Abstract
Six meat emulsion treatments were manufactured, depending on the addition of Trigonella seed powder. Meat emulsions were prepared with starch as control treatment, Trigonella seed powder (Tfg) or defatted Trigonella seed powder (Dtfg) in two percentages 2% and 4%. Cooking losses, lipid oxidation degree, meat emulsion stability through measurement of total released fluid, water released, fat released, TPA attributes and color parameters were evaluated. Cooking loss and fat, fluid releases were lower in Tfg and Dtfg samples related to Starch. Also, lipid oxidation was higher (P < 0,05) in Tfg samples than Dtfg or Starch. Hardness, chewiness and gumminess were lower (P < 0,05) in Tfg and Dtfg samples than Starch samples. The a* values in samples with starch were reduced rapidly (P < 0,05) than Tfg and Dtfg values during preservation. These results show that Trigonella Foenum-Graecum seed powder is an efficient candidate for improving quality of emulsion type meat products with vegetable oils as animal fat replacers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trigonella foenum-graecum, commonly known as Fenugreek, is an herbaceous plant, which belongs to the family of Leguminosae or Fabaceae plants (Ahmad et al. 2016). One of the oldest medicinal plants, its name comes from the triangle shape of its leaves and it is being commercially grown in many countries of Middle East, Mediterranean basin and Europe (Ahmad et al. 2016). Despite the interest for the whole plant, Trigonella foenum-graecum seeds obtained huge interest because of the variety of nutritional and medicinal activities, known even from ~ 4000 BC (Ahmad et al. 2016).
Seed and more specifically endosperm of Trigonella foenum-graecum is very rich in terms of functional chemical constituents. It contains high amount of carbohydrates (~ 46%), mainly fibers (~ 45%), which mainly consist of Fenugreek gum, a soluble fiber which represent almost 20% of the raw seed weight (Ahmad et al. 2016; Wani et al. 2016). This distinctive fenugreek gum, which is mainly galactomannan (> 75%), consists of D-mannopyranose and D-galactopyranose residues with a molar ratio of 1.2:1.0. B-(1,4)-linked D-mannopyranose residues comprise the main chain of the polysaccharide which is mainly substituted (83.3%) at C-6 with a single residue of a-(1,6)-D-galactopyranose. This formation is commonly found in plants of Leguminosae family (Jiang et al. 2007). This formation results to effects on physicochemical properties, such as increasing of water holding capacity and better solubilization. Fenugreek gum, also, and other gums with lower G:M ratio, have more substituted formation (~ 85% for fenugreek gum) which leads to enhanced stability against enzymic depolymerase processes (Jiang et al. 2007). Also, seeds contain high protein amount (~ 25%), which gives stabilizing and emulsifying properties in various food systems (Isikli and Karababa 2005; Kaltsa et al. 2016).
As discussed above Trigonella Foenum-Graecum (fenugreek) seed powder has potential of widespread use in food industry due a distinctive combination of functional ingredients like dietary fiber and protein that gives a perspective of efficient and feasible application in food technology with plenty of advantages as thickening, textural, emulsifying, gelling and encapsulating agent (Wani et al. 2016). Traditionally, fenugreek seed powder is used for Cemen preparation, a paste from different herbs and spices, mainly fenugreek, intended to cover the meat for Pastirma production, a very popular meat product in Europe and Eastern countries (Isikli and Karababa 2005). Recently, addition of the seed powder on bakery products leaded to bread products supplemented with fenugreek seed powder at levels between 8 and 10% with better nutritional and physicochemical attributes (Roberts et al. 2014). Additionally, fenugreek seed powder has been incorporated also in extruded snack products in levels < 2% with improved quality characteristic (Wani et al. 2016). Also, incorporation of fenugreek seed powder in O/W nanoemulsions increased emulsifying activity of whey protein isolate in various conditions (Kaltsa et al. 2016), where in the same time emulsifying activity of soy protein isolate found four times higher in olive oil emulsions when fenugreek seed powder was incorporated (Kasran et al. 2013).
