Abstract
The present study aims to evaluate the quality of chemical, sensory properties and antioxidant potential of mulberry wine using selenium-enriched yeasts employing eight different methods (MW1–MW8). The selenium-enriched yeast significantly (p < 0.05) increased phytochemical profiles, flavor, quality and antioxidant capacity. The most effective method for raising the selenium level of mulberry wine was using L-seMC (MW5). Mulberry wine color was attributed to the anthocyanins and phytochemical composition with selenium content. DPPH and ABTS radical scavenging activity varied with change in treatment methods suggesting their impact on antioxidant activity. Total selenium content on L-SeMC supplementation proved a significant correlation between selenium content with total anthocyanin content, total polyphenol content and flavonoid content. Sensory analysis by electronic nose exhibited MW2 with high response value in the W2S sensor showing high alcohol concentration. GC–MS analysis showed the presence of 57 volatile aromatic compounds comprehended by esters and alcohol (isoamyl alcohol, 2-methylbutanol, 2,3-butanediol, and phenethyl alcohol). Principal component analysis affirms the response values for four categorical score values with reliability and consistency for all the parameters, significantly. Thus, the workflow demonstrates a simpler, cost-effective traditional methodology for rationalized outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mulberrry acts as the principal component for food as medicine and is usually referred to as "folk sacred fruit". Mulberry possesses several health benefits owing to the presence of phenolic substances and its antioxidant nature. The rich flavonoids in mulberry effectively reduce lipid peroxidation in the human body and improve the antioxidant status in the liver and blood. Augmentation of human health is attributed to the presence of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) enzymes (Yang et al. 2010). Further, rutin and isoquercitrin prevent inflammation by reducing the level of inflammatory cytokine IL-6. Subsequent reactions correlate to the inhibition of nitric oxide synthase through inducible synthesis and increased expression of anti-inflammatory cytokine IL-10. Adequate research has demonstrated that anthocyanins and flavonoids have potent anti-inflammatory effects by inhibiting cytokines, nitric oxide synthase, and NF-kB against pathogens such as, Escherichia coli, Staphylococcus aureus, etc. (Chen et al. 2017). The prominent health impact of mulberry wine has been coerced to the presence of bioactive compounds along with nutritional benefits. (Yuan and Zhao 2017). The chemical constituents in mulberry wine by alcohol fermentation yield carbonyl compounds, alcohols, esters, acids and acetals, and ethanol production (Feng et al. 2015). Thus the by-products improves the flavor of mulberry wine with increased palatability and compatibility.
The essential micronutrient, selenium (Se) shows multiple health implications and various biological functions encompassing antioxidant activities (Grasemann and Holguin 2021), immune enhancement (Sena et al. 2013) and anti-ageing properties (Liu et al. 2016; Cai et al. 2019). Se deficiency is associated with several diseases, including Keshan disease, cancers, diabetes, thyroid disease, atherosclerosis, asthma, hearing failure, and skin diseases (Liu et al. 2018a, b, c). Therefore, organic selenium-fortified wines, foods and other functional foods are recommended for humans (approximately 55 µg/day). However, the toxic nature of inorganic Se, selenate and selenite requires microbial biotransformation of Se into organic forms, including selenocysteine, selenomethionine, and selenomethyl selenomethionine (Kieliszek et al. 2015). Numerous studies have demonstrated that selenium-loaded Saccharomyces cerevisiae is capable of converting inorganic selenium to organic selenium through biotransformation (Kieliszek et al. 2015), and probiotic-based nutraceuticals (Palomo-Siguero 2016). The emphasis on emerging trends of fermented Se-enriched yeasts mulberry wine of non-dairy origin for increased health benefits, probiotics and antioxidant profiles is necessitated. The purpose of this study was to evaluate the quality of chemical, sensory properties and antioxidant profile of mulberry wine fermented with selenium-enriched yeasts using different methods (MW1–MW8). The goals were (a) preparation of Se-enriched yeast and mulberry fruit Juice (b) perform mulberry wine fermentation by Se-enriched yeast with different methods (c) evaluate sensory properties, chemical and antioxidant profiles during fermentation (d) Identification and quantification of volatile aroma compounds during fermentation (e) correlation of sensory properties, aroma components and antioxidant profiles using principal component analysis (PCA). Thus a simpler protocol for screening various plethora of selenium incorporations is assessed through a concrete and cost-effective methodology.
Materials and methods
The methodology utilizes a simple protocol that can be rationalized for applications in enhancing a variety of organic selenium sources (Figure S1).
Preparation of mulberry fruit sample
Black-colored mulberry fruits—Morus nigra was mixed to maintain Brix value of 19 with pH of 4.7 and 6.2 g/L acidity. Mulberry fruits were obtained from the mulberry farm at Zhenjiang, Jiangsu, China. After washing the fruits with 0.02% sodium hypochlorite, the preparation of wine was preceded by rinsing with sterile water and was stored at -20 °C.
Chemicals and reagents
PC-3 pectinase was purchased from Nuo Enzyme Productivity Promotion Co., Ltd (Tianjin, China). Potassium metabisulfite was purchased from DAL CIN, Italy. L-Selenium-Methylselenocysteine (L-SeMC) was procured from Chuanqi Pharmaceutical Co., Ltd (Jiangxi, China). Chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. included Folin-Ciocalteu reagent, rutin, 2,2-diazobis-3-ethylbenzene, thiazoline-6-sulfonic acid diamine salt, 1,1-diphenyl-2-trinitrophenylhydrazine, etc.
Preparation of samples
Preparation of selenium-enriched active yeast
The seed culture was prepared according to several manifested protocols. Precisely, a ring of Saccharomyces cerevisiae stored in CECA (Angel Yeast Co., Ltd., Hubei, China) was picked up and streaked onto the sterilized YPD solid medium. The yeast was cultured and incubated in an incubator (25 °C for two days). Then, a single colony was selected from the solid medium and was inoculated into 100 mL of YPD liquid medium. Subsequent culturing in a shaker at 28 °C and 150 rpm for 24 h was performed. The optimized fermentation parameters include the addition of seed solution inoculum with 4.6%, sodium selenite (Na2SeO3) for 4 h of fermentation (40 μg/mL) with selenium enrichment time for 58 h.
Preparation of selenium-enriched yeast extract
The extract of Angel Selenium Enriched Yeast Nutritional Additive (Angey Yeast Co., Ltd., Hubei, China) was prepared as per the following methodology. First, the compound protease (obtained from Nanning Donghenghua Road Biotechnology Co., Ltd., Guangxi, China) was added and final composition of 0.32% was obtained. The enzymatic hydrolysis temperature and the enzymatic hydrolysis time were 54 °C and 12 h, respectively. The final concentration of the yeast suspension was made up 4.6%.
