Abstract
The use of animal fats as raw material for shortening production has been avoided because of low supply, and religious restrictions of certain beliefs. The use of hydrogenated vegetable oils is also avoided because that may induce cardiovascular diseases. Palm oils and soya bean oil are theoretically potentials to be used as raw materials for shortening manufacturing due to their triacylglycerols composition and these oils can be easily modified to achieve desirable plasticity. In this study, shortening was produced by formulating a blend of palm stearin and soya bean oil in varying proportions. Physicochemical properties, product stability, and sensory acceptability of the processed shortening were determined. Stability tests of the processed shortening were determined for 6 months at two months intervals. The acidity, peroxide value, and free fatty acid values were increased with storage time and storage temperature. The physicochemical properties of the processed shortening samples were within the requirements of the food domain. The samples stored at 37 °C exhibited the highest acid, peroxide, and free fatty acid values throughout storage time. In conclusion, shortening produced from 60% palm stearin (S60) and stored at room temperature has shown a good physicochemical characteristic and is well accepted for different sensory attributes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal fat has been used for cooking since the beginning of civilization. Due to the limited supply of animal shortening, efforts have been placed to develop the technology to substitute animal shortening with vegetable fat. Shortening is defined as an edible fat used to shorten or tenderize baked products. Being insoluble in water, fat prevents the cohesion of gluten strands during mixing, thus literally shortening them and making the product tender. Shortening is used mainly in the production of flour-based foodstuffs such as cake, cookies, bread, and frying, in addition to shortening or tenderizing, such fats impart other important functional characteristics such as aeration to the finished product. Nowadays, attribute to the limited supply of animal shortening, and because of restrictions in some religions as well as high trans fatty acid content in the product other types of shortenings are required. The use of hydrogenated vegetable oils is also avoided because it contains trans fat content that may induce cardiovascular diseases (Mensink and Katan, 1990).
Soybean oil is widely accepted as a healthy oil, low in saturated acids and rich in polyunsaturated acids, especially linoleic acid. However, these oils are also easily oxidized, leading to rancidity and quality deterioration (Chen et al. 2011; Waraho et al. 2011). The oil represents 53% of total oilseed production and is the second most consumed (28%) in the world (Karasulu et al. 2011). It is a rich source of protein 38–46% and 18–20% oil (Mesfin and Abush, 2018) and it accounts for 75% of vegetable oil used in commercial and consumer cooking and is the primary ingredient in many processed foods such as salad dressings, sandwich spreads, margarine, bread, mayonnaise, non-dairy coffee creamers and snack foods, including dairy product substitutes. The physical properties of shortening are depending on factors such as plasticity, consistency, and structure. The Hardening of fats is produced by the addition of hydrogen to double bonds in the chains of fatty acids in triacylglycerol (Hamm, 2005). This process has a vital role in the fats and oils industry because it permits the transformation of liquid oils into semisolid fats and results in materials with improved stability (Pollock, 2004; ISEO, 2006). Hydrogenation, interesterification, and crystallization are distinct processes that can be applied to modify the physical or chemical properties of fats and oils to improve their usefulness. Crystallization is the process of forming solid material from a liquid solution or melt, where the solid material formed has a crystalline (as opposed to amorphous) structure. Shortening can be produced by formulating a blend, solidifying and plasticizing the blend, and packing and tempering (Gunstone, 2002). Even though, there are two shortening processing companies in Ethiopia; none of them are used soya bean oil as a raw material. Therefore, the objective of this study was to produce shortening by blending soybean oil with palm stearin. Since the habit of using vegetable fat is very low due to low accessibility as well as knowledge gap for the consumers. So, it is necessary to give clear information about the product. And also for the substitution of animal shortening with vegetable fat due to the limited supply of animal shortening and the restriction in some religions. Availability of formula to produce soya bean oil-palm oil shortening may improve the viability of the soya bean oil industry and at the same time provide more acceptable food products in terms of technical quality, health, and religion.
Materials and methods
Study area and raw material collection
The study was conducted at Addis Ababa University, Center for Food Science and Nutrition Laboratory. Refined, Bleached, and Deodorized Soybean Oil (RBDSBO) samples were collected from KUNIFIRA Agro-Processing PLC, supermarkets, shops, and retailers in Addis Ababa. The palm stearin was obtained from AFHA Food Processing Plant (Addis Ababa). The oil samples were collected, coded, and, preserved in a dry and cool place in the food science and nutrition laboratory. Commercial shortening (as a control) was obtained from shortening processing industries.
