Abstract
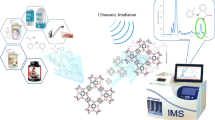
In the present study, a magnetic nano gel as the sorbent which is the combination of octatonic acid: cumarin as eutectic solvent and Fe3O4@SiO2 was introduced as the sorbent in ultrasound-assisted dispersive µ-solid phase extraction process coupled with high performance liquid chromatography with photo diode array detector for simultaneous separation and determination of tetracyclines residues in food samples. FT-IR, SEM, VSM were used for the characterization of the synthetized magnetic nano gel. Under obtained optimum conditions, the obtained linear ranges were 1.5–500 (µg L−1), 2.5–750 (µg L−1), 2–750 (µg L−1), and 2.5–500 (µg L−1) for tetracycline, oxytetracycline, chlortetracycline, and doxycycline, respectively. Moreover, the below level of quantification (BLQ) (based on S/N = 3) of 0.47 µg L−1, 0.11 µg L−1, 0.85 µg L−1, 0.66 µg L−1, 0.81 µg L−1 and the limit of quantification (based on S/N = 10) of 1.61, 2.74, 2.23 (µg L−1), and 2.66 were achieved for tetracycline, oxytetracycline, chlortetracycline, and doxycycline, respectively. The intra-day and inter-day precision (%) of the procedure were less than 3.2 and 3.8, respectively. The recoveries over 95% confirmed high sufficiency of the proposed method for application in complex matrices such as honey, milk, and egg.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics as important antibacterial medications are extensively applied in human and domestic animals for the main purposes including prophylactics and therapy of disease and also growth stimulant (Lian et al. 2018). Tetracycline family (TCs) more noticeably tetracycline (TC), doxycycline (DC), oxytetracycline (OTC), and chlortetracycline (CTC), are one of the most used antibiotics category due to their high accessibility and significant antimicrobial activity especially in veterinary medicine (Huang et al. 2019). TCs are not only used for infection treatment but also they are commercially applied for growth improvement in cattle and poultry as food-producing animals which is a serious concern for food scientists (Zhou et al. 2018). The presence and accumulation of TCs residues in foodstuff such as cow meat, milk, egg, chicken meat, and etc. is a huge concern because their consumption by human leads to irreparable and harmful effects (Khaled et al. 2020). Despite the imposed strict limitations over the TCs usage by European Union (EU) Council Regulation owing to their high potential risk, the indiscrimination to these rules are still problematic. Hypersensitivity, yellow–brown discoloration of teeth, skin photosensitivity, microvesicular fatty liver, gastrointestinal disturbance are some of the side effects of TCs over-intake during a period of time (Arabsorkhi et al. 2019). Therefore, the maximum residue limits (MRLs) of 100 μg Kg−1 in food has been inaugurated by the European Union (EU) (Gissawong et al. 2019). Considering the food safety and control importance, in order to determine TCs concentrations in animal food products, application of an efficacious analytical method is essential. Although, various analytical methods including spectrophotometric (Palamy and Ruengsitagoon 2017), fluorescence (Li et al. 2018), chromatographic (Lian et al. 2018), and electrochemical (Taghdisi et al. 2016) techniques have been applied for determination of TCs, due to the trace levels of these antibiotics in complex matrices and also to enhance the sensitivity of determination, application of sample treatment techniques such as extraction methods is essential prior to instrumental analysis. To this purpose, different sample treatment methods including liquid–liquid extraction (LLE) (Tang et al. 2020), solid phase extraction (SPE) (Li et al. 2022), solid phase microextraction (SPME) (Dong et al. 2021), dispersive solid-phase extraction (D-µSPE) (Cherkashina et al. 2020), single drop microextraction (SDME) (Li and Row 2022), and dispersive liquid–liquid microextraction (DLLME) (Faraji et al. 2020; Sereshti et al. 2021) have been reported for separation and preconcentration of TCs in various matrixes. Obviously, all the reported methods have some advantages and disadvantages. Dispersive solid-phase extraction (D-µSPE) as a developed type of SPE, can be categorized as one of the easiest and simplest methods which can be carried out by adding specific amount of sorbent to the media and analyte separation process occurs after several minutes of shaking or sonication. Then, the process follows by desorption of analyte from the sorbent. Separation process of the sorbent in D-µSPE is the main drawbacks of this method which led to the introduction of magnetic dispersive µ-solid phase extraction (MD-µSPE). Application of magnetic sorbents in D-µSPE intensifies the attractiveness of this method due to the facile separation of the magnetic sorbent by a simple magnet from the media which eliminates the centrifugation step at magnetic D-µSPE method (Isazad et al. 2022). Magnetic dispersive µ-solid phase extraction is based on the dispersion of low amount of a magnetic sorbent in an aqueous solution. However, MSPE method requires external source of energy such as vortexing, vibration, and ultrasound assistance, short pretreatment time, extremely low consumption of organic solvents, and high extraction efficiencies are the advantages of MD-µSPE (Ding et al. 2019).
