Abstract
Fish (Engraulis encrasicolus) oil was successfully microencapsulated using sage essential oils prepared in three different concentrations as 1% (S1), 2% (S2) and 3% (S3). The microencapsulated fish oil powders fabricated with spray drying were stored at room temperature (24 ± 1 °C) in order to determine the oxidative deterioration for 12 weeks. The highest microencapsulation efficiency was observed in the S3 (60.17%) as compared with other groups. Although the changes in free fatty acid (FFA) values were defined between 6.04 and 9.29% at the end of the storage period, the lowest FFA value was found in S2 samples (p < 0.05). Among the microencapsulated samples, the highest peroxide value (PV) was measured as 20.24 meq O2/kg for S1 at the 11th week of the experimental period. Moreover, statistical differences between the control (25.93 meq O2/kg) and S1 samples were observed (p < 0.05). The rapid increase in the thiobarbituric acid (TBA) value of fish powders was delayed by microencapsulation technique fabricated with spray drying. The use of sage essential oils within this combination effectively retarded the oxidation in fish oil powders at ambient storage, indicating cost-effective for the food industry. Therefore, encapsulation of fish oils with sage oil using the spray drying technique has improved oxidation stability of fish oil and can be used for food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food is a vital source for humans because of the fact that it contains essential nutritional materials for obtaining a sustainable and healthy life. In this respect, food safety and maintenance of nutritional values in foods play an important role for the consumers. In order to provide food safety, conventional (food additives, chilling technology) and recently novel technologies (microencapsulation and nanoencapsulation) are being used in the food industry. The mentioned technologies can limit the rapid microbiological spoilage but also rapid deterioration in sensory, chemical, and physical quality of foods (Durmuş et al. 2020). Incorporation of bioactive compounds (such as essential oils, polyphenols, vitamins, minerals, omega-3-fatty acids, bioactive proteins or peptides, probiotics etc.) into micro or nanocapsules have been paid attention since bioactive compounds have poor stability and low bioavailability during storage/processing, which limits their applications in food industry (Ozogul 2019). Thus, encapsulation has been regarded as an smart method to entrap bioactive compounds within a polymer material for protecting and delivering bioactive compounds at the right time and site by using several techniques such as nano-precipitation, spray drying, freeze drying, emulsification, and liposome preparation (Wen et al. 2017). For example, thyme, carvacrol, curcumin, rosemary, and wheat germ oil could be effectively used for fatty acid stability of fish oils, which have a key role in the protection of human health (Ceylan et al. 2020a, b).
Intake of omega-3 (ω-3) fatty acids, EPA (eicosapentaenoic acid), DPA (docosapentaenoic acid) and DHA (docosahexaenoic acid), classified in long-chain polyunsaturated can limit the occurring of cardiovascular matters and serious diseases like cancer. Moreover, for infants, DHA and EPA synthesized C18:3 ω-3 possess a role in the improvement of brain function (Durmuş 2019). However, even in western countries, intake of EPA and DHA is low (Innes and Calder 2020). Therefore, the incorporation of PUFAs like EPA and DHA into the mass-public diet by obtaining fortified foods has been recently considered by the food/fish oil industry (Šimat et al. 2020). On the other hand, food industry can face some challenges for the effective incorporation of PUFAs into food matrix. EPA and DHA contain of bis-allylic hydrogens that significantly improve the susceptibility to oxidation. As a results of the oxidation of EPA, and DHA, development of some off-odor and flavour compounds are produced (Ceylan et al. 2017). Strategies, in order to decline lipid oxidation in foods enriched with PUFAs, are needed in the food industry. Thus, encapsulation of different oil droplets in a cover material is mostly utilized (Cetinkaya et al. 2021). The shell material can effectively provide a barrier system around the oil droplets, limiting the contact of oxygen, being a kind of prooxidant, and also increasing the oxidative stability in the oil samples (García-Moreno et al. 2018).