Formulation of shortenings
The oil samples were mixed and homogenized and the palm stearin was melted at 90 °C before use. Fat blends were formulated by mixing a highly saturated fat (palm stearin) with native vegetable oil (soybean oil) in varying proportions as indicated in Table 1. The formulation of fat blends was done according to Hadi (2013) with slight modifications. Six different fat blends were made for shortening preparation and the selection of the blend proportions was used for the analysis of physicochemical characteristics, shelf life, and, sensory acceptability studies.
The blended sample as shown (Table 1), was processed into vegetable fat (shortening) at the AFHA food processing plant by crystallization process with continuous mixing. After the mixing process was completed, the blended samples were left for at least 24 hrs at 13–14 °C for settling a purpose. Based on the above formulations (Table 1), six samples were produced, but the desired physical quality or characteristics of the processed shortening was not displayed for (20:80, 40:60, and 50:50) Ps and SBO formulations respectively. Thus, these products could not form crystals or expected shortening for further applications. Therefore, vegetable shortening was prepared by a combination of 60% and 70% palm stearin and 40% and 30% soya bean oil respectively, because, these products exhibited the desired physicochemical characteristics of vegetable shortening.
Physicochemical analysis
Physicochemical measurements like, moisture content, acid value, free fatty acid, peroxide value, saponification value, unsaponifiable matter, and iodine value and sensory evaluation test were carried out as follows after the formulated shortening was processed.
Moisture determination
The moisture content of shortenings was determined by oven drying following the method of AOCS (Method Ca 2b-38). All determinations were carried out in duplicate.
Moisture and volatile matter (%):
where
W1 = weight of (gm) of the metal dish
W2 = Weight of the sample (gm) and
W3 = weight of dried sample and metal dish
Determination of specific gravity
The specific gravity of the shortening samples was determined according to the method by (Akinola et al. 2010). An empty bottle was cleaned, dried, and weighed. About 10 ml of the sample was filled in a cleaned and dried empty bottle and weighed. Then, the density of the sample was calculated. Specific gravity was calculated as follows.
where
W1 = weight of the sample
W2 = weight of water
Determination of melting point
The melting point of the product was determined by (ISO 6321: 1997). A portion of the test sample was melted as rapidly as possible to at least 5 °C, but not more than 10 °C above which is completely melted. Then cooled the melted test sample with occasional stirring until its temperature is 32–34 °C and then stirred continuously with a stirrer allowing the fat to cool until the first signs of cloudiness appeared. Stirring was continued by hand until the fat had a pasty consistency and then transferred the fat to a 100 ml beaker at room temperature. The fat was stored at this temperature for a minimum of 24 hours. Four capillary tubes were pushed into the conditioned fat until the column of fat 10 mm + 2 mm long was obtained in each tube and removed any fat adhering to the outer surfaces of the tubes and the temperature was adjusted so that it could raise 10 C/minute. The capillary tubes were put in the apparatus with adjusted temperature. Lastly, the temperature was taken at which the first fat droplet was observed and reported the average of the capillary tubes as one determination.
Determination of acid value and free fatty acid
The oil or melted fat was mixed thoroughly before weighing. The mass of the test sample has been taken based on the color and expected acid value. The acid value was determined by using an official method of AOCS Method Ca 5a-40. Based on (ISO, 660) the expected maximum acid value for edible oils and fats must be less than 0.6. Thus, about 20 gm of the sample was placed in a 250 ml conical flask. Then 50ml of hot ethanol with 0.5 ml of 1% phenolphthalein was added and mixed. Lastly, the sample was titrated with 0.1N potassium hydroxide solution, until light pink color appeared. The acid value was calculated with the Eq. (3):
where, AV represents acid value, V - the volume of standard potassium hydroxide solution, (mL) (volume of KOH solution used for sample – blank reagent); N - normality of the potassium hydroxide solution; m - the weight of the test sample (gm).
Determination of free fatty acids (FFA):
The acidity is frequently expressed as the percentage of FFA in the sample. The percentage of FFA value was calculated using the following Eq. (3).