Till now, many sorbents such as molecularly imprinted polymers (MIPs) (Asfaram et al. 2018), multi wall carbon nanotubes (Jalilian et al. 2018), metal–organic framework (Amiri et al. 2019), and functionalized graphene oxide (Khiltash et al. 2021) have been used in MD-µSPE. The noticeable efforts of chemists have been to introduce highly efficient sorbents to attain higher recoveries. For instance, Ma et al. introduced novel Metal–Organic Frameworks/Molecularly Imprinted Nano-polymer for MD-µSPE of tetracyclines from chicken samples. However, short linearities were obtained and also the BLQs and theLOQs were not improved enough (Ma et al. 2020). Tang et al. proposed magnetic solid phase extraction in tandem with liquid–liquid extraction method for determination of four tetracyclines good linearity was attained but the detection limits were not acceptable. Therefore, introduction of highly efficient sorbents is necessary for MD-µSPE (Tang et al. 2020). Nanofluids (NFs) are significant colloidal dispersions consisting of a nano structure material and a fluid. Nanofluids are considered as one of the most attractive extraction phases in recent years in sample treatment methods owing to their high mass transfer coefficient. However, the requirements of all chemical processes to get along with green chemistry, made a great change in the structure of NFs to use ionic liquids and deep eutectic solvents as the fluids which named as “nanogel or bucky gel”(Yousefi et al. 2017). In this research study, the novel magnetic nano gel which is the combination of Fe3O4@SiO2 nanocomposite as the nanostructure and a deep eutectic solvent (oceanic acid: cumarine) was synthetized and applied in MD-µSPE for separation and preconcentration of tetracycline family in some food samples (cow milk, honey, and egg) followed by HPLC–DAD.
Experimental
Chemicals
All the applied reagents in the entire set of experiments were of analytical grade with the highest purity. To provide the standard stock solution (100 mg L−1) of the target analytes including tetracycline (Sigma Aldrich), oxytetracycline (Sigma Aldrich), doxycycline (Sigma Aldrich), and chlortetracycline (Supleco Anaytical Standard), appropriate calculated amount of analytes were dissolved in methanol and stored at -20 °C. The standard solutions were prepared daily by the dilution of the stock solution with deionized water and stored at 4 °C. HPLC grade methanol, acetonitrile, acetone and water were purchased from Merck. The chemical structure and pKa values of TCs are shown in Table S1 (Chico et al. 2012). Tetraethylorthosilicate (TEOS), iron (III) chloride hexahydrate, iron (II) chloride tetrahydrate, ammonia solution (28%) were all purchased from (Sigma Aldrich, St. Louis, MO, USA) to prepare Fe3O4@SiO2. The routine applied solvents including Methanol, acetone, acetonitrile were all purchased from Merck Chemicals Co., (Darmstadt, Germany).
Instrumentation and chromatographic conditions
Analytical HPLC (Instrument ID: 8112, KNAUER, Germany) with Auto sampler (model AZURA 3950), quaternary pump (AZURA, P 6.1 L), PAD detector (model AZURA DAD 6.1 L), C18 column (25 cm length and 4.6 mm inner diameter) was used for chromatographic analysis of tetracycline family.
The HPLC program consists of two steps: The first 10 min: The mobile phase was methanol: acetonitrile: oxalic acid (0.01 mol L−1) with volume ratio of 3:12:85, and the second 10 min: the mobile phase volume ratio changed methanol: acetonitrile: oxalic acid (0.01 mol L−1) with volume ratio of 5:18:77 (Xu et al. 2018).