Recent advances in science and technology suggest new novel non-meat ingredients for meat industry, for the purpose of animal fat lowering, but in the same time providing functional compounds, which both could be beneficial for human health (Vasquez-Mejia et al. 2019). Meat emulsion-based products from comminuted meat like frankfurters or mortadella type sausages, are wide consumed and popular products, but the fact that they contain around 25–30% fat, 10% of it as saturated animal fat (USDA 2016), makes them a risk for human health for cholesterol problems and CVD (Kouzounis et al. 2017).
Basic strategy for fat reducing in meat emulsion products is the approach of reducing and/or replacing animal fat, with lipids whose properties are healthier. Vegetable oils, for example, tend to have smaller percentages of saturated fatty acids (SFAs) and larger proportions of unsaturated fatty acids, namely monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) and better n−6/n−3 PUFA and PUFA/SFA ratios (Choi et al. 2009). Vegetable oils comprise a source of unsaturated fatty acids and are known for their low cholesterol levels (Choi et al. 2009). From vegetable oils, olive oil has gained vast attention for animal fat reduction in meat products, mainly due to its worth noticing combination of MUFA, mainly oleic acid (C18:1) and natural antioxidants (Hur et al. 2008). Although various studies have been conducted with addition of olive oil in emulsion type meat products (Choi et al. 2009; Vasquez-Mejia et al. 2019; Youssef and Barbut 2011), percentage of addition presents limitations and animal fat cannot be fully replaced due to physicochemical and sensory problems that are generated by reduction of animal fat (Jimenez-Colmenero et al. 2010).
Incorporation of dietary fibers, which are low-glycemic index hydrocolloids (Ahmad et al. 2016) in low fat meat emulsion systems with vegetable oils gathered attention recently, because they might counteract the negative physicochemical, microbiological and sensory effects of vegetable oil addition. Many studies that have been conducted with addition of fibers from various sources (Choi et al. 2009; Eyiler-Yilmaz et al. 2016; Morin et al. 2004; Vasquez-Mejia et al. 2019) confirm the development of favorable physicochemical and texture properties of low-fat meat emulsion products with olive oil. However, the available literature on dietary fibers incorporation on low fat meat emulsions with olive oil is limited, due to several important restrictions. These are the difficulties of total replacement of animal fat, the inefficiency of one type of dietary fiber alone to induce stability in the meat emulsion system and the low threshold of dietary fiber percentage of inclusion without texture and quality defects (Vasquez-Mejia et al. 2019).
Considering the above, aim of this study was to evaluate the physicochemical, texture and color effects of the incorporation of Trigonella foenum-graecum seed in a meat emulsion system with olive oil with replacement of starch content.
Materials and methods
Materials
Fresh minced pork ham (M. biceps femoris, M. semitendinosus, M. semimembranosus), were purchased from a local market at 48 h postmortem. Trigonella foenum-graecum seeds were purchased from a local market and then it was grounded using a laboratory grinder (Analytische Mühle, IKA). Sodium chloride (Kallas klassiko), corn starch (Bioygeia) and virgin olive oil (Altis Klassiko, ELAIS) were purchased from local markets. Sodium nitrite was obtained from CG Chemicalien (CG Chemicalien, Belgium).
Defatting
Defatting of Trigonella foenum-graecum seeds was carried out based on the method of lipid extraction (Aburezq et al. 2008). In this method for the defatting of raw seeds a Soxhlet apparatus was used utilizing Diethylether as solvent. More specifically, a certain amount of Trigonella foenum -graecum seed powder was weighed and placed in a cellulose extraction cartridge. The cartridge was plugged with cotton wool and then placed in the Soxhlet chamber which was fitted to a pre-tared distillation flask containing dielthylether and 2–3 glass regulators. After extraction of 2 h in 50–60 °C the cartridge allowed to cool and then the cartridges were placed in an oven at 105 °C for 12 h, following cooling in a desiccator and weighing. Placing in the oven for specific time, cooling in a desiccator and weighing was repeated until difference between two consecutive weights was smaller than 2 mg.