Mulberry wine preparation
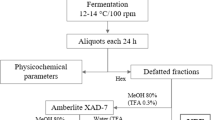
The mature and non-spoiled mulberries were selected as the raw material. After the mulberries were beaten, 0.2% pectinase was added. The mulberry juice was centrifuged at 4000 rpm for 15 min and heated in a water bath (40 °C) for 90 min. Mulberry juice (1000 mL), 4.6% (v/v) yeast inoculum (107 CFU/mL), and potassium metabisulfite (120 mg/L) were added to eight 2-L fermentation vessels (MW1–MW8) to initiate alcohol fermentation. These sample mixture was fermented for 7 days at 25 °C and kept in a orbital shaker at 160 rpm. Then 200 μg/L of other selenium nutritional additives were added before and after the fermentation of mulberry wine. The detailed fermentation and addition methods are shown in Fig. 1a.
Evaluation of enological parameters
Physicochemical parameter analysis
LIDA Instrument PHS-3C Precision pH/mV meter was utilized for pH estimation. WYT 0–80° Brix Meter was used to estimate Soluble solids (TSS), Total Acidity (TTA), Total Volatile Acidity (TVA), and Total Alcohol Content (TAL). Physicochemical parameters were done according to the Chinese National Standard GB/T 15038–2006 (General Analysis Method for Wine and Fruit Wine).
Color evaluation
The color detection (TCD) was evaluated using the method of Zhang et al. (2017). Mulberry wine was prepared to detect the absorbance of the sample at wavelengths 420, 520 and 620 nm. The color intensity and hue angle of mulberry wine was calculated according to Eqs. (1) and (2). The contribution of each color (yellow, red and blue) to the overall color of mulberry wine were calculated by dividing the absorbance at 420 nm (Ye%), 520 nm (Re%) and 620 nm (Bl%). The color intensity was calculated using the formula.
Phytochemical composition
Determination of total anthocyanin content
Dai et al.(2009) method was used to determine the total anthocyanin concentration (TAC). In brief, 1 mL sample was placed in two 10 mL volumetric flasks. The volume was adjusted with pH 1.0 (0.2 mol/L KCl: 0.2 mol/L HCl = 25: 67 (v/v)) buffer solution and pH 4.5 (1 mol/LNaAc: 1 mol/L HCl: H2O = 100: 60: 90 (v/v/v)) buffer solution, respectively. The solutions were allowed to stand at room temperature for 2 h. The absorbance of the two samples was measured at wavelengths of 510 nm and 700 nm, respectively. Anthocyanins were calculated using Eq. (1) and expressed using corn color cyanidin 3-glucoside (C3G).
where MW refers to the molecular weight of C3G = 449.2 mol/L, DF corresponds to the dilution factor, ε—The molar absorbance (26,900 L/mol cm), and L accounts for the optical path length of the cuvette (1.0 cm).
Determination of total polyphenol content
The Folin-Ciocalteu method described by Bajčan et al. (2013) was used to estimate total polyphenol content. Briefly, 0.2–1.2 mL of the gallic acid standard solution (100 μg/mL) was taken in six test tubes and the volume was made up to 25 mL with distilled water. Without sample served as the blank. Subsequently, 1.0 mL of Folin-Ciocalteu reagent was added. Then, the tubes were permitted to stand for 5 min after vortexing at room temperature. After that, 4.0 mL of Na2CO3 (12%), the solution was added and kept at 25 ℃ in the dark for 2 h. The absorbance was measured at 760 nm in a UV spectrophotometer (UV-1600) against a blank. Similarly, 1 mL of mulberry wine was diluted 50 times and the polyphenol content was calculated. Finally, total phenol content was estimated in the samples and expressed as mg gallic acid equivalents/g sample.
Determination of total flavonoids
The aluminum chloride method was employed to evaluate the flavonoid content in mulberry wine Magalhães et al. (2012). Initially, a standard rutin solution with a mass concentration of 0.2 mg/mL was prepared. Distilled water and reagents mixture served as a blank. Briefly, different volumes of the standard solution (2, 4, 6, 8, 10 and 12.0 mL) were taken in a 50 mL volumetric flask and the final volume was made up to 20 mL of distilled water. Further, 2 mL of sodium nitrate (5%) solution was added, mixed and allowed to stand for 6 min. Then, 2.0 mL of AlCl3 (10%) solution was added and the mixture was allowed to stand for 6 min. To the above mixture, 4 mL of NaOH solution was added and the volume was adjusted to 50 mL with distilled water. The absorbance was measured at 510 nm using a UV spectrophotometer (UV-1600) against a blank (distilled water and reagents) to prepare a standard curve. Similarly, a 1 mL mulberry wine was measured and the content of total flavonoids was calculated according to the standard curve.
Antioxidant assays
2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging
Kwaw et al. (2018a) adopted a modified method for estimating DPPH radical scavenging activities. Briefly, 2 mL of ethanolic DPPH (0.1 mM) solution was added with 2 mL of 20-fold diluted mulberry wine sample. The mixture was vortexed well and kept in the dark for 30 min. The absorbance (As) of the sample was measured at a wavelength of 517 nm using a UV–VIS spectrophotometer. The solution mixture of 2 mL of ethanol added with 2 mL of DPPH solution served as control. The DPPH radical scavenging ability of the sample was expressed by the formula (4).
ABTS radical cation scavenging activity
The ABTS radical scavenging activity was determined by the method of Chaves et al. (2020). ABTS solution (7.4 mmol/L) and the potassium persulfate solution (2.6 mmol/L) were mixed in equal volumes and stored in the dark at an ambient temperature for the generation of cation radical. The solution prepared are optimized with double distilled water to set 0.700 OD at 734 nm. Further, 2 mL of mulberry wine sample was mixed with 2 mL of freshly prepared ABTS solution. The solution mixture was shaken well and allowed stand for 6 min. The absorbance (As) of the sample was measured at 734 nm by a UV–VIS spectrophotometer. The absorbance (A0) of the without sample ABTS working solution was measured under the same conditions. The ABTS radical scavenging ability was measured according to formula (5).
Determination of hydroxyl radical scavenging
The hydroxyl radical scavenging ability (HRSA) was determined by the method described by Çelik et al. (2010). Alcohol salicylate solution (6 mM), ferrous sulfate solution (6 mM) and hydrogen peroxide solution (6 mM) were prepared. The 20-fold diluted mulberry wine sample (2 mL), ferrous sulfate solution (2 mL) and hydrogen peroxide solution (2 mL) was added to the test tube 1. Further, it was mixed and allowed to stand for 10 min. Salicylic acid solution (2 mL) was added to the sample group (A1). Then, 2 mL each of ferrous sulfate, salicylic acid, and mulberry wine samples were added to test tube 2 (A2). The solution mixture containing 2 mL of ferrous sulfate, salicylic acid, and hydrogen peroxide was served as the blank control group (A0). Each test tube was placed in a water bath (37 °C for 30 min), and the absorbance value was measured at 510 nm. The HRSA of the sample was expressed by formula (6).