The column temperature was set at 25 ℃, and the analytical wavelength of HPLC–PDA was set at 350 nm with the injection volume of 10 µL. The flow rate degassed mobile phase was adjusted at 1.0 ml min−1.
Sample preparation
Milk, egg, and honey were purchased from a local supermarket in Jiroft, Iran. To reduce the matrix effect, milk sample was deprotonated by adding a 1:0.5 volume ratio of milk: acetonitrile and overtaxed for 3 min. Then, the mixture was centrifuged at 4000 rpm for 5 min and the upper phase was separated and filtered by 0.22 μm syringes filter. The obtained solution was dilute to 30 ml with deionized water to be used for the analytical process (Al-Afy et al. 2018). For egg sample; 1 ml HCl (0.1 mol L−1) and 3 ml acetonitrile (70%) was added to 2 mL homogenized egg sample and after overtaxing for 3 min, the mixture was centrifuged at 4000 rpm for 7 min. The upper phase was filtered and the filtrate was dried under nitrogen mild flow rate. The residue was diluted to 30 ml with deionized water (Du et al. 2019). For honey sample; 4 g of honey was diluted to 30 mL with deionized water and vortexed for 2 min. then, the mixture filtered and the filtrate used for the analytical process (Gissawong et al. 2019).
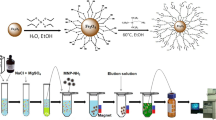
Synthesis of magnetic nanogel based Fe3O4@SiO2
In order to prepare DES, octatonic acid and cumarin with the mole ratio of 2: 1 was mixed and stirred for 5 min at 50 °C. The Fe3O4@SiO2 nanocomposite was synthetized according to the reported literature (Du et al. 2006). In brief, tetraethyl orthosilicate (TEOS) was added into a mixture of ammonium hydroxide and TMAOH dispersed Fe3O4 in the solution of water: ethanol (1:10). TEOS solution containing 1 ml of TEOS in 49 ml of anhydrous ethanol, was added gradually in into the solution A and stirred for 24 h. Due to the hydrolysis of TEOS, the mixture changed to gelatinized form. Finally, the obtained product was dried under vacuum condition at 110 °C for 48 h. The dried synthetized nanocomposite was mixed with the prepared DES to gain the magnetic nanogel.
MNF-USA-CL-CPE procedure
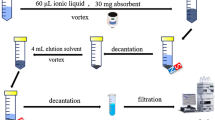
The pH of 30 ml sample solution containing tetracycline, oxytetracycline, chlortetracycline, doxycycline was set at 7 using HCl or NaOH (0.01 mol L−1). Then, 50 µL MNG as the sorbent was rapidly injected to the solution. The mixture was ultsonicated for 3 min to accelerate the analytes mass transfer into the MNG. The MNG containing the target analytes was separated and settled down with an external magnet without any centrifugation and the upper phase was discarded. The analyte were back-extracted from the MNG by adding 75 µL methanol and sonication for 90 s. Then, the MNG was settled down by a magnet and the methanol phase was transferred to the HPLC vial for injection.
All the optimizations experiments were carried out in triplicate. The calculation of the Extraction efficiency (EE) (%) follows Eq. (1) (Bidabadi et al. 2009):
where, \({C}_{O}\) and \({C}_{aq}\) are the analyte concentrations in the organic phase (eluent solvent) and in aqueous solution (initial concentration of analyte) and \({V}_{O}\) and \({V}_{aq}\) are the organic phase and aqueous phase volumes respectively. In order to reveal the peconcentration potential of the method, Enrichment factor (EF) was also evaluated which follows Eq. (2) (Shirani et al. 2022):
Experimental design
At this research study, the analytical procedure was carried out using one-at-the-time (Single factor) method in which each factor was studied at the time and the others kept constant. In order to attain the most suitable extraction efficiencies, the influence of substantial factors including pH (in the range of 3–9), the volume of MNF (20–80 µL), type and volume of dispersive solvent, extraction time (1–5 min), type and volume of desorption solvent (25–125 µL), desorption time (30–150 s), ionic strength (0–5% (w/v)), and sample volume (10–50 mL) were considered.
Results and discussion
Bidabadi et al. (2009).