For encapsulation of materials, electrospray, electrospinning, freeze-drying, melt injection (extrusion), spray-dry, and spray-chilling are widely preferred (Drosou et al. 2017). Among them, spray drying used for microencapsulation of bioactive materials and defined as cost-effective is the most experienced encapsulation method in the food industry (Özyurt et al. 2020). The solvent and liquid materials like water are removed during encapsulation process by spray drying (Drosou et al. 2017). Moreover, spray-drying has been effectively utilized technique in order to microencapsulate fish oil (Li et al. 2015). In order to microencapsulate fish oil by spray drying, hydrophilic carbohydrates for example dextran could be used. In addition, sage oil as bacterial agents on some food-borne pathogens could be used in nanoemulsion systems (Moghimi et al. 2016). The sage herb is rich in secondary metabolites (flavonoids, polyphenols, and terpenes) that indicate significant antioxidant capacity. However, despite abundant literature data on the antioxidant activity and bioactive components of sage tea oils, there are not enough studies on their viable uses to contribute to food safety in potential industrial uses such as microencapsulation of functional oils with a spray dryer. Novel hydrophilic carbohydrates together with essential oils can be employed for the improvement of the stability of fish oil.
The use of essential oils in the food industry is restricted due to their low water solubility, high volatility and intensive flavour. Therefore, to prevent essential oils from degradation, micro/nanoencapsulation is considered as an alternative technique. Therefore, the aim of the study was to determine to potential effect of the integration of sage essential oil into fish oil in different concentrations as an alternative micro-sized material to delay the rapid oxidation of fish oils.
Materials and methods
Oils and wall materials
The raw material of the current study, anchovy oil, was obtained from a fish oil company operating in the province of Trabzon (Kobyalar Group Co., Trabzon, Türkiye) and it was suitable for human consumption. Maltodextrin (DE:18-20) and sodium caseinate (Alfasol, Turkey) as wall materials for microencapsulation of anchovy oil (Hamasol, Turkey) were used. Sage tea (Salvia officinalis) essential oil was obtained from BIOMESI Bioagrotechnology R&D located in Adana, Turkey.
Preparation of emulsions
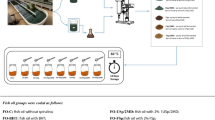
The ingredients of the emulsions are demonstrated in Table 1. The emulsion was prepared by dissolving 1:1 sodium caseinate and maltodextrin (DE:18‐20) in 55 °C water for 1 h and then left them outside to cool down. Fish oil was added to this mixture and homogenized in ultra-turrax (IKA T25, Baden-Württemberg, Germany) at 14.000 rpm for 10 min in a cold environment (in iced box) and thus the control group was formed. Different concentrations of sage oil were added to fish oil at 1% (S1), 2% (S2), and 3% (S3) ratios to form treatment groups. The ratio of fish oil and coating materials (sodium caseinate + maltodextrin, 1:1) was 1:2. Then, this mixture was homogenized in different tubes to form an emulsion, and treatment groups were formed. In the control group, sage oil was not used and other procedures were applied exactly.
Spray drying process and storage conditions
The emulsions were dried and pulverized in a 0.7 mm diameter spray nozzle laboratory spray dryer (Buchi Mini Spray Dryer B-290, Switzerland). 0.5 L/h as flow rate was used by continuous homogeneous mixing under the magnetic stirrer before and during drying. Spray dryer (SD) inlet temperature is 160 °C and outlet temperature is 90 ± 5 °C. The aspirator flow rate is 35 m3/h and pump speed are set to 20%. At the end of the process, powdered products were placed in dark glass bottles and stored at room temperature (24 ± 1 °C) for 12 weeks. For triplicated chemical analyses, three bottles of microencapsulated fish oil for each group were randomly selected on the 0, 2, 4, 5, 6, 7, 8, 9, 10, 11, and 12th weeks of storage.
Determination of volatile constituents and total phenolic content (TPC) of sage tea oil
A GC–MS (Clarus 500, Perkin Elmer, Waltham, MA, USA) equipped with a silica capillary SGE column (BPX5, 60 m Å to 0.25 mm, ID 0.25 um, Perkin Elmer, Shelton, CT, USA) was used to define the volatile compounds of sage tea oil. During the GC operating, the oven temperature was adjusted from 60 °C for 10 min and increased continuously to 250 °C at 4 °C/min and kept for 10 min in the last temperature. The carrier gas (helium at 1.5 mL min−1 and 220 °C) was used. 1 μL of diluted oil in hexane with splitless was adjusted for injection. At the same time, the energy of ionization, the temperature of the ion source, the range of the scanned mass, and the temperature of the interface line were defined as 70 eV, 200 °C, m/z 35–425, and 250 °C, respectively. Identification of volatile compounds was provided by comparing their retention index, mass spectra with those of commercial spectra databases (NIST-MS, Wiley-MS, and the flavor library of essential oil constituents). A method as described by Singleton and Rossi (1965) was performed to determine the TPC of sage tea oil. 0.4 mL of the gallic acid (GA as standard) solution and 1.6 mL of sodium carbonate (7.5 g/L) were added into 2 mL of 0.2 N Folin-Ciocalteu's phenol reagent. For homogenization with the same reagents, sage tea oil (0.4 mL) was used. Following the incubation carried out at room temperature for an hour, the absorbance value of the mentioned samples was defined at 760 nm. Obtained results were calculated according to GA equivalent (GAE)/g.