Ferric reducing antioxidant power (FRAP) capacity
The FRAP assay was performed by Haida and Hakiman (2019) method. The mulberry wine sample (2 mL) was diluted 50-fold, and phosphate-buffered saline (2.5 mL, 0.2 mol/L, pH 6.6) and potassium ferricyanide solution (2.5 mL, 1% (w/v)) were added to the test tube. The mixture was incubated in a water bath at 50 °C for 20 min. After cooling, 2 mL of 10% trichloroacetic acid solution (w/v) was added and centrifuged at 4000 rpm for 10 min. Ferric chloride solution (0.5 mL 0.1% (w/v)) was added to the supernatant (2 mL) and made up to 6 mL with distilled water. After mixing, the absorbance was measured at a wavelength of 700 nm. The absorbance value reflected the reducing power. Strong reducing power detected was corroborated to greater absorbance.
Determination of total selenium content (TSC)
The selenium-enriched yeast sample (0.2–0.5 g) was placed in a microwave digestion tank, and nitric acid (5–10 mL) was added and placed for 1 h or overnight. The completion of the reaction between the sample and nitric acid was regarded as the endpoint. Microwave digestion refers to the national standard GB 5009.268–2016. The heating program corresponds to the digestion employing temperature at 120 ℃ for 5 min, 150 ℃ for 10 min, and 190 ℃ for 20 min (the temperature rise in each stage was controlled at 5 min). After the digestion was completed, ultrasonic degassing was performed for 5 min. The volume was adjusted to 25 mL for later use.1000 μg/mL selenium standard solution was used to prepare 0.0, 0.1, 1.0, 5.0, 10.0, 30.0, 50.0, 100.0 μg/L corresponding to 8 gradients of selenium standard solution. The solution was kept on the machine to be tested, a standard selenium concentration curve was drawn, and the response value was calculated. ICP-MS detection conditions encompass radiofrequency power of 1500 W, plasma gas flow rate of 15 L/min, carrier gas flow rate of 0.8 L/min, the auxiliary gas flow rate of 0.8 L/min, and helium gas flow rate of 4 mL/min. The atomization of the chamber temperature was 2 °C. The sample lifting rate was 0.3 r/s. The method was repeated thrice with the sample to assess reproducibility.
Determination of electronic nose
The instructions depicted in the machine was used to calibrate the PEN3 electronic nose with acetone, isopropanol and propanol. Initially, 5 mL of each mulberry wine sample was taken in a 20 mL headspace bottle. The sample was equilibrated for 5 min for headspace enrichment before analysis. Then, the measurement phase lasted for 250 s, long enough for the sensor to reach a stable signal value. Next, the clean gas was pumped into the sample gas channel for 300 s to normalize the sensor signal. The response value of the sensor was defined as G/G0, where G0 and G are the conductivity of the activated carbon filter gas and the volatile sample gas detected by the sensor, respectively. The PEN3 electronic nose detector was composed of 10 kinds of sensor elements, and the sensitive substances corresponding to each sensor are different, as shown in Fig. 1b (Ekumah et al. 2021).
Determination of volatile flavor substances
Briefly, 5 mL mulberry wine samples and 1 g NaCl were added into a 20 mL headspace bottle and heated and balanced at 40 ℃ for 2 min. The extraction needle (50/30 μm DVB/PDMS/CAV) was inserted into the headspace flask for extraction for 15 min, and the magnetic stirring speed was 250 rpm/min. After extraction, the sample was analyzed at 250 ℃ for 5 min at the injector.
The gas chromatographic column was sh-RXI-5SIL MS (30 m × 0.25 mm × 0.25 µm) with 5% phenyl and 95% dimethylpolysiloxane as stationary phase. Carrier gas flow rate (helium, 99.999%) was 1 mL/min without a shunt. The interface temperature of the MS was 250 ℃, electron energy bombardment ionization was 70 eV, ion source temperature was 230 ℃, mass scan range was 45–450 amu, and the detection start time was 3 min. Other parameters were set according to the software parameters. The programmed temperatures are initial temperature with escalations at 40 ℃ > 100 ℃ for 2 min at 5 ℃/min > 160 ℃ for 2 min at 6 ℃/min > 220 ℃ for 5 min.
The volatile compounds were compared with the data in nist17 (National Institute of Standards and Technology mass spectrometry Library). In addition, qualitative analysis was carried out by the material retention index and the sh-rxi-5sil MS column reported in the literature (Kieliszek et al. 2019). The quantitative method uses the area normalization method to express the relative percentage content of each volatile substance.
Result and discussion
Effects of different selenium sources on physicochemical parameters
Physical and chemical indexes of mulberry wine were assessed to estimate the effects of various selenium sources (Table 1). The results are affirmative that the TSS of MW2 and the pH decreased significantly compared with MW1. The Physicochemical changes may be accounted for the inherent effects of yeast after the treatment of inorganic selenium salts. Stimulus reaction mechanisms such as antioxidant, Kleberdril effect, and carbon catabolite inhibition were incorporated. Assessment of carbon sources in correlating metabolism efficiency in producing ethanol and various acidic metabolites defines the effective selenium source (Ekumah et al. 2021). The consistent chemical reactions correlate to a significant negative correlation (R2 = − 0.812) between TSS and TAL. Furthermore, the correlation was supported by the highly substantial negative correlation between PH and TTA, TAL, and TVA. A significant positive correlation between TTA and TVA (R2 = 0.867), shows significant variations. A similar trend was observed between TAL and TVA (R2 = 0.886) (Table 1). No significant difference in indicators (except pH), between the other groups and the blank MW1 except MW2 was observed. The addition of a selenium source before fermentation significantly impacted the physical and chemical properties of mulberry wine. The effect of the indicator may be due to the low amount of selenium addition. Hence optimization of the amount of selenium will have profound implications in upscaling from laboratory to industrial scale applications and associated variations in quality, yield and aroma of the wine. A recent study showed the usage of optimized Se-enriched mulberry wine (OpSeMW) over ordinary wine after fermentation optimization by BBD—Box-Behnken design and RSM—Response surface methodology (Johnson et al. 2023). However, the present work is simpler in design, execution and cost-effective, when compared to the above study. Nano-Se based soy sauce showed increased antioxidant profiles and was reported as a novel method for segmenting organic selenium content. However, the aroma quality and taste of the soy sauce has not been reported (Chen et al. 2022). Furthermore, research reports have stressed the utility of Se-biofortified plants and foods with increased nutraceutical and bioactivity profiles (D’Amato et al. 2020). Pueraria lobata plant roots fortified with Se have been shown to possess anti-cancer proteins and polysaccharides rich in Se content (Zou et al. 2014), Thus the selenium incorporation and fortification in wine and other foods require a cost-effective simpler protocol for augmented applications in nutraceutical and therapeutic screening strategies. The present preliminary assessment depicts the usage that can act as Launchpad for fermentation biologists. Further, determining the largescale production of Se-enriched mulberry wine production with S. cerevisiae for further toxicity studies and enhanced associative nutraceutical efficacy will reinvigorate the biomedical context.