Characterization of the MNG
The main force for the formation of DES is intermolecular hydrogen bonding between octanoic acid, as the hydrogen bond donor (HBD), and coumarin, as the hydrogen bond acceptor (HBA). FTIR is a confirmed method to illustrate these interactions. A shift in the stretching vibration band of the O–H group in HBD is occurred due to the hydrogen bonding that confirmed the successful formation of DES. Fig. S1 (a) shows the FT-IR spectrums of octanoic acid, coumarin, and coumarin/octanoic acid DES. According to the FT-IR spectrums, the broad bands at around 3600 cm−1 related to the stretching vibration of the O–H groups in octanoic acid as well as the DES. The peaks at 2944.19 cm−1 and 2917.19 cm−1 are related to the symmetric and asymmetric vibrational state of C–H bonds in methyl groups. In addition, the peaks at 1702 cm−1 and 1620 cm−1 are attributed to the C=O and C=C stretching in the carboxylic acid and aromatic ring, respectively. Also, the peak at 1412 cm−1 is attributed to the alkoxy (C–O) stretching in the carboxylic acid and coumarin, respectively. Both the characteristic peaks of octanoic acid and coumarin are present in the DES as well as board band of O–H group which these results indicate that successful preparation of DES.
The functional groups on the surface of magnetic gel were studied by ATR spectra (Fig. S1 (b)). A broad and intensive peak appeared at 3470 cm−1 is assigned to –OH stretching band. Besides, peaks at 2920.34 cm−1, 2895.26 cm−1, 1650 cm−1, 1550 cm−1, 1380 cm−1, 1090 cm−1 and 450 cm−1 are corresponded to the vibration frequencies of methyl (C–H), carbonyl (C=O), aromatic C=C, alkoxy (C–O), Si–O and Fe–O bonds, ATR spectrum of magnetic gel indicated both the characteristic peaks of DES and the characteristic peaks of the Fe3O4@SiO2.
The SEM image of magnetic nano gel (DES- Fe3O4@SiO2) is shown in Fig. S2 (a) which reveals the nanostructure and morphology of the synthetized MNG. Magnetization of the Fe3O4@SiO2 was evaluated by VSM. According to VSM curve in Fig. S2 (b), Fe3O4@SiO2 shows superparamagnetic behavior due to zero magnetic remanence when an applied external magnetic (H) is zero. In addition, from plateau part of the VSM curve, saturation magnetization (Ms) is around 50 emu g−1. Such excellent Ms for core/shell material is completely sufficient for magnetic separation in practical applications.
The effect of pH solution
pH of the solution is an important factor in the physico-chemical interaction of the aqueous solution and the solid phase of the sorbent (i.e. MNG). Since, TCs are amphoteric compounds, the acidity and basicity of the media directly affects their protonation or deprotonation (Althumayri et al. 2023). As shown in Table S1, the pKa values of TCs are in the range of 3.0–9.6. TCs are positively charged at pH values below pKa1 (pH < pKa1), zwitterion at pH values between pKa1 and pKa2, and finally negatively charged at pH values over pKa3. Therefore, the effect of pH in the range of 3–9 was investigated. As the results in Fig. 1a reveal, the extraction efficiencies of four TCs increased from 3 to 7 and then decreased. Therefore, the optimum pH of 7 was chosen as optimum. It seems that at this pH, the target analytes are in neutral form and can make п-п interaction between the phenyl ring of cumarin in MNG structure and tetracyclines and also the hydrogen bond interactions between octanoic acid and nitrogen and oxygen atoms in tetracycline molecules (Erdem et al. 2021). Also, at this pH there are efficient electrostatic interactions between protonated amine groups of the TCs and carboxylic group of octanoic acid in composition of the MNG.
The effect of MNG amount on extraction efficiencies of tetracyclines
In sorbent based techniques, the separation of analyte from the solution occurs through the adsorption of the analyte on the active cites of the sorbent (Ghorbani et al. 2020). In order to have maximum extraction efficiencies, appropriate amount of sorbent should be applied. To this aim, various volumes of MNG in the range of 20–80 µL was investigated and as indicated in Fig. 1b, by increasing the amount of MNG from 20 to 50 µL, the available active sites for analytes and also the contact surface between the analytes and the solution would be increased and as a consequence the extraction efficiencies enhances. Since, the volume of 50 µL MNG provides sufficient empty cites for the analyte molecules in the solution, the extraction efficiencies almost become constant for volumes higher than 50 µL. Accordingly, the volume of 50 µL MNG was sufficient and used for further studies.