Measurement of microencapsulation efficiency (ME)
Each group of microencapsulated fish oil powders (2 g) was added into a 250 mL volumetric flask, and 15 mL hexane was put to each sample. Whatman no. 1 filter paper was used to obtain suspension following the rinsing of the papers twice using 20 mL hexane (Bae and Lee 2008). The solvent was evaporated using a rotary evaporator (Buchi R210, Rotavapor, Flawil, Switzerland) at 60 °C until the volumetric flask reaches a constant weight. The surface oil was designed from the weight differences. The given equation below was used to reveal the ME.
Microcapsule morphology by using scanning electron microscopy
The characterization of surface morphology of microencapsulated fish oils was done using scanning electron microscope (SEM). Microencapsulated fish oil samples were coated with gold for 30 s to a thickness of 2 nm using a Q150R ES Coater (Quorum Technologies, UK). The coated samples were observed using the SEM (Quanta FEG650, USA) at voltage of 20 kV with a magnification of 1000 and 5000 and working distance of 10.8–10.9 mm. Carbon tape is used for the adhesion of the material and for conductivity. Morphological structure of microcapsules was characterized by a scanning electron microscope (SEM) in Central Research Laboratory of Cukurova University (CUMERLAB, Adana, Turkey). The particle sizes of the microencapsulates were also measured using SEM. The number of images analyzed for size determination was seven.
Oxidation parameters
A method as described by Bligh and Dyer (1959) was used to measure the lipid content. 2 g of sample was homogenized with the mixture of chloroform and methanol (1:2), and then the samples were left in a dark place after CaCl2 was added. Chloroform was vaporized from the samples by using an evaporator. The differences among the flask weights revealed the lipid content of the samples (n = 3). AOCS PV method Cd 8-53 (1994) was performed in order to reveal the peroxide value (PV). Thiobarbituric acid reactive substances (TBARs) were determined a method as described by AOCS method Cd 19-90 (1998). TBARs value of the samples was given as mg MA/g of oil in the present study.
Statistical analyses
All analysis was done in triplicate. All collected data were analyzed by one-way ANOVA (p < 0.05) confidence level using the Duncan multiple range test.
Results and discussion
Microencapsulation efficiency (ME)
Microencapsulation efficiency values of fish oil samples containing sage essential oils with different ratios are presented in Table 2. While the sage essential oil ratio was increased from 1 to 3%, ME values were in the range of 50.65 and 60.17. It was observed that using 1% (S1) and 2% (S2) sage oil were significantly decreased the ME (p < 0.05). Ahn et al. (2008) were reported similar differences in their study on the antioxidant effect of natural plant extracts on the microencapsulated high oleic sunflower oil (MEHS). They reported that while the ME value of 1% (w/w) citrus extract (MEHS-C) was 45.02%, ME value of a mixture of 1% citrus mixture extract and 0.05% rosemary extract (MEHS-CR) was found as 51.25%. On the other hand, the use of 3%-sage essential oil in fish oil provided similar encapsulation efficiency as compared to control group (p > 0.05). As stated by Prieto and Lagaron (2020), the selected encapsulation process and composition of the wall play an important role in the definition of encapsulation efficiency. The encapsulation of fish oil into carbohydrate particles sized between 0.1 and 1.5 µm, was also determined ~ 70% as described by García-Moreno et al. (2017). According to Kamdem and Boulet Ntsama (2014), the microencapsulation efficiency of microencapsulated fish oil using Gelatin/SDS/NaCMC and secondary coating application with sodium polyphosphate after spray drying was found to be 53.2% and 56.63%, respectively. The encapsulation efficiency of the microparticle or microsphere or microcapsule depends upon different factors like concentration of the polymer, solubility of polymer in solvent, rate of solvent removal, solubility of organic solvent in water, etc. (Jyothi et al. 2010). In this study, especially the integration of (3%) sage essential oil with fish oil had a key role in the determination of microencapsulation efficiency.