Effects of different selenium sources on mulberry wine color characteristics
The influence of different concentrations of selenium sources on the TCD of mulberry wine was expressed by color intensity (CI), hue angle (CH), yellow (Ye%), red (Re%) and blue (Bl%). The results indicate that except for the mulberry wine with selenium source added after fermentation, the CI and Re% of the other groups increased, and the Ye% decreased compared with the blank MW1 (Table 2). The color of mulberry wine was mainly affected by anthocyanins (TAC) (Kwaw et al. 2018b). The significant positive correlation between CI and Re% and TAC (R2 = 0.978) was similar to the results of Tao et al. (2017) (Table 1). The significant negative correlation between Ye% and TAC also supported our assertion, consistent with the results of Kelebek (2010). The o-hydroxyphenols of phenols are easily oxidized into o-quinone or semiquinone free radicals. These quinone free radicals were unstable and prone to condensation reactions to form colored macromolecule substances (Kwawet al. 2018), affecting the color of fruit wine. The color of the wine is enhanced due to the decrease in CH and the increase in TAC (Zhang & Wang 2017) (Table 3). The variability patterns correspond to the significant negative correlation between CH and TAC (R2 = − 0.958) (Table 4). A similar trend was consistent with the results of earlier reports with similar statistical significance (Kwaw et al. 2018c). TFC and TAC had a very significant positive correlation (R2 = − 0.919) (Table 4), as evident from the present assessment. Anthocyanins are regarded as an essential flavonoid component amongst other flavonoids including flavonols, flavanones, isoflavones, anthocyanins and flavans (Liu et al. 2018a, b, c). Various concentration of selenium supplementation improves the organic characteristics as revealed by the increased flavonoid content in the present study. Significantly, the color of the mulberry wine enhances the wine quality apart from discrepancies in low-level/high-level supplements correlating to the selenium supplementation, prior to fermentation.
Effects of different selenium sources on phytochemical components
The results of the TAC, TPC, and TFC of mulberry wine with different selenium sources are represented in Fig. 2. Phytochemicals in mulberry wines supplemented with selenium exhibited an increasing trend (p < 0.05) compared to those in the blank MW1. Significant variations were observed between the selenium-enriched yeast fermentation (MW2) and the addition of L-SeMC selenium source (MW5) before fermentation (MW5). After fermentation, there was no discernible difference when various selenium sources were added (MW6, MW7, MW8). Thus, the quality of mulberry wine is dependent on the supplementation of selenium before and after fermentation corroborating with microbial biotransformation. The range of TAC was obtained with 32.99–44.45 mg/L, wherein MW5 had the highest TAC with a growth rate of 26.72%. Pearson's analysis (Table 5) showed a significant positive correlation between anthocyanin and selenium content. Similar findings have already been documented and are consistent with our present findings (Barbulescu et al. 2012). Thus, selenium supplementation during wine fermentation substantially enhances anthocyanin levels. The inclusion of selenium with Saccharomyces cerevisiae cells increases TAC levels, consistently. Hence, the improved activity of intracellular antioxidant enzymes such as glutathione peroxidase (GSH-Px) and glutathione reductase (GR) (Kieliszek et al. 2019) are affirmative to the obtained results. Thus, yeast secretes potential antioxidant enzymes into the fermentation broth, which degrade anthocyanins when dissolved in the media.
There was a significant difference between the TPC of MW3 and MW4 (p < 0.05). The impact mentioned may be caused by the wall-breaking treatment that increases the solubility rate of selenium-containing substances. Further, the concentration of selenium-containing enzymes in the selenium-enriched yeast reveals variable profiles. Pearson’s analysis demonstrates that the phenolic compounds of mulberry wine are preserved during fermentation (Table 5). Thus, the TPC and selenium contents were substantially correlated (p < 0.05), indicating the presence of protective phenolic compounds. The results corroborate the hypothesis that selenium supplementation enhanced the amount of glucosidase enzyme produced by yeast (Ekumah et al. 2021). Thus, selenium supplementation hydrolyzes complex phytochemicals, increasing the solubility of phenolic compounds. Additionally, Saccharomyces and Lactobacillus-produced esterases contribute to the hydrolysis of glycoside ester bonds, thereby releasing soluble and insoluble phenolic substances attached to phytoconstituents (Kwaw et al. 2018c; Minnaar et al. 2019). The effect of selenium content on TFC (R2 = 0.996) was consistent with the impact of TAC and TPC (Gąsecka 2015). Holistically, selenium supplementation improves quality, color, and phenolic compounds before fermentation.
Effects of different selenium sources on antioxidant activity
The antioxidant activities that define the beneficial health effects of red wines in each sample are tabulated in Table 3. MW1 exhibited a considerable amount of DPPH radical scavenging activity (45.02%), indicating a strong antioxidant potential (Hur et al. 2014). The antioxidant effect of MW2 (48.34%) and MW5 (48.50%) was significantly greater than that of MW1. The DPPH radical scavenging ability of MW5 was substantially higher than MW8 (46.27%) (p < 0.05). According to Irondi et al. (2019), the results indicate that the antioxidant potential of DPPH•-SA with L-SeMC addition is minimal after fermentation. Recent studies demonstrated that selenium supplementation enhanced the bioavailability of selenium compounds such as selenomethioinine and selenocysteine. The phenolic compounds with H+ donating ability obtained from mulberry wine fermented by selenium-supplemented yeast neutralize the DPPH free radicals (Kieliszek et al. 2019).
Additionally, the results indicated that samples treated with selenium exhibited higher antioxidant activity in the presence of ABTS radical scavenging electron transfer (Table 3). MW2 showed a significant ABTS radical scavenging capacity (55.71%), which was greater than that of blank MW1 (35.79%). Previous research indicates that the acidic or phenolic hydroxyl groups present in phenolic substances are flavonoids with associated antioxidants. The resonance between the aromatic benzene ring of the phenolic substance and the free electron pair on the phenolic oxygen leads to increased electron delocalization and enhanced antioxidant activity. Therefore, the antioxidant activity of a substance may vary based on its chemical properties (Aydın & Mammadov 2017). The above research corroborates the significant positive association shown in this study between ABTS· + SA and TFC (R2 = 0.916) and TAC (R2 = 0.916) (Table 5). Thus molecular mechanisms underlying the positive results from the present study are ascertained.
Hydroxyl radical scavenging ability was detected in the variation range of 47.61–58.91%. Significant differences between MW2, MW5, and MW7 and blank MW1 were observed (p < 0.05); however, the blank control was comparatively non-significant. It has been demonstrated that the reducing power of mulberry wine is correlated to electron transfer in reducing Fe3+ to Fe2+ and the termination of the free radical chain reaction (Kwawet al. 2018; Kieliszek 2019). The reducing power varies from 0.64 to 0.77. In vitro antioxidant activity possesses the one-electron transfer reaction or the hydrogen atom transfer reaction of antioxidants or oxidants to free radicals. (Lü et al. 2010). According to Pearson analysis, a significant relationship is found between phenolics and the four antioxidant properties. The antioxidant activity of phenolic compounds is determined by the group attached to the base aglycone, which defines the strength of the antioxidant capacity. Glycosides associated with flavonoid groups, such as flavonols or anthocyanins, disrupt the coplanarity resulting in decreased electron delocalization and diminished antioxidant activity (Pérez-Gregorio et al. 2011).