Meat emulsion preparation
Meat emulsion systems were prepared according to the methodology described by Vasquez-Mejia et al. (2019) with modifications in formulations and methodology. Six 500-g base emulsions were prepared (without artificial food dyes, preservatives, spices and seasonings), containing pork (54.86%), water (according to the level of Trigonella foenum graecum seed powder or starch incorporated), olive oil (26.14%) and sodium chloride (2%). Trigonella foenum-graecum seed powder (Tfg), Deffated Trigonella foenum-graecum seed powder (Dtfg) and Starch were included at two independent inclusion levels of 2%, 4%. Samples containing starch at the two inclusion levels (2%, 4%) were considered as control samples. The different meat emulsion system formulations which were produced for the different hydrocolloids used and their quantities are shown in Table 1.
Firstly, for the preparation of meat emulsion systems all ingredients (Ground pork, Olive oil, salts and Tfg, Dtfg, starch powders were weighed. The second step of preparation was the mixing of ground pork with sodium chloride and 50% of water (ice) in a 4-knife food processing machine (MCM3100W, 800 W, Bosch) for approximately 1 min for the purpose of salt-soluble meat proteins solubilization. Directly after this, the corresponding Tfg, Dtfg or starch powder level and the remaining 50% of water were added on the mixture and another 20 s of mixing took place. This step was immediately followed by the addition of olive oil and a final mixing for 30 s until obtaining a homogeneous mass. Throughout the mixing and cutting process, the temperature was below 10 °C, as ensured by monitoring the temperature and the use of ice instead of half the quantity water. Finally, each sample was stuffed into polyethylene bags, vacuum packaged and stored under refrigeration at 4 °C ± 0.5 °C until future analysis. The whole experiment was repeated in triplicate.
Proximate composition
Proximate composition properties of raw meat emulsions were evaluated using AOAC methods. More specifically, moisture content was determined by weight loss after drying for 12 h at 105 °C in a drying oven (AOAC, 2012). Fat content was determined in ground pork, meat emulsions and Trigonella foenum-graecum powder using the method 991.36 (AOAC 2016a). This method was performed with Soxhlet extraction using programmable heating mantle equipped with Pyrex glassware using diethyl ether as extraction solvent. Protein was determined using Kjeldahl method (AOAC 2016b) with an automatic Kjeldahl nitrogen analyzer.
Cooking loss
Evaluation of cooking loss was conducted gravimetrically according to the method proposed by Alvarez and Barbut (2013) with some modifications. Specifically, 30 g of raw samples was stuffed and weighed into 50 ml falcon polypropylene tubes and centrifuged (Hermle Z300K, Wehingen, Germany) for 2 min at 2000 rpm to remove any remaining air bubbles. Then, the tubes were placed in a waterbath (Memmert, Germany), which was fixed to 60 °C and when the internal temperature of the samples reached 50 °C, the temperature of the waterbath was increased to 80 °C and the samples were cooked until reaching an internal temperature of 72 °C. Internal temperature was measured with a thermocouple, which was inserted inside of one sample. The test tubes were cooled in a cold-water bath for 5 min and inverted for 14 h to release the exudate. Then, the tubes were weighed again, and cooking loss was expressed as the ratio between final weight and raw batter weight. Cooking loss test was performed in triplicate for each formulation.
TBARS analysis
2-thiobarbituric acid (TBA) test was used to evaluate the lipid oxidation in raw meat emulsions stored for 7 days at 4 °C, according to the method proposed by Hernandez et al. (2008) with modifications. Specifically, 10 g of raw meat emulsion of each formulation was transferred in a Duran glass vial and homogenized with 25 ml distilled water using an Ultra Turrax T18 Basic electric homogenizer (IKA Works Inc. Wilmington USA) in 14,000 g for 1 min. Directly after this step, each sample was transferred to distillation bottles and every Duran glass, previously containing the homogenated raw meat emulsion, was rinsed with 5 ml HCl 2 N and transferred into the distillation bottle. For the purpose of avoiding foam formation during distillation, 5 drops of silicon antifoam were transferred into the distillation bottle. Hydrodistillation was performed until collecting 50 ml of distillate. Then, 5 ml of the distillate were transferred to test tube with 5 ml of TBA 0.02 M and placed to boiling waterbath for 35 min. After cooling in a cold-water bath, absorbance was measured at 532 nm with a spectrophotometer (UV-1800, Shimadzu Co., Kyoto, Japan). The results were expressed as 2-thiobarbituric acid reactive substances (TBARS) in mg MDA (Malondialdehyde equivalents)/kg of raw meat emulsion and represent the average of two measurements for each sample. TBARS analysis was performed in duplicate for each formulation.