Microcapsule morphology
Figure 1 shows SEM images of microcapsules of prepared at different concentrations of sage tea essential oil groups. Although a weak aggregation was present in all groups, it was generally homogeneous. The particle size of the microencapsulated for control, S1, S2, and S3 groups were 5.77–20.84, 8.79–37.49, 5.94–28.82, and 5.83–24.44 μm, respectively. Microencapsules had generally irregular cylindrical shapes, and there was not any cracking in the particles. It is important for the microcapsules to be round without any cracks or pores, to protect the core material from oxygen and to undesired release of oil droplets to the surface of particles. Microcapsule morphology exerts variable depending on the coating material, drying conditions, and the level and viscosity of the encapsulated substance (Wyspiańska et al. 2019).
Determination of volatile compounds and TPC of sage tea essential oil
Identification of essential oil is given in Fig. 2. In total, 20 compounds contained about (90.38 ± 0.340%) of total GC peak areas in the oil. The major compounds were α-pinene, camphene, β-pinene, eucalyptol, camphor, bornyl acetate, borneol, caryophyllene, and caryophyllene oxide. This essential oil was full of hydrocarbonated monoterpenes and oxygenated monoterpenes.The highest concentrations of compounds were α-pinene (14.11%), camphor (14.38%), and eucalyptol (14.56%), a terpene group that gives the odor of the plants. Borneol (8.14%), camphene (7.59%), β-pinene (6.31%), bornyl acetate (4.43%), caryophyllene (3.42%), and caryophyllene oxide (3.84) were determined as secondery components. Other components represented less than 3% (Fig. 2). Alizadeh and Shaabani (2012) reported that the essential oil composition of Salvia officinalis L. cultivated in Iran included borneol (8.33%), camphene (3.46%), α-pinene (3.24%), α-humulene (2.64%), and β-pinene (2.25%). Bouaziz et al. (2009) reported that the most abundant components (> 4%) of the sage tea essential oil cultivated in Tunisia were β -thujone (17.76%), 1,8-cineole (16.29%), camphor (14.19%), α-thujone (7.41%), transcaryophyllene (5.45%), viridiflorol (4.63%), β-pinene (4.41%), α-humulene (4.37%) and camphene (4.07%). Also, Delamare et al. (2007) reported that the main constituents (> 2%) of the essential oil of sage tea cultivated in South Brazil were α-thujone (24.8%), 1,8-cineole (14.8%), camphor (10.9%), β-pinene (9.87%), δ-gurjunene (8.20%), camphen (4.40%), β-thujone (3.97%), α-pinene (3.07%) and β-caryophyllene (2.89%). These differences can be explained by the fact that the extraction methods used are different as well as the geographies where the samples are taken. In addition, parameters such as soil, air, water conditions, sampling times where plants grow can cause differences in essential oil compositions (Russo et al. 2013).
Phenolics are the most important secondary metabolites acting as natural antioxidants in plants, and there is a close relationship between the total amount of antioxidant activity and phenolic content. The sage tea oil used in this study has a high phenolic content (26.86 mg GAE/g) and can be used as a natural source of phenolics and antioxidants. Alizadeh and Shaabani (2012) reported that the phenolic content of the same species was 25.13 mg GAE/g DW. Sage tea oil from the Lamiaceae family posses high level of phenolic compounds like phenolic acids, flavonoids and phenolic diterpenes, having antioxidant activity. These compounds can limit or inhibit the oxidative damage caused by free radicals and can protect people against critical diseases like coronary and heart problems and cancer diseases (Kris-Etherton et al. 2002).
