18 days, due to its enriched antimicrobial and antioxidant activities. Similarly, the C-NHC film effectively preserved meat quality until the 18th day. Overall, these biomass-derived nanoparticle-reinforced chitosan films have great potential for packing fresh meat. Thus, by extending shelf life and improving preservation, they offer an environmentally friendly alternative to non-degradable packaging films used in the food industry. This research opens up new possibilities for reducing food spoilage and creating sustainable packaging solutions that can positively impact the perishable food sector.
Availability of data and material (data transparency)
Not applicable.
Code availability (software application or custom code)
Not applicable.
References
Ashfaq A, Khursheed N, Fatima S et al (2022) Application of nanotechnology in food packaging: pros and cons. J Agric Food Res 7:100270. https://doi.org/10.1016/j.jafr.2022.100270
Barbosa MA, Pêgo AP, Amaral IF (2011) 2.213—Chitosan. Compr Biomater 221–237. https://doi.org/10.1016/B978-0-08-055294-1.00072-6
Chandra Mohan C, Radhakrishnan K, Babuskin S et al (2017) Active compound diffusivity of particle size reduced S. aromaticum and C. cassia fused starch edible films and the shelf life of mutton (Capra aegagrus hircus) meat. Meat Sci 128:47–59. https://doi.org/10.1016/j.meatsci.2017.02.001
Conrad Z, Niles MT, Neher DA et al (2018) Relationship between food waste, diet quality, and environmental sustainability. PLoS ONE 13:1–18. https://doi.org/10.1371/journal.pone.0195405
Crouvisier-Urion K, Bodart PR, Winckler P et al (2016) Biobased composite films from chitosan and lignin: antioxidant activity related to structure and moisture. ACS Sustain Chem Eng 4:6371–6381. https://doi.org/10.1021/acssuschemeng.6b00956
Dave D, Ghaly A (2011) Meat spoilage mechanisms and preservation techniques: a critical review. Am J Agric Biol Sci 6:486–510. https://doi.org/10.3844/ajabssp.2011.486.510
Gil M, Rudy M (2023) Innovations in the packaging of meat and meat products—a review. Coatings 13(2):333. https://doi.org/10.3390/coatings13020333
Jacob Rani BS, Venkatachalam S (2022) Cleaner approach for the cascade production of nanocellulose, nanohemicellulose and nanolignin from Prosopis juliflora. Carbohydr Polym 294:119807. https://doi.org/10.1016/j.carbpol.2022.119807
Karwowska M, Łaba S, Szczepański K (2021) Food loss and waste in meat sector—why the consumption stage generates the most losses? Sustain. https://doi.org/10.3390/su13116227
Kearney J (2010) Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci 365:2793–2807. https://doi.org/10.1098/rstb.2010.0149
Khan A, Ezati P, Rhim J (2023) Chitosan/starch-based active packaging film with n, p-doped carbon dots for meat packaging. ACS App Bio Mat 6(3):1294–1305. https://doi.org/10.1021/acsabm.3c00039
Lambert AD, Smith JP, Dodds KL (1991) Shelf life extension and microbiological safety of fresh meat—a review. Food Microbiol 8:267–297. https://doi.org/10.1016/S0740-0020(05)80002-4
Louis ACF, Venkatachalam S, Gupta S (2022) Innovative strategy for rice straw valorization into nanocellulose and nanohemicellulose and its application. Ind Crops Prod 179:114695. https://doi.org/10.1016/j.indcrop.2022.114695
Nassu RT, Juárez M, Uttaro B, Aalhus JL (2010) Fresh meat packaging: trends for retail and food service. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour. https://doi.org/10.1079/PAVSNNR20105055
Ncube LK, Ude AU, Ogunmuyiwa EN et al (2020) Environmental impact of food packaging materials: a review of contemporary development from conventional plastics to polylactic acid based materials. Materials 13:1–24. https://doi.org/10.3390/ma13214994
Noshirvani N, Ghanbarzadeh B, Fasihi H, Almasi H (2016) Starch-PVA nanocomposite film incorporated with cellulose nanocrystals and MMT: a comparative study. Int J Food Eng 12:37–48. https://doi.org/10.1515/ijfe-2015-0145