%FFA value (as oleic acid) =\(\frac{28.2*V*N}{w}\)
Where V: volume of the standard KOH solution,
N: normality of KOH
W: weight of the sample
Determination of iodine value
The iodine value was determined according to AOAC (2000) and 0.25 gm of the sample was placed into a 250 ml conical flask. Then 10 ml of chloroform and 30 ml of Hanus reagent (18.2 gm of iodine dissolved in 1L of glacial acetic acid with 3ml of bromine water) were added. Then, the solution was placed in the dark through mixing for 30 min. At the end of the specified time, 10 ml of potassium iodide (15%) was added and diluted with 100 ml of distilled water to prevent loss of the free iodine. Then the solution was titrated with 0.1N sodium thiosulfate, and 2–3 drops of starch solution where blue solutions formed and then continued with titration till the blue color disappeared.
The iodine value was calculated with the equation:
Where IV represents iodine value, (g I2/100 g sample);
0.127 - number of grams of iodine corresponding to 1mL of sodium thiosulfate solution, (g);
B - the volume of sodium thiosulfate solution used for the blank reagent, (mL);
S- the volume of sodium thiosulfate solution used for the sample, (mL);
N- Concentration of sodium thiosulfate solution; m – a mass of sample (gm).
Determination of peroxide value
The peroxide value was determined by AOAC (2000). About 5gm of the sample was placed in a 250 ml conical flask. Then, 30 ml of glacial acetic acid-chloroform solution (3:2) and 0.5 ml of saturated potassium iodide (KI) solution were added and left for a minute in a dark at ambient temperature. Then, 30 ml of distilled water was added to stop the reaction and to prevent free iodine loss. 2 ml of the saturated starch solution was used as an indicator and the mixture formed dark blue color and titrated with standardized 0.01N sodium thiosulfate solution until the color of the mixture disappeared. The peroxide value was calculated using the below equation:
Where, PV: Represents peroxide value, (m Eq O2/kg sample);
V1: Volume of standard sodium thiosulfate solution used for the blank reagent, (mL);
V2: Volume of standard sodium thiosulfate solution used for the sample, (mL);
m: Weight of the sample (gm).
Determination of saponification value
The saponification value was determined by AOAC (920.160) method. About 2 gm of the filtered sample was put on a 250 ml Erlenmeyer flask. Then, 25 ml of alcoholic KOH (mixture of 40 gm KOH and 45 gm CaO dissolved in 1L of ethanol) solution was added to the flask. The flask was connected with the condenser and boiled until the fat was completely saponified for 1hr. After a specified time, the sample was cooled and titrated with 0.5 M HCL (dilute 42.5 ml of 37% HCL with distilled water) phenolphthalein was used as an indicator. The saponification value was calculated using the below equation:
where SV represents saponification value, (mg KOH/ g sample); the 56.1-molecular weight of HCL; M- exact concentration of HCL used for titration; V1 - the volume of standard hydrochloric acid required for the sample, (mL); V2 - the volume of standard hydrochloric acid required for blank reagent, (mL); m - the weight of the oil sample (gm).
Determination of unsaponifiable matter
The unsaponifiable matter was determined according to ISO 3596 (2000). The unsaponifiable matter is expressed as a percentage by mass of the sample.
where
m0 = mass of test portion, gm
m1 = mass of residue, gm
m2 = mass of residue from the blank.gm
m3 = mass of free fatty acid (if any), gm
Preparation of cookies using the formulated shortenings
Homemade cookies were used as a baked product for the sensory acceptance test and those cookies were processed as follows according to the (Ayodele and John, 2015) procedure. The raw materials used include wheat flour 100 g, sugar 33.3 g, shortening 33.3 g, 5 gm baking powder, and 2.8 gm salt. All the ingredients were mixed thoroughly in a mixer for 15 minutes until smooth dough and they were baked at 180 °C for 20–25 minutes. After baking, the hot cookies were removed from the pan and placed on a clean tray to cool down. The cookies were then packed after cooling in polyethylene bags
Sensory evaluation
Sensory evaluation of cookies was evaluated through a customer acceptance test. This acceptance test involved 20 Food science and nutrition students from the center for food science and nutrition. All cookie samples were coded with three-digit random numbers and presented randomly to the panelists. The panelists were asked to rate their degree of liking or disliking of the cookies sample using 9-point hedonic scales that rated from 1 (extremely dislike) to 9 (extremely like) and they were provided water to rinse the mouth between evaluations. The sensory attributes were evaluated based on color, texture, aroma, taste, and overall acceptability.