Effects of different selenium sources on the selenium content
Different selenium sources on the total selenium content (TSC) effect of mulberry wine are shown in Fig. 3. The highest TSC of MW8 was 54.54 μg/mL, which was 3.75 times higher than the blank control MW1. Consequently, L-SeMC supplementation had the most significant impact on improving the selenium content of mulberry wine. The TSC of mulberry wine was lower than that of mulberry juice, with a loss rate of 38.88%, demonstrating that fermentation had a greater effect on the selenium content. The selenium content of MW8 was significantly higher than that of MW5, confirming variable patterns of selenium supplementation. The correlation between selenium content and phenols showed that selenium significantly correlated with total anthocyanin content (R2 = 0.922). Moreover, the results correlate significantly with total polyphenol content (R2 = 0.971) and flavonoid content (R2 = 0.996). Saccharomyces cerevisiae had a high bioconversion rate of selenium (Adadi et al. 2019). Hence the use of selenium-enriched yeast to ferment mulberry juice is proven as an innovative and optimal method for selenium enrichment. Further, L-SeMC as a selenium-enriched active yeast fermentation shows prominent implications for cost-effectiveness and economic feasibility.
Analysis of mulberry wine samples by electronic nose
The electronic nose equipped with ten metal oxide semiconductor sensors estimated the flavor of mulberry wine with different selenium sources. The response value of each substance tends to stabilize in a smaller duration of 50 s. The radar chart (Fig. 4) structurally illustrates the difference in response values between 8 samples. In previous study, W2S was sensitive to alcohol aromatic compounds (Xu et al. 2019). Similarly, the MW2 sample had the most considerable response value in the W2S sensor, indicating the higher alcohol concentration in the MW2 sample (Table 1; Fig. 1b). In a similar study, the W1W sensor responded to sulfur compounds such as H2S and was sensitive to terpenes and organic sulfur compounds (Xu et al. 2019). All groups of mulberry wine showed the most significant response value to the W1W sensor. SO2 in the fermentation of mulberry wine inhibits the growth of miscellaneous bacteria attributing to prominent response values. Mulberry wine is sensitive to W1W sensors due to a series of enzymatic reactions during the brewing process and the yeast’s catabolism of sulfur-containing amino acids (cysteine) in the fermentation broth (Yu et al. 2014). Additionally, each group of mulberry wine exhibits a more excellent response value to the W5S sensor, as previously demonstrated by Xu et al. (2020). Moreover, the W5S sensor was sensitive to nitrogen and oxygen compounds (Xu et al. 2019). During mulberry wine fermentation, amino acids and proteins are hydrolyzed and a portion of the pectin is dissolved and increases the amount of soluble nitrogen in the fermentation broth (Zhao et al. 2020). Through chemical and electronic study of the nose, it was revealed that MW2 includes the class of more ethanolic, highly acidic and a higher concentration of aromatic compounds.
Analysis of mulberry wine samples by GC–MS
Volatile flavor compounds significantly influence the aroma quality of mulberry wine. The entire aroma of fruit wine constitutes fruity volatiles, fermentation volatiles, and volatiles formed during aging (Table 6) (Liu et al. 2018a, b, c). Approximately, 57 volatile aroma components were identified and quantified using GC–MS in eight different mulberry wines. The cataloguing includes different alcohols (six types), esters (36), acids(3), aldehydes (2), ketones (1), ethers (1), and phenols (2), alkanes (3) and others (3) (Figure S2).
Mulberry wines with different selenium sources revealed the presence of various aroma components. Yeast converts sugar into ethanol during the fermentation process, producing various by-products. The inclusive products are composed of esters, acids, aldehydes, ketones, terpenes and other volatile compounds, which contribute to the aroma of wine (Gamero et al. 2015; Cao et al. 2020). As shown in Table 6, the most prevalent volatile flavoring compounds were alcohols and esters. The relative percentage change of alcohol content between each group of mulberry wine ranged from 18.71 to 38.16%. Alcohols contained a relatively high proportion of isoamyl alcohol, 2-methylbutanol, 2,3-butanediol, and phenethyl alcohol. The relative content of isoamyl alcohol in each group of mulberry wine was higher and consistent with the results of Wang et al. (2015). Isoamyl alcohol was generated from phenylalanine through decarboxylation and reductive deamination reaction. Wherein, isoamyl alcohol poses the important volatile flavor substance in wine, with a pleasant rose and honey aroma (Fiorini et al. 2014). The relative content of phenylethyl alcohol in mulberry wine showed aroma variations. Phenylethyl alcohol was produced by yeast using L-phenylalanine as a precursor and was made through various metabolic pathways (Pentose phosphate pathway, Ehrlich pathway, and shikimic acid pathway) (Bolat et al. 2013). Thus, the pathways mentioned above impart a distinctive rise in aroma with the copious flavor compounds of MW2 and MW4 mulberry wine (Fig. 5).
The formation of esters includes the synthesis and metabolism of esters and the esterification between alcohols and organic acids. Esters are essential for enriching the aroma of fruit wine (Niu et al. 2019; Belda et al. 2017). There were a total of 36 esters found in various mulberry wines, and their relative content varied from 60.96 to 79.90%. Among them, the relative content of ethyl caprylate in each group of mulberry wine esters was the largest, accounting for the total aroma content variation range of 11.59–42.75%. According to earlier studies, the formation of ethyl caprylate (with a sour apple flavor) was easily influenced by temperature, and high temperatures were advantageous to its production. (Saerens et al. 2008). The large-scale growth and reproduction of yeast during fermentation generate yeast heat. The actual temperature of the fermentation broth higher than the control temperature results in a high content of ethyl caprylate. The relative content of other esters was relatively small but increased the complexity of the aroma of mulberry wine. For example, ethyl caproate gives an aroma to the mulberry wine of banana and pineapple, and ethyl caproate with a fruity aroma similar to grapes (Bolat et al. 2013). The reaction between isobutanol and acetic acid in fruit wine produces a floral aroma, which is similar to the flavor of isobutyl acetate. Whereas isoamyl acetate in fruit wine had a stronger aroma than bananas (Peng et al. 2015). Alcohols and esters significantly impact the production of mulberry wine flavor and are the fundamental building blocks of mulberry wine aroma. The results obtained affirms the chemical constituents and their corresponding impact in aroma of mulberry wine.