Extraction time defines as the contact time between the sorbent surface and analytes. Proper extraction time provides enough time for analytes to be adsorbed on the active sites of the sorbent which leads to the best extraction efficiencies (Marzi Khosrowshahi et al. 2022). The sonication time was explored in the range of 1–5 min and the time of 3 min was selected as optimum extraction time (Fig. 1c).
The effect of type and volume of desorption solvent and desorption time
Desorption conditions have direct effect on the performance of the method because the desorbed analytes from the sorbent would be determined to obtain the extraction efficiencies. Therefore, an appropriate desorption solvent with proper volume can completely desorb the preconcentrated analytes from the sorbent. To this aim the effect of methanol, acetone, and acetonitrile as desorption solvents were investigated and as shown in Fig. 1d, methanol can more efficiently elute the tetracyclines from the MNG. Moreover, the solubility of target tetracyclines in methanol is more than two other solvents and can make strong hydrogen bond with tetracyclines to elute them from the sorbent. The volume of methanol in the range of 25–125 µL was examined and as presented in Fig. 1e, the volume of 75 µL methanol was selected as the best desorption solvent volume. Since, the preconcentration factor defines as the ratio sample volume and desorption solvent volume, with the optimum sample volume of 30 mL and methanol volume of 75 µL, the preconcentration of 400 would be achieved.
To obtain better desorption results the duration of desorption condition is really important. Therefore, the desorption time in the range of 30–150 s was explored and the desorption time of 90 s was used for the rest of studies.
The effect of ionic strength and sample volume
The process of ionic strength is commonly considered by the addition of NaCl to the solution in which the solubility of organic analytes will decrease and the extraction efficiencies increases due to the salting-out effect (Noormohammadi et al. 2022; Shirani et al. 2022). Therefore, the concentration of NaCl in the range of 0–5% (w/v) was studied and as shown in Fig. 2a, from 0 to 3% (w/v) the extraction efficiencies increased and then decreased owing to the increment of the solution viscosity.
Sample volume is a substantial factor in due to its direct impact on the preconcentration factor. The effect of sample volume was explored in the range of 10–50 mL. According to the obtained results in Fig. 2b, the extraction efficiencies of tetracyclines were almost constant from 10 to 30 mL and then decreased. Therefore, the sample volume of 30 mL was chosen as optimum and with sample volume of 30 mL and the desorption solvent volume of 75 µL, the preconcentration factor of 400 was achieved.
Analytical merits of the proposed method
The evaluation of the analytical performance of the DES-MNG-UA-DµSPE coupled with HPLC–DAD was accomplished under the optimum conditions to achieve the linear ranges, below level of quantification (BLQ), limits of quantifications (LOQs), and relative standard deviations (RSDs). Calibration curves were attained by the external standard approach using least-squares linear regression analysis of the peak areas versus analyte concentrations. The linear ranges of 1.5–500 (µg L−1), 2.5–750 (µg L−1), 2–750 (µg L−1), and 2.5–500 (µg L−1) were obtained for tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC), and doxycycline (DC), respectively. Moreover, the BLQ (S/N = 3) of 0.47, 0.11, 0.85 (µg L−1), 0.66, 0.81 and the LOQ (S/N = 10) of 1.61, 2.74, 2.23 (µg L−1), and 2.66 were achieved for TC, OTC, CTC, and DC respectively. The intra-day and inter-day precision (%) of the procedure were less than 3.5 and 3.2, respectively. The results are tabulated in Table 1.
Analysis of food samples via DES-MNG-UA-DµSPE method
The application of DES-MNG-UA-DµSPE coupled with HPLC–DAD for the separation and determination of trace amount of tetracyclines was investigated in egg, honey, cow milk. The food samples were prepared according to Section “Real sample preparation”. The target antibiotics were not detected in food samples. To consider the accuracy of the method, the analytes were spiked in samples in two levels of 50 and 200 µg L−1. The relative recoveries (%) of 95.2–99.3 were obtained with RSDs (%) less than 3.5 for three replicates. The results are presented in Table 2. The HPLC chromatograms of blank honey sample and also with two spiked levels of 50 and 200µg L−1 of the standard solution of target analytes are indicated in Fig. 3.