Results and discussion
Physicochemical analysis
Physicochemical characteristics were determined for all processed shortening (S60 & S70) and commercial shortening at 0-month storage indicated in Table 2.
Shortening processed from 70% palm stearin had the highest melting point (44. 7 °C) as shown in Table 2, similar to values reported by Hadi (2013). Melting point changes with the chain length of fatty acids, unsaturation ratios, trans fatty acid content, and the position of the fatty acids in the glycerol backbone (Karabulutet al. 2003; Hadi 2013). Siddique et al, (2010) also reported a substantial decrease in the melting point of the palm olein when blended with other oils. The low melting point of S60 may be attributed to a higher portion of the low melting point oil soybean oil blended with palm stearin resulting in a further decrease in the melting point of the blends. The iodine value of the produced shortenings decreased from 49.7 to 45 when the amount of palm stearin increased in the fat blends from 60 to 70% respectively. Soybean oil has higher IV than PS due to the high degree of unsaturation, thus becoming more vulnerable to oxidation (Siddique et al. 2010). The iodine values for the blends increased significantly (p < 0.05) with the increasing amount of SBO in the blends because of an increasing amount of unsaturated fatty acids in SBO which are linoleic acid and oleic acid. Commercial shortening was found to have the highest peroxide value and saponification value followed by shortening produced from 70% palm stearin while shortening containing 60% palm stearin had the lowest PV and SV. But these values were not significantly different in terms of density, specific gravity, and acid value (Table 2). On the other hand, the FFA, peroxide value, and saponification value of the product were increased when the percent of palm stearin was increased during processing. FAO/WHO Joint Committee standard for shortenings products indicated that peroxide value should not exceed 10 mEq/kg; the free fatty acid should also be less than 0.3% and the melting point between 41 and 51 °C (Eshetu, 2007). Based on (ISO, 660) the expected maximum acid value for edible oils and fats must be less than 0.6. Based on the above table the finding of this study also agreed with FAO/WHO standards.
Oxidative stability tests
Oxidation stability is one of the most important quality parameters of edible vegetable oils and fats. It determines their usefulness in technological processes as well as shelf life. Determination of peroxide value (PV) and acid value (AV) is the most frequent method for the determination of oxidative products (Maszewska et al, 2018). Shortenings and vegetable ghee relatively contain no water which makes them have better stability during storage. They can undergo autoxidation and hydrolytic rancidity due to poor raw materials, processing, packaging, and storage conditions (Teklit, 2015).
Table 3, shows the comparison of chemical properties of shortening during different storage times. The result indicated that the moisture content of commercial shortening was higher at the initial time (0-month storage) but there was no significant difference between storage time and temperature changes. The acidity, FFA, and PV were increased significantly when the storage time and storage temperatures were increased for all products. The acidity, free fatty acid, and peroxide value were higher when the products were stored at 37 °C. The lowest acid value was found in all (0,3, and 6) month storage for S60 stored at room temperature, while in both 3rd & 6th -month storage the highest acid value was found for S70 at 37 °C storage temperature. The lowest free fatty acid was found in all (the 1st ,3rd, and 6th ) month storage for S60 stored at room temperature, but in the 3rd -month storage, the highest FFA value was found for commercial shortening, while in the 6th -month storage S70 was the highest at 37 °C storage temperature. Peroxide was found the highest in commercial shortening, while the lowest in S60 for all storage months in both storage conditions. Peroxide is the primary oxidation product and peroxide concentration may fluctuate over time since peroxide turns into other oxidation products with time (Thomaidis & Georgiou, 2000). Bukola, et al. 2015 also reported that peroxide value and acid value were increased with storage time for different vegetable oils. However, the acid value, peroxide value, and free fatty acid values were increased during storage for processed shortening (S60 & S70) after 6 months of storage, none of the tested samples exceeded the maximum permissible values as recommended by WFP (World Food Program), but peroxide value of commercial shortening at 6-month storage was above the recommended level.