Measurement of antioxidative potential
Total phenols content (TPC)
Total phenols content in meat emulsion samples was determined according to the method proposed by Okarini et al. (2013) for evaluation of antioxidative potential in meat samples. More specifically, raw meat emulsion samples for each treatment were weighed and freeze dried for 24 h for total moisture removal. In the next step, 1 g of freeze-dried meat paste was extracted with 10 ml methanol (Chem-Lab, Zedelgem, Belgium) in an ultrasonic waterbath for 15 min and then filtrated. Total phenols content in filtrate was estimated by the Folin–Ciocalteau method. An 0.01 aliquot of each filtrate was added to 0.79 ml of distilled water. Directly after this 0.05 ml of Folin–Ciocalteau reagent were added to the solution (Merck KGaA, Darmstadt, Germany) followed by the addition of 0.15 ml of sodium carbonate solution (20% w/v). The reaction mixture was vortexed and the absorbance was measured at 750 nm with a spectrophotometer (UV-1800, Shimadzu Co., Kyoto, Japan) after kept in dark for 120 min at room temperature. Results were expressed as mg GAE (Gallic Acid Equivalents), based on the standard curve generated with GA (Gallic Acid) and represent the average of three measurements for each sample. TPC was performed in triplicate for each formulation.
1-Diphenyl-2-Picrylhydrazyl (DPPH˙) radical scavenging assay
DPPH˙ radical scavenging activity was estimated based on the method of Arnous et al. (2002) with modifications, on the meat emulsion extracts prepared with the method previously described. More specifically, in 50 μl of properly diluted sample 1950 μl of methanolic DPPH˙ solution (100 μM) was added. The reaction mixture was vortexed, and the absorbance was measured at 515 nm with a spectrophotometer (UV-1800, Shimadzu Co., Kyoto, Japan) after kept in dark for 30 min at room temperature. Control sample was prepared and measured using only methanolic DPPH˙ solution. Results were expressed as mM Trolox Equivalents (mM), after proper calculations and based on the standard curve generated with Trolox and represent the average of three measurements for each sample. DPPH˙ assay was performed in triplicate for each formulation.
Emulsion stability
Emulsion stability parameters were evaluated based on the method proposed by Horita et al. (2011) with some modifications. Briefly, certain amount of raw meat emulsions of each formulation was stuffed into falcon polypropylene tubes and weighed with subsequent centrifugation at 3,000 rpm for 3 min. Directly after this, the samples were heated at 50 °C in a waterbath for 15 min followed by 75 °C for 20 min. Then, after cooling in a cold-water bath the tubes were left upside down with the attachment of pre-weighed porcelain crucibles under every tube for exudate collection for 12 h in a desiccator. The total amount of released fluid (TFR) was expressed as a percentage of the sample weight. Water released (WR) was determined by the difference in the total liquid released after drying of exudates in an oven at 105 °C for 12 h and was expressed as a percentage of the sample weight. The content of released fat (FR) was determined by the difference between total released fluid and water released (WR). Emulsion stability tests were performed in triplicate for each formulation. Equations used for determination of emulsion stability parameters are given below:
Texture profile analysis (TPA)
Texture properties of the cooked meat emulsions were determined using a Texture Analyzer (Model XT2i, Stable Micro Systems, England). Meat emulsion samples of each treatment were cooked with the same method described previously in chapter 2.5. Analysis was conducted on the first day after meat emulsion manufacture. A 75 mm cylindrical cell was used to compress the meat emulsion samples. Six cores (2.5 cm height) were cut from each of six meat emulsion replicates per treatment and each one axially compressed to 75% of its original height in a two-cycle compression. Force–time curves were recorded at 1 mm/s. All tests were performed at room temperature (20–22 °C). The calculation of TPA values was obtained by graphing a curve using force and time plots. Values for hardness (N), springiness, cohesiveness, gumminess (N), and chewiness (N) were determined as described by Bourne et al. (1978). Collection and processing of TPA data was conducted with the Exponent Pro software.