Comparison with other studies
The potential and analytical performances of the proposed method were compared with some reported studies. The proposed method was compared with some recent reported literatures for the extraction and determination of TCs in the same matrices. As indicated in Table 3, the analytical performance including linearity, detection limit, and repeatability of the proposed method are better comparable to the other methods However, Ma et al. obtained better sensitivity and also with higher preconcentration factor in comparison with other studies in Table 3. It is also necessary to mention that with the same instrument, the selective adsorbents such as MIP-MOF in comparison with magnetic nanogel usually provides better efficiency in the complex matrices. However, magnetic feature of the extraction sorbent in our work same as method provides easy separation and consequently shorter extraction time. Moreover, the sensitivity and the determination resolution of UPLC (Ma et al. 2020) and UPLC-QTOF/MS (Xu et al. 2016) techniques are much better than HPLC–DAD and also sensitivity, reliability and selectivity is outstanding in the case of UPLC-QTOF/MS. Considering the simplicity and cost of instrumentation, the proposed method could be a good substitute to LC–MS based techniques and besides, the accessibility to high-tech instruments is limited. Simplicity, eco-friendly of the solvent, high speed of analysis, low cost, accessibility and high sensitivity are the main advantages of the proposed method.
Conclusion
This work represents a high performance magnetic nano gel based cumarine: octanoic acid—Fe3O4@SiO2, used in dispersive µ-solid phase extraction coupled with HPLC–DAD for efficient extraction of tetracycline, doxycycline, oxytetracycline, and chlortetracycline in honey, egg, and cow milk samples. Application of deep eutectic solvent as a green solvent make this method as a green and environmentally friendly process. Facile separation of MNG from the media, facile and easy extraction process, low detection limit, ecofriendly extarction phase, proper precision and accuracy are the significant advantages of the proposed method which was successfully applied for determination of tetracyclines in food samples.
References
Al-Afy N, Sereshti H, Hijazi A, Rashidi NH (2018) Determination of three tetracyclines in bovine milk using magnetic solid phase extraction in tandem with dispersive liquid-liquid microextraction coupled with HPLC. J Chromatogr B 1092:480–488
Althumayri K, Guesmi A, El-Fattah WA, Houas A, Hamadi NB, Shahat A (2023) Enhanced adsorption and evaluation of tetracycline removal in an aquatic system by modified silica nanotubes. ACS Omega 8(7):6762–6777
Amiri A, Ghaemi F, Maleki B (2019) Hybrid nanocomposites prepared from a metal-organic framework of type MOF-199(Cu) and graphene or fullerene as sorbents for dispersive solid phase extraction of polycyclic aromatic hydrocarbons. Microchim Acta 186(3):131
Arabsorkhi B, Sereshti H, Abbasi A (2019) Electrospun metal-organic framework/polyacrylonitrile composite nanofibrous mat as a microsorbent for the extraction of tetracycline residue in human blood plasma. J Sep Sci 42(8):1500–1508
Asfaram A, Arabi M, Ostovan A, Sadeghi H, Ghaedi M (2018) Simple and selective detection of quercetin in extracts of plants and food samples by dispersive-micro-solid phase extraction based on core–shell magnetic molecularly imprinted polymers. New J Chem 42(19):16144–16153. https://doi.org/10.1039/C8NJ03349H
Bidabadi MS, Dadfarnia S, Shabani AMH (2009) Solidified floating organic drop microextraction (SFODME) for simultaneous separation/preconcentration and determination of cobalt and nickel by graphite furnace atomic absorption spectrometry (GFAAS). J Hazard Mater 166(1):291–296
Cherkashina K, Voznesenskiy M, Osmolovskaya O, Vakh C, Bulatov A (2020) Effect of surfactant coating of Fe3O4 nanoparticles on magnetic dispersive micro-solid phase extraction of tetracyclines from human serum. Talanta 214:120861
Chico J, van Holthoon F, Zuidema T (2012) Ion suppression study for tetracyclines in feed. Chromatogr Res Int 2012:135854