Sensory test
Color, texture, flavor, taste, and overall acceptability of the cookies prepared using the developed shortenings were analyzed by using a nine-point hedonic scale that are presented in Table 4. Analysis of variance (ANOVA) was performed on the data gathered to determine differences by using SPSS Software. The sensory evaluation of cookies parameters was identified by comparing the addition of shortening samples during baking concerning different ratios of palm stearin and soya bean oil blends. The scored values of baked cookies were given in Table 4 and it can be seen that there was no significant difference in sensory attributes such as color, flavor, texture, taste, and overall acceptability among three different types of cookies prepared with newly formulated shortening (S60 and S70) and commercial shortening (Table 4). Therefore, according to these findings, we can conclude that the processed products have equal sensory acceptance by the consumers compared with that commercial shortening.
Conclusions
The technology for the production of shortening using palm stearin and soya bean oil as raw materials using the crystallization process reveals that it is possible to develop products with the desired quality. The study also assessed the shelf-life stability of processed shortening on the physicochemical properties for six (6) months at different storage temperatures. During the storage period, changes took place in the values of quality parameters. The physicochemical properties of the product were within the requirements of the food domain, but the peroxide value of commercial shortening was above the requirement during 6-month storage in both storage temperatures. All these vegetable shortenings can exist as semi-solid at room temperature. Products stored at 37 °C have poor physicochemical characteristics than that stored at room temperature especially the peroxide value and acid value were increased with storage temperature and storage time. All shortening products had equal sensory acceptance for different sensory attributes. The physicochemical property and oxidative stability of S60 was good compared with S70 and commercial shortening. Therefore, shortening produced from 60% palm stearin and 40% soya bean oil was found preferable product for its preferred quality. The results from physicochemical properties, baking performance, and sensory evaluation indicated that S70 and S60 shortening formulation was the most suitable fat blends to be used as bakery shortening.
Gap in the research
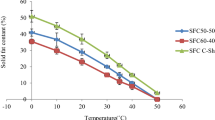
The gap in this research was, that the solid fat content of the product was not analyzed due to un functionality of the instrument, due to this I was trying to complete the work without SFC determination so, so I recommended that the solid fat of processed shortening will be measured for giving full information about this product.
Data availability
Data will be made available on the request
Abbreviations
- ANOVA:
-
Analysis of variance
- AOAC:
-
Association of Official Analytical Chemists
- AOCS:
-
American Oil Chemist Society
- AV:
-
Acid value
- AV:
-
Iodine value
- FAO:
-
Food and Agricultural Organization
- FFA:
-
Free fatty acid
- ISO:
-
International Standard Organization
- MP:
-
Melting point
- Ps:
-
Palm stearin
- PV:
-
Peroxide value
- RBDSBO:
-
Refined, bleached and deodorized Soya bean oil
- RT:
-
Room temperature
- SBO:
-
Soya bean oil
- SFC:
-
Solid fat content
- SV:
-
Saponification value
- WFP:
-
World food program
- WHO:
-
World Health Organization
References
AOAC (2000) 17th and, official method 920.159. Iodine absorption number of oils and fats / I.S.I. Handbook of food analysis. Part XIII). 1984 page 76)
AOAC (2000) 17th ed, Official method 920.160. Saponification number of oils and fats / IUPAC 2. 202 / I.S.I Handbook of food analysis (Part XIII) 1984, page 78)
AOAC (2000) Official method of analysis Arlington, 17th edn. Association of Official Analytical Chemists, VA USA
AOCS Official Method Ca 2b-38 (Reapproved, 2017) Moisture and Volatile Matter in Butter, Fats, Margarine, and Oils. Hot Plate Method
AOCS Official Method Ca 5a-40 (2017) Free fatty acids in crude and refined fats and oils
Akinola FF, Oguntibeju OO, Adisa AW, Owojuyigbe OS (2010) Physico-chemical properties of palm oil from different palm oil local factories in Nigeria. J Food Agric Environ 8(34):264–269