Analysis of mulberry wine samples by principal component analysis (PCA)
Analysis and evaluation of various quality indicators of different groups of mulberry wine were deciphered. The results of basic physical and chemical indicators (pH, TSS, TTA, TAL and TVA), color indicators (CI, CH, Ye%, Re% and Bl%), phytoactive ingredients (TAC, TPC and TFC), antioxidant activities (DPPH, ABTS, HRSA and FRAP), TSC, and GC–MS components were subjected to PCA. The characteristic values of PCA of various indexes of mulberry wine in different groups are shown in Supplementary Table S1. The scattered biplot (Fig. 6) further demonstrated that the samples differed significantly from one another. The cumulative contribution rate of the first four principal components is 91.953% (Supplementary Table S1). Hence the first four principal components are affirmative in evaluating the comprehensive quality of different mulberry wines. The comprehensive mulberry wine quality score with varying fermentation methods was calculated. The first two principal components (PC) PC1, 44.19% and PC2, 18.24, accounted for the majority (62.43%) of the sample variations. PC1 demonstrated that the samples MW2 and MW5 were highly correlated with the parameters including physico-chemical, chromatic indicators, phytochemicals, antioxidant activities and certain GC–MS compounds (Fig. 6). Additionally, the PC2 categorized the different samples based on indicators of wine quality. Based on the strength of the relationship between the quality attributes and the principle components, it may be concluded that a cluster of variables properly characterized each sample. The control MW1 and MW3 are situated on the negative terminal of PC1, whereas the positive axis of PC2 is characterized by a pH, TSS, and alcohols that indicate the poor quality of mulberry wine. Similarly, the samples MW6, MW7 and MW8 were characterized by alkanes, phenols, ethers and ketones complemented with Ye% and CH. The sample MW4 is located on the positive terminal of PC1 and is characterized by phytochemicals, selenium content, antioxidant activities and some color attributes indicating the medium quality of mulberry wine. The samples MW2 and MW2 are situated on the positive axis of PC1, characterized by high phytochemicals (TAC, TFC, TPC, aldehydes, acids, esters and other chemicals) and selenium content. The PCA values complementthe presence of TAA, TTA, TVA, total aroma, RE%, BI% and high association antioxidant activities suggesting the higher quality of mulberry wine (Fig. 6). The primary components of several groups of mulberry wine were classified as MW2, MW5, MW4, MW3, MW7, MW6, MW8, and MW1 in order of high to low quality (Supplementary Table S2). The sensory evaluation results indicate that the evaluation model has a certain degree of reliability with consistency. The main composition distribution points among eight groups of mulberry wine reveal four categorical score values (Fig. 6). The present study provides authenticated mulberry wine fermentation with different selenium sources based on a simpler and cost-effective methodology. However, future research on economic feasibility and largescale applications will benefit fermentation optimization with reduced toxicity in produing selenium based mulberry wine as a neutraceutical component.
Conclusion
The preparation of mulberry wine using various selenium sources and analysis of physical and chemical indicators revealed that selenium-enriched active yeast improves carbon source utilization. The quality of mulberry wine fermented with selenium-enriched active yeast (MW2) and mulberry wine fermented with L-SeMC added before fermentation (MW5) was evaluated. According to CI, selenium supplementation before fermentation resulted in higher quality and aroma than in addition after fermentation. Re%, total polyphenols, flavonoids, anthocyanin content, and selenium content weregreater in the experimental group than in the control group (p < 0.05). The correlation between the total anthocyanin content of mulberry wine and CI and Re% was highly significant (p < 0.01). Fermentation leads to the loss of selenium content, with a loss rate of 38.88%. The most effective method for raising the selenium level of mulberry wine was to use L-seMC. The significant antioxidant capability and the variety of aroma compounds indicate that MW2 was better quality. Although several fermentation-optimization and selenium incorporation studies have been reported, the present study renders a small-scale fermentation rational upscaling economical strategy. The strategy enumerated in the present approach involves screening various selenium sources for total selenium content before fermentation inclusion. Phytochemical characteristics, aroma, quality, antioxidant profiles, sensory analysis GC–MS profiling and principal component analysis. The preliminary assessment showed sufficient evidence for supplementing with various selenium sources. Nevertheless, toxicology experiments and fermentation optimization coupled with sensory evaluation should be affirmative for future commercialization aspects.
Data availability
All datasets generated for this study are included in the article/Supplementary Material.
Code availability
Not applicable.
Abbreviations
- PC-3:
-
Pectinase
- L-SeMC:
-
L-selenium-methylselenocysteine
- DPPH:
-
1,1-Diphenyl-2-trinitrophenylhydrazine
- ABTS:
-
2,2-Diazobis-3-ethylbenzene, thiazoline-6-sulfonic acid diamine salt
- Na2SeO3 :
-
Sodium selenite
- KCl:
-
Potassium chloride
- HCl:
-
Hydrochloric acid
- C3G:
-
Cyanidin 3-glucoside
- Na2CO3 :
-
Sodium carbonate
- AlCl3 :
-
Aluminum chloride
- NaCl:
-
Sodium chloride
- CFU:
-
Colony forming unit
- TSS:
-
Soluble solids
- TTA:
-
Total Acidity
- TVA:
-
Total volatile acidity
- TAL:
-
Total alcohol content
- CI:
-
Color intensity
- CH:
-
Hue angle
- TAC:
-
Total anthocyanin concentration
- TPC:
-
Total phenolic concentration
- TFC:
-
Total flavonoid concentration
- PCA:
-
Principal component analysis
- pH:
-
Potential of hydrogen
- UV:
-
Ultra violet
- VIS:
-
Visible
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- PEN3:
-
Portable electronic nose
- GC–MS:
-
Gas chromatography–mass spectrometry
References
Adadi P, Barakova NV, Muravyov KY, Krivoshapkina EF (2019) Designing selenium functional foods and beverages: A review. Food Res Int 120:708–725. https://doi.org/10.1016/j.foodres.2018.11.029
Aydın C, Mammadov R (2017) Phenolic composition, antioxidant, antibacterial, larvacidal against Culex pipiens, and cytotoxic activities of Hyacinthella lineata steudel extracts. Int J Food Prop 20(10):2276–2285. https://doi.org/10.1080/10942912.2016.1236271