Color evaluation
Instrumental color was conducted by taking a direct reading of raw meat emulsions with a colorimeter (Konica Minolta CR-400, USA). Standard observer was 2° (Closely matches CIE 1931 Standard Observer [(x2λ, yλ, zλ)]), 8 mm diameter circular aperture and d/0 (D65,Diffuse illumination / 0 < ° viewing angle) illuminate were used. The CIE-L*, a*, b* parameters were evaluated according to the methodology proposed by American Meat Science Association (AMSA 2012). Calibration of colorimeter performed using a white plate, L* = 97.58, a* = 0.03, b* = 1.08. For the color analysis of raw meat emulsions, 10 g of each sample was placed on a 9 cm diameter petri dish at 1 cm thickness. The average of nine readings was reported. All tests were performed at room temperature on the first day after manufacture and then five days after manufacturing (Table 2).
Statistical analysis
All statistical analyses were performed with SPSS (Version 23, IBM, USA). Homogeneity of variances were tested with Levene test in the case of non-normal data with SPSS. Normality of the residuals was tested using UNIVARIATE of SPSS, and consideration was given to the Shapiro-Wilks test for normality. Once it was determined that the assumptions of ANOVA were met for these data, the GLM procedure of SAS with a fixed effect of treatment and a random effect of replication was used for statistical determination of all variables. Tukey’s test (P < 0,05) was used to determine the differences between the treatment means. Least squares means were separated using 3 single degree of freedom estimate statements to determine the difference between meaningful comparisons, which included: 1) Starch (2%, and 4% inclusion) vs. Tfg (2% and 4% inclusion), 2) Starch (2% and 4% inclusion) vs. Dtfg (2%, and 4% inclusion), and 3) Tfg (2%, 4% inclusion) vs. Dtfg (2% and 4% inclusion). Differences were considered statistically different at P < 0.05. This experiment was completed in its entirety three times.
Results and discussion
Cooking loss
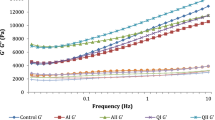
The meat emulsion’s ability to retain the moisture into the protein matrix before and after cooking process is an index of manufacturing efficiency and a quality factor in the same time (Jimenez-Colmenero et al. 2010; Horita et al. 2011). Temperature–time combination and method of thermal process, additives and binding agents incorporated in meat emulsion composition and fat content are the four more significant factors influencing weight losses during cooking. Among them, fat content and binding agents are the prevailing factors (Choi et al. 2009). Both hydrocolloid type (Tfg, Dtfg, Starch) and level of inclusion (2%, 4%) effect was observed for cooking loss (P < 0.05). Samples containing fenugreek in normal and defatted form had cooking losses ranging from 1–2%, while samples containing starch had cooking losses ranging from 5–6% (Table 3, Fig. 1). This reduction of cooking losses in the samples containing fenugreek seed powder in all forms, is mainly induced by the high amount of soluble dietary fiber, fenugreek galactomannan (Ahmad et al. 2016) whose chemical structure and G:M ratio (1:1.2) allow the high interaction of polymeric chains with water molecules leading to high water retention and increasing of viscosity (Jiang et al. 2007). Incorporation of dietary fibers in meat emulsion products are known to improve physicochemical characteristics (Eyiler-Yilmaz et al. 2016). Choi et al. (2009) observed lower cooking losses in meat emulsion products with dietary fiber from rice. Fat, from the other side, is also a major determinant of a meat emulsion’s protein matrix integrity and eventually moisture retainment extent. Thus, reduction of animal fat alone or with substitution with vegetable oils in meat emulsion systems is correlated with high cooking losses (Jimenez-Colmenero et al. 2010). In these studies, cooking losses were ranged between 7–8% and process yield was ranged between 80 and 85% for low fat emulsion type meat products with olive oil. However, in the present study, cooking loss values ranged between 1–2% in the samples containing fenugreek seed powder. This finding could be also attributed to the high protein content (~ 30%), which leads to olive oil emulsification and finally higher meat emulsion stability and moisture retaining through thermal process, despite the fact that direct comparison with results other studies is not favorable due to composition alterations.