Ding W, Wang X, Liu T, Gao M, Qian F, Gu H, Zhang Z (2019) Preconcentration/extraction of trace bisphenols in milks using a novel effervescent reaction-assisted dispersive solid-phase extraction based on magnetic nickel-based N-doped graphene tubes. Microchem J 150:104109
Dong Z-M, Cheng L, Sun T, Zhao G-C, Kan X (2021) Carboxylation modified meso-porous carbon aerogel templated by ionic liquid for solid-phase microextraction of trace tetracyclines residues using HPLC with UV detection. Microchim Acta 188(2):43
Du F, Sun L, Tan W, Wei Z, Nie H, Huang Z, Ruan G, Li J (2019) Magnetic stir cake sorptive extraction of trace tetracycline antibiotics in food samples: preparation of metal–organic framework-embedded polyHIPE monolithic composites, validation and application. Anal Bioanal Chem 411(10):2239–2248
Du GH, Liu ZL, Xia X, Chu Q, Zhang SM (2006) Characterization and application of Fe3O4/SiO2 nanocomposites. J Sol-Gel Sci Technol 39(3):285–291
Erdem S, Öztekin M, Sağ AY (2021) Investigation of tetracycline removal from aqueous solutions using halloysite/chitosan nanocomposites and halloysite nanotubes/alginate hydrogel beads. Environ Nanotechnol Monit Manag 16:100576
Faraji M, Mahmoodi-Maymand M, Dastmalchi F (2020) Green, fast and simple dispersive liquid-liquid microextraction method by using hydrophobic deep eutectic solvent for analysis of folic acid in fortified flour samples before liquid chromatography determination. Food Chem 320:126486
Feng MX, Wang GN, Yang K, Liu HZ, Wang JP (2016) Molecularly imprinted polymer-high performance liquid chromatography for the determination of tetracycline drugs in animal derived foods. Food Control 69:171–176
Ghorbani M, Aghamohammadhassan M, Ghorbani H, Zabihi A (2020) Trends in sorbent development for dispersive micro-solid phase extraction. Microchem J 158:105250
Gissawong N, Boonchiangma S, Mukdasai S, Srijaranai S (2019) Vesicular supramolecular solvent-based microextraction followed by high performance liquid chromatographic analysis of tetracyclines. Talanta 200:203–211
Huang L, Yu W, Guo X, Huang Y, Zhou Q, Zhai H (2019) Chip-based multi-molecularly imprinted monolithic capillary array columns coated Fe3O4/GO for selective extraction and simultaneous determination of tetracycline, chlortetracycline and deoxytetracycline in eggs. Microchem J 150:104097
Isazad M, Amirzehni M, Akhgari M (2022) Highly efficient dispersive liquid–liquid microextraction assisted by magnetic porous carbon composite-based dispersive micro solid-phase extraction for determination of tramadol and methadone in urine samples by gas chromatography-mass spectrometry. J Chromatogr A 1670:462989
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2018) Determination of acidic, basic and amphoteric drugs in biological fluids and wastewater after their simultaneous dispersive micro-solid phase extraction using multiwalled carbon nanotubes/magnetite nanoparticles@poly(2-aminopyrimidine) composite. Microchem J 143:337–349
Khaled A, Belinato JR, Pawliszyn J (2020) Rapid and high-throughput screening of multi-residue pharmaceutical drugs in bovine tissue using solid phase microextraction and direct analysis in real time-tandem mass spectrometry (SPME-DART-MS/MS). Talanta 217:121095
Khiltash S, Heydari R, Ramezani M (2021) Graphene oxide/polydopamine-polyacrylamide nanocomposite as a sorbent for dispersive micro-solid phase extraction of diazinon from environmental and food samples and its determination by HPLC-UV detection. Int J Environ Analyt Chem 1–16
Li G, Row KH (2022) Single-drop microextraction technique for the determination of antibiotics in environmental water. J Sep Sci 45(4):883–895
Li W, Zhu J, Xie G, Ren Y, Zheng Y-Q (2018) Ratiometric system based on graphene quantum dots and Eu3+ for selective detection of tetracyclines. Anal Chim Acta 1022:131–137
Li P, Rao D, Wang Y, Hu X (2022) Adsorption characteristics of polythiophene for tetracyclines and determination of tetracyclines in fish and chicken manure by solid phase extraction-HPLC method. Microchem J 173:106935
Lian L, Lv J, Wang X, Lou D (2018) Magnetic solid–phase extraction of tetracyclines using ferrous oxide coated magnetic silica microspheres from water samples. J Chromatogr A 1534:1–9