Changes in oxidative stability
FFA is produced as a result of enzymatic hydrolysis of esterified lipids (Pacheco-Aguilar et al. 2000). It is known that there is a relationship between the amount of FFA and fish freshness depending on the storage period (Özogul et al. 2005). Moreover, as the amount of FFA increases, it can adversely affect the taste of the oil and increase rancidity. Therefore, monitoring changes in the FFA content is an important parameter for lipid hydrolysis of fish oils. The FFA value of the crude fish oil before processing was determined as 2.81 ± 0.15%. After the microencapsulation process, it changed in the range of 3.98–14.01% for the C group. While the FFA values at the beginning of the storage period were in the range of 3.98–5.23%, it increased to 6.04–9.29% at the end of storage (Table 3). There were no statistically significant differences among the groups at the beginning of the storage. However, fluctuations were observed in FFA values of all groups during storage. The highest FFA values were determined for the control, S1 and S3 groups at 2-week storage (14.93, 14.09 and 11.08%, respectively) and for S2 group at 10-week storage (12.43%). In general, during storage period, while the highest FFA values were observed in the control and S1 groups, the lowest values were found in the S2 and S3 groups. This suggests that increasing sage tea oil concentration minimizes FFA changes in fish oil during storage. Lipid hydrolysis developed at a slower rate in the treated groups, especially in the high concentration. This might be associated with the inhibition effect of phenolic compounds in sage tea. Bimbo (1998) reported that the FFA content is normally between 1 and 7% in raw fish. Skelbaek and Andersen (1994) found that the FFA content of microencapsulated oils or fats is below 5%. FFA values at the beginning of storage were close to this reported value (Table 3) whereas they exceeded this reported value (between 1 and 7% in raw fish) towards to end of the storage period.
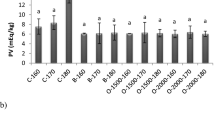
The formation of peroxides is considered to be the indicator of the first step of lipid oxidation. They provide information about the degree of oxidation of the oil. The changes in PV of the samples stored at room temperature (24 ± 1 °C) are given in Table 4. Statistical differences were observed in PV among the groups (p < 0.05) during the storage period. PV changed between 11.77 and 15.81 meq O2/kg oil immediately after the microencapsulation process. There were no statistical differences between treatment groups in terms of PV values at the 4th, 5th, 9th, 10th, and 12th weeks of storage (p < 0.05). PV levels in the samples tended to increase at the end of the storage. The PV value of the crude fish oil before processing was determined as 2.71 ± 0.26 meq O2/kg. After the microencapsulation process, it changed in the range of 11.77–25.93 meq O2/kg for the C group. Previous studies also showed a similar pattern of hydroperoxide content in fish oil (Ozogul et al. 2017; Rezaei et al. 2007). Although the control gave the highest PV during storage, among the treatment groups, S1 at 11 weeks (20.24 meq O2/kg), S3 at 2 weeks (13.91 meq O2/kg), and S2 at 10 weeks (12.50 meq O2/kg) also gave high PV. At the end of storage period the highest PV was observed in S1 group (18.05 meq O2/kg) while the lowest value was found in S3 group (17.27 meq O2/kg). The lowest PV were observed in S2, S3 and S1 groups (7.46, 8.01, and 8.61 meq O2/kg, respectively) at the 9th week of storage. Yeşilsu and Özyurt (2019) reported that the PV of microencapsulated fish oil with rosemary, thyme, and laurel extracts were lower than those of the control group containing commercial antioxidants. They found that the addition of rosemary extract delays the formation of peroxide in microencapsulated anchovy oils, and the addition of 1500 ppm rosemary provides better protection in terms of preventing the formation of peroxide from 250 ppm of synthetic antioxidant BHT. Bakry et al. (2016) investigated the PV of the microencapsulated tuna oil stored at 45 °C for 28 days and they reported that the PV of the group with peppermint oil was two times less than that of the non-added group. Kolanowski et al. (2004) reported that fish oil that was microencapsulated using antioxidant-free and modified cellulose for 36 days at room temperature increased peroxide value above 60 meq. Hogan et al. (2003) reported that PV of microencapsulated fish oil samples stored at room temperature for 28 days increased to 85.6 meq O2/kg oil. However, in the current study, PV were considerably low. It can be concluded that the addition of sage tea EOs in the wall material positively affected the lipid stability of microcapsules.