Bajčan D, Harangozo Ľ, Hrabovská D, Bončíková D (2013) Optimizing conditions for spectrophotometric determination of total polyphenols in wines using folin-ciocalteu reagent. J Microbiol Biotechnol Food Sci 2021:1271–1280
Barbulescu ID, Ferdes M, Bejan C, Rodica A, Teodorescu R (2012) Improving wine-making products with selenium and total polyphenols. Rom Biotechnol Lett 17(5):10
Belda I, Ruiz J, Esteban-Fernández A, Navascués E, Marquina D, Santos A, Moreno-Arribas M (2017) Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 22(2):189. https://doi.org/10.3390/molecules22020189
Bolat I, Romagnoli G, Zhu F, Pronk JT, Daran J-M (2013) Functional analysis and transcriptional regulation of two orthologs of ARO10, encoding broad-substrate-specificity 2-oxo-acid decarboxylases, in the brewing yeast Saccharomyces pastorianus CBS1483. FEMS Yeast Res 13(6):505–517. https://doi.org/10.1111/1567-1364.12051
Cai Z, Zhang J, Li H (2019) Selenium, aging and aging-related diseases. Aging Clin Exp Res 31(8):1035–1047. https://doi.org/10.1007/s40520-018-1086-7
Cao Y, Wu Z, Weng P (2020) Comparison of bayberry fermented wine aroma from different cultivars by GC–MS combined with electronic nose analysis. Food Sci Nutr 8(2):830–840. https://doi.org/10.1002/fsn3.1343
Çelik SE, Özyürek M, Güçlü K, Apak R (2010) Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, Abts/persulphate and FRAP methods. Talanta 81(4–5):1300–1309. https://doi.org/10.1016/j.talanta.2010.02.025
Chaves N, Santiago A, Alías JC (2020) Quantification of the antioxidant activity of plant extracts: analysis of sensitivity and hierarchization based on the method used. Antioxidants 9(1):76. https://doi.org/10.3390/antiox9010076
Chen H, Yu W, Chen G, Meng S, Xiang Z, He N (2017) Antinociceptive and antibacterial properties of anthocyanins and flavonols from fruits of black and non-black mulberries. Molecules 23(1):4. https://doi.org/10.3390/molecules23010004
Chen J, Feng T, Wang B, He R, Xu Y, Gao P, Zhang ZH, Zhang L, Fu J, Liu Z, Gao X (2022) Enhancing organic selenium content and antioxidant activities of soy sauce using nano-selenium during soybean soaking. Front Nutr 9:970206
D’Amato R, Regni L, Falcinelli B, Mattioli S, Benincasa P, Dal Bosco A, Pacheco P, Proietti P, Troni E, Santi C, Businelli D (2020) Current knowledge on selenium biofortification to improve the nutraceutical profile of food: a comprehensive review. J Agric Food Chem 68(14):4075–4097
Dai J, Gupte A, Gates L, Mumper RJ (2009) A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: extraction methods, stability, anticancer properties and mechanisms. Food Chem Toxicol 47(4):837–847. https://doi.org/10.1016/j.fct.2009.01.016
Ekumah J-N, Ma Y, Akpabli-Tsigbe NDK, Kwaw E, Jie H, Quaisie J, Manqing X, Johnson Nkuma NA (2021) Effect of selenium supplementation on yeast growth, fermentation efficiency, phytochemical and antioxidant activities of mulberry wine. LWT 146:111425. https://doi.org/10.1016/j.lwt.2021.111425
Feng Y, Liu M, Ouyang Y, Zhao X, Ju Y, Fang Y (2015) Comparative study of aromatic compounds in fruit wines from raspberry, strawberry, and mulberry in central Shaanxi area. Food Nutr Res 59(1):29290. https://doi.org/10.3402/fnr.v59.29290
Fiorini D, Caprioli G, Sagratini G, Maggi F, Vittori S, Marcantoni E, Ballini R (2014) Quantitative profiling of volatile and phenolic substances in the wine Vernaccia di Serrapetrona by development of an HS-SPME-GC-FID/MS method and HPLC-MS. Food Anal Methods 7(8):1651–1660. https://doi.org/10.1007/s12161-014-9802-1
Gamero A, Belloch C, Querol A (2015) Genomic and transcriptomic analysis of aroma synthesis in two hybrids between Saccharomyces cerevisiae and S. kudriavzevii in winemaking conditions. Microbial Cell Factor 14(1):128. https://doi.org/10.1186/s12934-015-0314-5
Gąsecka M, Mleczek M, Siwulski M, Niedzielski P, Kozak L (2015) The effect of selenium on phenolics and flavonoids in selected edible white rot fungi. LWT Food Sci Technol 63(1):726–731. https://doi.org/10.1016/j.lwt.2015.03.046
Grasemann H, Holguin F (2021) Oxidative stress and obesity-related asthma. Paediatr Respir Rev 37:18–21. https://doi.org/10.1016/j.prrv.2020.05.004
Haida Z, Hakiman M (2019) A comprehensive review on the determination of enzymatic assay and non-enzymatic antioxidant activities. Food Sci Nutr 7(5):1555–1563. https://doi.org/10.1002/fsn3.1012
Hur SJ, Lee SY, Kim Y-C, Choi I, Kim G-B (2014) Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem 160:346–356. https://doi.org/10.1016/j.foodchem.2014.03.112
Irondi EA, Adegoke BM, Effion ES, Oyewo SO, Alamu EO, Boligon AA (2019) Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci Hum Wellness 8 (2):142–148. https://doi.org/10.1016/j.fshw.2019.03.012
Johnson NA, Ekumah JN, Ma Y, Akpabli-Tsigbe ND, Adade SY, Manching X, Quaisie J, Kwaw E, Wang C (2023) Optimization of fermentation parameters for the production of a novel selenium enriched mulberry (Morus nigra) wine. LWT-Food Sci Technol 178:114608
Kelebek H, Canbas A, Jourdes M, Teissedre P-L (2010) Characterization of colored and colorless phenolic compounds in Öküzgözü wines from Denizli and Elazig regions using HPLC-DAD–MS. Ind Crops Prod 31(3):499–508. https://doi.org/10.1016/j.indcrop.2010.01.012
Kieliszek M (2019) Selenium–fascinating microelement, properties and sources in food. Molecules 24 (7):1298. https://doi.org/10.3390/molecules24071298
Kieliszek M, Błażejak S, Gientka I, Bzducha-Wróbel A (2015) Accumulation and metabolism of selenium by yeast cells. Appl Microbiol Biotechnol 99(13):5373–5382. https://doi.org/10.1007/s00253-015-6650-x
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kot AM (2019) Effect of selenium on growth and antioxidative system of yeast cells. Mol Biol Rep 46(2):1797–1808. https://doi.org/10.1007/s11033-019-04630-z
Kwaw E, Ma Y, Tchabo W, Apaliya MT, Wu M, Sackey AS, Tahir HE (2018a) Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem 250:148–154. https://doi.org/10.1016/j.foodchem.2018.01.009
Kwaw E, Ma Y, Tchabo W, Apaliya MT, Sackey AS, Wu M, Xiao L (2018b) Impact of ultrasonication and pulsed light treatments on phenolics concentration and antioxidant activities of lactic-acid-fermented mulberry juice. LWT - Food Sci Technol 92:61–66. https://doi.org/10.1016/j.lwt.2018.02.016
Kwaw E, Ma Y, Tchabo W, Sackey AS, Apaliya MT, Xiao L, Wu M, Sarpong F (2018c) Ultrasonication effects on the phytochemical, volatile and sensorial characteristics of lactic acid fermented mulberry juice. Food Biosci 24:17–25. https://doi.org/10.1016/j.fbio.2018.05.004