Orsuwan A, Shankar S, Wang LF et al (2016) Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll 60:476–485. https://doi.org/10.1016/j.foodhyd.2016.04.017
Palanichamy P, Venkatachalam S, Gupta S (2022) Improved recovery of cellulose nanoparticles from printed wastepaper and its reinforcement in guar gum films. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02516-y
Pirsa S, Shamusi T (2019) Intelligent and active packaging of chicken thigh meat by conducting nano structure cellulose-polypyrrole-ZnO film. Mater Sci Eng C 102:798–809. https://doi.org/10.1016/j.msec.2019.02.021
Romero Figueroa JR (2018) Improvement of physicochemical properties of starch films by blending it with poly(N-vinyl-2-pyrrolidone). Food Sci Nutr 4:1–8. https://doi.org/10.24966/fsn-1076/100036
Rubab M, Chelliah R, Saravanakumar K et al (2020) Phytochemical characterization, and antioxidant and antimicrobial activities of white cabbage extract on the quality and shelf life of raw beef during refrigerated storage. RSC Adv 10:41430–41442. https://doi.org/10.1039/d0ra06727j
Shaikh S, Yaqoob M, Aggarwal P (2021) An overview of biodegradable packaging in food industry. Curr Res Food Sci 4:503–520. https://doi.org/10.1016/j.crfs.2021.07.005
Singh MVP, Sanjuvikasini V, Shruthi S et al (2021) Value-added seafood products processing through novel enzyme. Value-Addition Food Prod Process through Enzym Technol 2022:309–320. https://doi.org/10.1016/B978-0-323-89929-1.00013-5
Siripatrawan U, Harte BR (2010) Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll 24:770–775. https://doi.org/10.1016/j.foodhyd.2010.04.003
Souza VGL, Pires JRA, Vieira ÉT et al (2019) Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: from in vitro assays to application in fresh poultry meat. Food Hydrocoll 89:241–252. https://doi.org/10.1016/j.foodhyd.2018.10.049
Souza VGL, Pires JRA, Rodrigues C et al (2020) Chitosan composites in packaging industry-current trends and future challenges. Polymers 12:1–16. https://doi.org/10.3390/polym12020417
Wardhono EY, Pinem MP, Kustiningsih I et al (2019) Cellulose nanocrystals to improve stability and functional properties of emulsified film based on chitosan nanoparticles and beeswax. Nanomaterials 9(12):1707. https://doi.org/10.3390/nano9121707
Wu F, Jia X, Yin L et al (2019) The effect of hemicellulose and lignin on properties of polysaccharides in lentinus edodes and their antioxidant evaluation. Molecules. https://doi.org/10.3390/molecules24091834
Yadav M, Chiu FC (2019) Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr Polym 211:181–194. https://doi.org/10.1016/j.carbpol.2019.01.114
Yadav M, Behera K, Chang YH, Chiu FC (2020) Cellulose nanocrystal reinforced chitosan based UV barrier composite films for sustainable packaging. Polymers. https://doi.org/10.3390/polym12010202
Zhang X, Wei Y, Chen M et al (2020) Development of functional chitosan-based composite films incorporated with hemicelluloses: effect on physicochemical properties. Carbohydr Polym 246:116489. https://doi.org/10.1016/j.carbpol.2020.116489
Funding
This work was supported by the Anna Centenary Research fellowship provided by Anna University.
Author information
Authors and Affiliations
Contributions
BSJR: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation and Writing—Original Draft; SV: Writing – Review & Editing, Visualization and Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jacob Rani, B.S., Venkatachalam, S. Biomass-derived nanoparticles reinforced chitosan films: as high barrier active packaging for extending the shelf life of highly perishable food. J Food Sci Technol 61, 990–1002 (2024). https://doi.org/10.1007/s13197-023-05896-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05896-9