The usage of PV as the only indicator of lipid oxidation can be misleading because of high inconstancy of lipid hydroperoxides. Break down of these products produces secondary products such as hydrocarbons, alcohols, carbonyls, and aldehydes, which cause the flavor deterioration of foodstuffs (Frankel 1987). The TBARs test, regarded as the index of secondary phase of lipid oxidation, determines the content of aldehydes. In this test, not only malondialdehyde reacts with TBA to produce red pigment but also other aldehydes might be participated in the reaction. Besides, TBARs represent malondialdehydes, which are secondary degradation products of lipid oxidation, and are one of the criteria showing the rancidity of oils. TBARs values of microencapsulated fish oils with sage tea EOs are given in Table 5. Statistical differences were observed among groups during the storage period (p < 0.05). The FFA value of the crude fish oil before processing was determined as 0.78 ± 0.06 mg MA/kg. After the microencapsulation process, it changed in the range of 0.54–2.14 mg MA/kg for the C group. TBARs values varied from 1.51 to 2.14 mg MA/kg after microencapsulation. At the beginning of the study, the TBARs value of the control group was determined as 2.14 mg MA/kg. The lowest value was determined for S2 group (1.51 mg MA/kg) while the highest TBARs value was determined in S1 group (1.84 mg MA/kg). Fluctuation was observed in all groups during the storage period. The highest TBARs values were also observed at the 2nd week of storage for S3 (1.90 mg MA/kg), S2 (1.89 mg MA/kg) and S1 (1.71 mg MA/kg). The lowest TBARs values were observed for both S1 and S3 groups at the 9th week (0.40 mg MA/kg) and for S2 group (0.54 mg MA/kg) at the 12th week. At the end of storage, the highest TBARs value was obtained for S3 group (0.93 mg MA/kg) among the treatment groups, while the lowest value was found for S2 group (0.54 mg MA/kg). TBARs values were observed to be lower than the control group from the beginning of the storage to the 7th week. However, higher TBARs values were observed in the S2 group compared to the control at the 8th and 9th weeks of storage, while TBARs values closer to the control were observed in the S3 groups at the 11th and 12th weeks of storage. Li et al. (2015) determined the TBARs value of microcapsules with blueberry extract as 0.487 mg MA/kg. Solval (2011) reported that the TBARs value that they could not detect in the crude oil varied between 0.11 and 0.12 mg MA/kg fat after microencapsulation. Binsi et al. (2017) reported that the TBARs values of microencapsulated fish oil samples with sage polyphenol exceeded the limit after 24 h when the samples were kept at 60 °C. It was reported that the product was unacceptable when the amount of 19 mmol MA exceeded in 1000 g fish oil (Kaitaranta 1992). In the current study, the TBARs value did not exceed 2.14 mg MA/kg, thus sage tea EOs for microencapsulation of fish oils were found to be a good oxidation retardant.
Conclusion
Anchovy oil enhanced with sage essential oil was microencapsulated and the oxidative stability of microencapsulated oil in different concentrations was investigated. Considering the oxidation parameters, it was determined that microencapsulated fish oil containing sage tea essential oil gave better results than the control group, especially group S2. Inclusion of sage essential oils into fish oil prior to spray drying process can protect highly sensitive omega-3 fatty acids from lipid oxidation.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code availability
Not applicable.
Abbreviations
- S1:
-
1% Sage oil with fish oil
- S2:
-
2% Sage oil with fish oil
- S3:
-
3% Sage oil with fish oil
- FFA:
-
Free fatty acids
- PV:
-
Peroxide value
- TBA:
-
Thiobarbituric acid
References
Ahn JH, Kim YP, Seo EM, Choi YK, Kim HS (2008) Antioxidant effect of natural plant extracts on the microencapsulated high oleic sunflower oil. J Food Eng 84(2):327–334
Alizadeh A, Shaabani M (2012) Essential oil composition, phenolic content, antioxidant and antimicrobial activity in Salvia officinalis L. cultivated in Iran. Adv Environ Biol 6(1):221–6