Liu M, Jing H, Zhang J, Che G, Zhou M, Gao Z, Li S, Ren Z, Hao L, Liu Y, Jia L (2016) Optimization of mycelia selenium polysaccharide extraction from Agrocybe cylindracea SL-02 and assessment of their antioxidant and anti-ageing activities. PLoS ONE 11(8):e0160799. https://doi.org/10.1371/journal.pone.0160799
Liu H, Yu F, Shao W, Ding D, Yu Z, Chen F, Geng D, Tan X, Lammi MJ, Guo X (2018a) Associations between selenium content in hair and Kashin-Beck disease/Keshan disease in children in northwestern China: a prospective cohort study. Biol Trace Elem Res 184(1):16–23. https://doi.org/10.1007/s12011-017-1169-x
Liu S, Liu E, Zhu B, Chai B, Liu R, Gao Q, Zhang B (2018b) Impact of maceration time on colour-related phenolics, sensory characteristics and volatile composition of mulberry wine: impact of maceration time on colour-related phenolics, sensory characteristics. J Inst Brew 124(1):45–56. https://doi.org/10.1002/jib.476
Liu Y, Tikunov Y, Schouten RE, Marcelis LFM, Visser RGF, Bovy A (2018c) Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: a review. Front Chem 6:52. https://doi.org/10.3389/fchem.2018.00052
Lü J-M, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14(4):840–860. https://doi.org/10.1111/j.1582-4934.2009.00897.x
Magalhães LM, Almeida MIGS, Barreiros L, Reis S, Segundo MA (2012) Automatic aluminum chloride method for routine estimation of total flavonoids in red wines and teas. Food Anal Methods 5(3):530–539. https://doi.org/10.1007/s12161-011-9278-1
Minnaar PP, du Plessis HW, Jolly NP, van der Rijst M, du Toit M (2019) Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: a strategy to improve the phenolic content of Syrah wine. Food Chem X 4:100070. https://doi.org/10.1016/j.fochx.2019.100070
Niu Y, Wang P, Xiao Z, Zhu J, Sun X, Wang R (2019) Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: an insight at the molecular level. Food Chem 275:143–153. https://doi.org/10.1016/j.foodchem.2018.09.102
Palomo-Siguero M (2016) Effect of selenite and selenium nanoparticles on lactic bacteria: a multi-analytical study. Microchem J. https://doi.org/10.1016/j.microc.2016.01.010
Peñas E, Martinez-Villaluenga C, Frias J, Sánchez-Martínez MJ, Pérez-Corona MT, Madrid Y, Cámara C, Vidal-Valverde C (2012) Se improves indole glucosinolate hydrolysis products content, Se-methylselenocysteine content, antioxidant capacity and potential anti-inflammatory properties of sauerkraut. Food Chem 132(2):907–914. https://doi.org/10.1016/j.foodchem.2011.11.064
Peng B, Li F, Cui L, Guo Y (2015) Effects of fermentation temperature on key aroma compounds and sensory properties of apple wine: effects of temperature on apple wine. J Food Sci 80(12):S2937–S2943. https://doi.org/10.1111/1750-3841.13111
Pérez-Gregorio MR, Regueiro J, Alonso-González E, Pastrana-Castro LM, Simal-Gándara J (2011) Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L.). LWT Food Sci Technol 44(8):1793–1801. https://doi.org/10.1016/j.lwt.2011.03.007
Saerens SMG, Delvaux F, Verstrepen KJ, Van Dijck P, Thevelein JM, Delvaux FR (2008) Parameters Affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol 74(2):454–461. https://doi.org/10.1128/AEM.01616-07
Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang C-R, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS (2013) Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38(2):225–236. https://doi.org/10.1016/j.immuni.2012.10.020
Tao Y, Wang Y, Yang J, Wang Q, Jiang N, Chu D-T, Han Y, Zhou J (2017) Chemical composition and sensory profiles of mulberry wines as fermented with different Saccharomyces cerevisiae strains. Int J Food Prop. https://doi.org/10.1080/10942912.2017.1361970
Wang X, Xie K, Zhuang H, Ye R, Fang Z, Feng T (2015) Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem 182:41–46. https://doi.org/10.1016/j.foodchem.2015.02.120
Xu J, Qi Y, Zhang J, Liu M, Wei X, Fan M (2019) Effect of reduced glutathione on the quality characteristics of apple wine during alcoholic fermentation. Food Chem 300:125130. https://doi.org/10.1016/j.foodchem.2019.125130
Xu X, Zhou S, Julian McClements D, Huang L, Meng L, Xia X, Dong M (2020) Multistarter fermentation of glutinous rice with Fu brick tea: effects on microbial, chemical, and volatile compositions. Food Chem 309:125790. https://doi.org/10.1016/j.foodchem.2019.125790
Yang X, Yang L, Zheng H (2010) Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol 48(8–9):2374–2379. https://doi.org/10.1016/j.fct.2010.05.074
Yu H, Dai X, Yao G, Xiao Z (2014) Application of gas chromatography-based electronic nose for classification of Chinese Rice wine by wine age. Food Anal Methods 7(7):1489–1497. https://doi.org/10.1007/s12161-013-9778-2
Yuan Q, Zhao L (2017) The mulberry (Morus alba L.) fruit—a review of characteristic components and health benefits. J Agric Food Chem 65(48):10383–10394. https://doi.org/10.1021/acs.jafc.7b03614
Zhang Q-A, Wang T-T (2017) Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem 234:372–380. https://doi.org/10.1016/j.foodchem.2017.05.022
Zhao C, Su W, Mu Y, Jiang L, Mu Y (2020) Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res Int 138:109800. https://doi.org/10.1016/j.foodres.2020.109800
Zou Y, Zhao T, Mao G, Zhang M, Zheng D, Feng W, Wang W, Wu X, Yang L (2014) Isolation, purification and characterisation of selenium-containing polysaccharides and proteins in selenium-enriched Radix puerariae. J Sci Food Agric 94(2):349–358
Acknowledgements
We thank the Zhenjiang City Key R&D Program-Modern Agriculture (SNY20200130052) project and the management of the Selenium Mulberry Beverage R&D team of Jiangsu University for their support of this study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
W.C.—Conducted the experiments, performed the data analyses and Writing-original draft; A.V.—performed the data analyses, Writing-original draft, review & editing; Y.M.—Conceptualization, Project administration, review & editing. H.J.—performed the data analyses; Writing-review & editing. A.K.R.—Review & editing; X.J. and L.D.—performed the data analyses and Writing-review & editing. The final manuscript was read and approved by all contributors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Congcong, W., Vinothkanna, A., Yongkun, M. et al. Production of mulberry wine using selenium-enriched Saccharomyces cerevisiae: implications from sensory analysis, phytochemical and antioxidant activities. J Food Sci Technol 61, 366–384 (2024). https://doi.org/10.1007/s13197-023-05847-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05847-4