Ma N, Feng C, Qu P, Wang G, Liu J, Liu JX, Wang JP (2020) Determination of tetracyclines in chicken by dispersive solid phase microextraction based on metal-organic frameworks/molecularly imprinted nano-polymer and ultra performance liquid chromatography. Food Anal Methods 13(5):1211–1219
Marzi Khosrowshahi E, Afshar Mogaddam MR, Farajzadeh MA, Nemati M (2022) Magnetic silicon carbide nanocomposite as a sorbent in magnetic dispersive solid phase extraction followed by dispersive liquid–liquid microextraction in the gas chromatographic determination of pesticides. Microchem J 181:107786
Noormohammadi F, Faraji M, Pourmohammad M (2022) Determination of aromatic amines in environmental water samples by deep eutectic solvent-based dispersive liquid-liquid microextraction followed by HPLC-UV. Arab J Chem 15(6):103783
Palamy S, Ruengsitagoon W (2017) A novel flow injection spectrophotometric method using plant extracts as green reagent for the determination of doxycycline. Spectrochim Acta Part A Mol Biomol Spectrosc 171:200–206
Sereshti H, Karami F, Nouri N (2021) A green dispersive liquid-liquid microextraction based on deep eutectic solvents doped with β-cyclodextrin: application for determination of tetracyclines in water samples. Microchem J 163:105914
Shirani M, Parandi E, Nodeh HR, Akbari-adergani B, Shahdadi F (2022) Development of a rapid efficient solid-phase microextraction: an overhead rotating flat surface sorbent based 3-D graphene oxide/lanthanum nanoparticles @ Ni foam for separation and determination of sulfonamides in animal-based food products. Food Chem 373:131421
Taghdisi SM, Danesh NM, Ramezani M, Abnous K (2016) A novel M-shape electrochemical aptasensor for ultrasensitive detection of tetracyclines. Biosens Bioelectron 85:509–514
Tang H-z, Wang Y-h, Li S, Wu J, Gao Z-x, Zhou H-y (2020) Development and application of magnetic solid phase extraction in tandem with liquid–liquid extraction method for determination of four tetracyclines by HPLC with UV detection. J Food Sci Technol 57(8):2884–2893
Wang R, Li C, Li Q, Zhang S, lv F, Yan Z. (2020) Electrospinning fabrication of covalent organic framework composite nanofibers for pipette tip solid phase extraction of tetracycline antibiotics in grass carp and duck. J Chromatogr A 1622:461098
Xu J-J, An M, Yang R, Tan Z, Hao J, Cao J, Peng L-Q, Cao W (2016) Determination of tetracycline antibiotic residues in honey and milk by miniaturized solid phase extraction using chitosan-modified graphitized multiwalled carbon nanotubes. J Agric Food Chem 64(12):2647–2654
Xu Y, Tang Y, Zhao Y, Gao R, Zhang J, Fu D, Li Z, Li H, Tang X (2018) Bifunctional monomer magnetic imprinted nanomaterials for selective separation of tetracyclines directly from milk samples. J Colloid Interface Sci 515:18–26
Yousefi SM, Shemirani F, Ghorbanian SA (2017) Deep eutectic solvent magnetic bucky gels in developing dispersive solid phase extraction: application for ultra trace analysis of organochlorine pesticides by GC-micro ECD using a large-volume injection technique. Talanta 168:73–81
Zhou Y, Yang Q, Zhang D, Gan N, Li Q, Cuan J (2018) Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens Actuators B Chem 262:137–143
Funding
This research has been supported by the University of Jiroft under Grant No. 3813–1400–1.
Author information
Authors and Affiliations
Contributions
MS Investigation, writing-original draft, conceptualization, methodology, MF, HRN, BA-a Conceptualization, Editing, SS conceptualization, methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable' for that section.
Consent to participate
All the authors have been participated at this research study.
Consent for publication
The data are not allowed to be published on line.
Data availability
The data will be available if request.
Code availability
'Not applicable'.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirani, M., Faraji, M., Rashidi Nodeh, H. et al. An efficient deep eutectic magnetic nano gel for rapid ultrasound-assisted dispersive µ-solid phase extraction of residue of tetracyclines in food samples. J Food Sci Technol 60, 2802–2812 (2023). https://doi.org/10.1007/s13197-023-05798-w
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05798-w