Bae EK, Lee SJ (2008) Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J Microencapsul 25(8):549–560
Bakry AM, Abbas S, Ali B, Majeed H, Abouelwafa MY, Mousa A, Liang L (2016) Microencapsulation of oils: a comprehensive review of benefits, techniques, and applications. Compr Rev Food Sci Food Saf 15(1):143–182
Bimbo AP (1998) Guidelines for characterizing food-grade fish oils. Int News Fats Oils Relat Mater 9(5):473–483
Binsi PK, Nayak N, Sarkar PC, Jeyakumarı A, Ashraf PM, Nınan G, Ravıshankar CN (2017) Structural and oxidative stabilization of spray dried fish oil microencapsulates with gum arabic and sage polyphenols: characterization and release kinetics. Food Chem 219:158–168
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Bouaziz M, Yangui TH, Sayadi S, Dhouib A (2009) Disinfectant properties of essential oils from Salvia officinalis L. cultivated in Tunisia. Food Chem Toxicol 47:2755–2760
Cetinkaya T, Mendes AC, Jacobsen C, Ceylan Z, Chronakis IS, Bean SR, García-Moreno PJ (2021) Development of kafirin-based nanocapsules by electrospraying for encapsulation of fish oil. LWT-Food Sci Technol 136:110297. https://doi.org/10.1016/j.lwt.2020.110297
Ceylan Z, Meral R, Kose YE, Cavidoglu I (2020a) Wheat germ oil nanoemulsion for oil stability of the cooked fish fillets stored at 4 °C. J Food Sci Technol 57(5):1798–1806. https://doi.org/10.1007/s13197-019-04213-7
Ceylan Z, Meral R, Kose S, Sengor G, Akinay Y, Durmus M, Ucar Y (2020b) Characterized nano-size curcumin and rosemary oil for the limitation microbial spoilage of rainbow trout fillets. LWT-Food Sci Technol 134:109965. https://doi.org/10.1016/j.lwt.2020.109965
Ceylan Z, Sengor GFU, Yilmaz MT (2017) A novel approach to limit chemical deterioration of gilthead sea bream (Sparus aurata) fillets: coating with electrospun nanofibers as characterized by molecular, thermal, and microstructural properties. J Food Sci 82(5):1163–1170. https://doi.org/10.1111/1750-3841.13688
Delamare APL, Moschen-Pistorello IT, Artico L, Atti-Serafini L, Echeverrigaray S (2007) Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem 100(2):603–608
Drosou CG, Krokida MK, Biliaderis CG (2017) Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: a comparative assessment of food-related applications. Drying Technol 35(2):139–162. https://doi.org/10.1080/07373937.2016.1162797
Durmuş M, Ozogul Y, Köşker AR, Ucar Y, Boğa EK, Ceylan Z, Ozogul F (2020) The function of nanoemulsion on preservation of rainbow trout fillet. J Food Sci Technol 57(3):895–904. https://doi.org/10.1007/s13197-019-04122-9
Durmuş M (2019) Fish oil for human health: omega-3 fatty acid profiles of marine seafood species. Food Sci Technol 39:454–461
Frankel EN (1987) Secondary products of lipid oxidation. Chem Phys Lipid 44(2–4):73–85
García-Moreno PJ, Özdemir N, Stephansen K, Mateiu RV, Echegoyen Y, Lagaron JM, Chronakis IS, Jacobsen C (2017) Development of carbohydrate-based nano-microstructures loaded with fish oil by using electrohydrodynamic processing. Food Hydrocolloids 69:273–285. https://doi.org/10.1016/j.foodhyd.2017.02.013
García-Moreno PJ, Pelayo A, Yu S, Busolo M, Lagaron JM, Chronakis IS, Jacobsen C (2018) Physicochemical characterization and oxidative stability of fish oil-loaded electrosprayed capsules: combined use of whey protein and carbohydrates as wall materials. J Food Eng 231:42–53. https://doi.org/10.1016/j.jfoodeng.2018.03.005
Hogan SA, O’riordan ED, O’sullivan M (2003) Microencapsulation and oxidative stability of spray-dried fish oil emulsions. J Microencapsul 20(5):675–688
Innes JK, Calder PC (2020) Marine omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci 21(4):1362. https://doi.org/10.3390/ijms21041362
Jyothi NVN, Prasanna PM, Sakarkar SN, Prabha KS, Ramaiah PS, Srawan GY (2010) Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul 27(3):187–197
Kaitaranta JK (1992) Control of lipid oxidation in fish oil with various antioxidative compounds. J Am Oil Chem Soc 69(8):810–813
Kamdem EP, Boulet Ntsama IS (2014) Optimization process by complex coacervation of fish oil using Gelatin/SDS/NaCMC and secondary coating application with sodium polyphosphate. Int J Sci: Basic Appl Res 17(1):74–94