American Oil Chemists’ Society (2003) Sampling and analysis of commercial fats and oils. AOCS official method, Ca 5a-40, Surplus Free Fatty Acids. AOCS Cold Spring Harbour, New York, NY, USA
Bukola AOC, Francis GA, Patience A, Olalekan O (2015) Effects of different storage temperatures on the physicochemical properties of cooking oils available in Nigeria markets. Eur J Acad Essays ISSN 2:7–14
Chen B, Han A, Laguerre M, McClements DJ, Decker EA (2011) Role of reverse micelles on lipid oxidation in bulk oils: impact of phospholipids on antioxidant activity of α-tocopherol and Trolox. Food and Function 2:302–309
Eshetu Gizaw (2007) Evaluation of hydrogenation process, trans fatty Acid level, and some qualities of selected hydrogenated food products in the markets of Addis Ababa
Gunstone FD (2002) Vegetable oils in food technology: composition, properties and uses. Blackwell Publishing, NewYork, p 41
Hadi N (2013) Physico-chemical properties of palm stearin, soybean oil, and their binary blends (Doctoral dissertation, Universiti Teknologi MARA)
Hamm W (2005) Fractionation technology.38 the uplands, Harpenden, Herts AL5 2NZ, UK.Institute of shortening and edible oils. 2006. Food fats and oils, 9th edn. ISEO Inc, Washington DC, USA
ISO 3596 (2000) :Animal and vegetable fats and oils.Determination of unsaponifiable matter method using diethyl ether extraction
Institute of Shortening and Edible Oils (2006) Food fats and oils, Ninth edition. ISEO Inc, Washington DC, USA
International Standard Organization (ISO) (1997) Animal and Vegetable fats and oils. Determination of melting point, Switzerland, ISO 6321:1997(E)
Karabulut I, Kayahan M, Yaprak S (2003) Determination of changes in some physical and chemical properties of soybean oil during hydrogenation. Food Chem 81(3):453–456
Karasulu HY, Karasulu E, Büyükhelvacıgil M, Yıldız M, Ertugrul A, Büyükhelvacıgil K, …, Gazel N (2011) Soybean oil: production process, benefits and uses in pharmaceutical dosage form
Maszewska ID, Anna Florowska ID (2018) ElzbietaDłu ˙ zewska, MałgorzataWroniak ˙ ID, KatarzynaMarciniak-Lukasiak and Anna Zbikowska Oxidative stability of selected edible oils
Mensink RP, Katan MB (1990) Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. New Engl J Med 323:439–445
Hailemariam M, Tesfaye A (2018) Progress of soybean [Glycine max (L.) Merrill] breeding and genetics research in Ethiopia: A Review. J Nat Sci Res 8(13):2224–3186
Pollock T (2004) The Optimization of a vegetable oil hydrogenation reactor. Chemical engineering department, university of Queensland, Australia
Siddique BM, Ahmad A, Ibrahim MH, Hena S, Rafatullah M (2010) Physico-chemical properties of blends of palm olein with other vegetable oils. Grasas Aceites 61(4):423–429
Aderinola TA., Allikura OJ (2015) Qulaity evaluation of cookies from wheat and breadfruit composite flour. Ann NY Acad Sci 16:354–358
Teklit GebregiorgisAmabye (2015) The effect "Trans fatty acid level and some qualities of selected hydrogenated food products in the markets of Mekelle. World Journal of Pharmaceutical and Life Sciences WJPLS
Thomaidis NS, Georgiou CA (2000) Direct parallel flow injection multichannel spectrophotometric determination of olive oil iodine value. Analytica chimica acta 405(1–2):239–245
Waraho T, McClements DJ, Decker EA (2011) Mechanisms of lipid oxidation in food dispersions. Trends in Food Science and Technology 22(1):3–13
Acknowledgements
The authors would like to acknowledge Center Food Science and Nutrition, AFHA Food Processing Plant (Addis Ababa) and Kunifira Agro-Processing PLC (Addis Ababa) for the financial and technical support.
Funding
This work was supported by Southern Agricultural Research Institute, Kunifera Agro Processing Plant and Addis Ababa University.
Author information
Authors and Affiliations
Contributions
MG conceived the study and was involved in the study design, reviewed the article, analyzed and interpreted the data, reported writing, drafted and revised the manuscript. KU: was my advisor and gave constructing comments and suggestions and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belachew, M.G., Badhadha, K.U. Physicochemical characteristics and shelf-life stability of soya bean oil-based shortening. J Food Sci Technol 60, 2393–2400 (2023). https://doi.org/10.1007/s13197-023-05760-w
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05760-w