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF et al (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 113(9):71–88
Kolanowski W, Laufenberg G, Kunz B (2004) Fish oil stabilisation by microencapsulation with modified cellulose. Int J Food Sci Nutr 55(4):333–343
Li J, Xiong S, Wang F, Regenstein JM, Liu R (2015) Optimization of microencapsulation of fish oil with gum Arabic/casein/beta-cyclodextrin mixtures by spray drying. J Food Sci 80(7):C1445–C1452. https://doi.org/10.1111/1750-3841.12928
Moghimi R, Aliahmadi A, McClements DJ, Rafati H (2016) Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT-Food Sci Technol 71:69–76. https://doi.org/10.1016/j.lwt.2016.03.018
Ozogul Y, Yuvka İ, Ucar Y, Durmus M, Kösker AR, Öz M, Ozogul F (2017) Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT-Food Sci Technol 75:677–684
Ozogul Y (ed) (2019) Innovative technologies in seafood processing. CRC Press
Özogul Y, Özyurt G, Özogul F, Kuley E, Polat A (2005) Freshness assessment of European eel (Anguilla anguilla) by sensory, chemical and microbiological methods. Food Chem 92(4):745–751
Özyurt G, Durmuş M, Uçar Y, Özoğul Y (2020) The potential use of recovered fish protein as wall material for microencapsulated anchovy oil. LWT-Food Sci Technol 129:109554. https://doi.org/10.1016/j.lwt.2020.109554
Pacheco-Aguilar R, Lugo-Sánchez ME, Robles-Burgueño MR (2000) Postmortem biochemical and functional characteristic of Monterey sardine muscle stored at 0 °C. J Food Sci 65(1):40–47
Prieto C, Lagaron JM (2020) Nanodroplets of docosahexaenoic acid-enriched algae oil encapsulated within microparticles of hydrocolloids by emulsion electrospraying assisted by pressurized gas. Nanomaterials 10(2):270. https://doi.org/10.3390/nano10020270
Rezaei M, Montazeri N, Langrudi HE, Mokhayer B, Parviz M, Nazarinia A (2007) The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss) stored in ice. Food Chem 103(1):150–154
Russo A, Formisano C, Rigano D, Senatore F, Delfine S, Cardile V et al (2013) Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem Toxicol 55:42–47
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Skelbaek T, Andersen S (1994) A microencapsulated oil or fat product. Int Pat Appl WO94/01001-A1:1–21
Solval KEM (2011) Spray drying technology for the production and processing of microencapsulated omega-3 fish oil with egg powder. Doctoral dissertation, Louisiana State University
Šimat V, Elabed N, Kulawik P, Ceylan Z, Jamroz E, Yazgan H, Čagalj M, Regenstein JM, Özogul F (2020) Recent advances in marine-based nutraceuticals and their health benefits. Mar Drugs 18(12):627. https://doi.org/10.3390/md18120627
Wen P, Zong MH, Linhardt RJ, Feng K, Wu H (2017) Electrospinning: a novel nano-encapsulation approach for bioactive compounds. Trends Food Sci Technol 70:56–68
Wyspiańska D, Kucharska AZ, Sokół-Łętowska A, Kolniak-Ostek J (2019) Effect of microencapsulation on concentration of isoflavones during simulated in vitro digestion of isotonic drink. Food Sci Nutr 7(2):805–816
Yeşilsu AF, Özyurt G (2019) Oxidative stability of microencapsulated fish oil with rosemary, thyme and laurel extracts: a kinetic assessment. J Food Eng 240:171–182
Acknowledgements
Not applicable.
Funding
This study was supported by Cukurova University (Project Number: FBA-2019-11949).
Author information
Authors and Affiliations
Contributions
Mustafa Durmus: Original draft preparation, review and editing; Yilmaz Ucar: Conceptualization, Software (chemical analyses), Writing; Ali Riza Kosker: Software (statistical analyses), Writing; Yesim Ozogul: Conceptualization; Writing; Gulsun Ozyurt: Conceptualization; Writing; Zafer Ceylan: Conceptualization; Writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Ethics approval was not required for this research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Durmus, M., Ucar, Y., Kosker, A.R. et al. Formulation with sage tea-loaded fish oil-based microcapsules to delay oxidation. J Food Sci Technol 60, 474–483 (2023). https://doi.org/10.1007/s13197-022-05629-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-022-05629-4