TBARS analysis
Analysis was conducted in 7th day after manufacturing, by the means of microbiological growth in high population above this period. Generation of MDA and detection through analysis, is a time-consuming process, because of the protective activity of protein matrix to fat globules against oxidation through repulsion between peroxides and prooxidant compounds (Hernandez et al. 2008). Eventually, degradation of protein matrix, through physiological microbial deterioration, leaves fat globules exposed to oxidative activity of peroxides which leads to oxidation and MDA generation. Significant differences were observed for TBARS between Tfg samples and Starch, Dtfg samples, although differences between Starch and Dtfg samples were not significant (P > 0.05) (Table 3). Increasing of MDA values in Tfg samples is mainly attributed to the fat quantity and composition of fenugreek seed, which ranges between 5 and 8% and fatty acid composition comprises by approximately 80% unsaturated fatty acids, whose double C-bonds are more susceptible to oxidation processes than saturated fatty acids (Jo et al. 2020). Dtfg samples, however, presented significantly (p < 0.05) lower MDA values, which can be attributed to the antioxidant compound of fenugreek seed (Ahmad et al. 2016). Still, fenugreek seed contains high amount of glycosylated flavonoids of mainly 3 aglycones, namely apigenin, kaempferol and luteolin (Wani et al. 2016) and glycosylation is known to increase polarity of compounds, which is correlated with increased antioxidant activity (Hernandez et al. 2008).
Antioxidant Capacity (AC): Total phenolic content (TPC) and radical scavenging activity
In order to quantify AC and TPC of meat emulsion samples, DPPH* and Folin–Ciocalteau assays were used respectively. Both hydrocolloid type and level of inclusion had effect (P < 0.05) on these factors. The lowest values for DPPH* and TPC were observed in Starch 4% sample (0.085 ± 0.059 mg GAE/g sample, 0.0005 ± 0.00002 mg TRE/g sample), while the highest values for DPPH* were observed in Dtfg 4% sample (0.0013 ± 0.0001 mg TRE/g sample) and in Tfg 4% for TPC (0.631 ± 0.043 mg GAE/g sample) (Table 4). High DPPH* and TPC values for samples containing fenugreek seed powder can be attributed to the high quantity of compounds with antioxidant activity, which are mainly polyphenols 1–3% (Hozzein et al. 2019). Also, higher DPPH* values generally than TPC, can be attributed to the presence of variety of compounds with antioxidant activity except from polyphenols in fenugreek, such as steroid compounds (Diosgenin) (Ahmad et al. 2016). Thus, according to Table 4, starch samples obtained also high values for TPC and DPPH, that could be attributed to the high percentage of olive oil (15%) on meat emulsions composition. Olive oil is known as a rich source of antioxidants, mainly polyphenols ranging between 0.1–0.7 mg GAE/g olive and are comprised mainly of hydroxytyrosol, tyrosol, oleuropein, and oleocanthal (Bulotta et al. 2014).
Emulsion stability
Meat emulsion samples with incorporation of fenugreek had significantly (P < 0.05) lower values either for TFR%, WR% or FR% (Table 3, Fig. 1). More specifically, TFR for Tfg and Dtfg samples ranged between 1 and 5%, while for Starch samples ranged between 8–9%. These findings are in agreement with literature, where beta-glycan (Choi et al. 2009; Vasquez-Mejia et al. 2019) increased fluid retaining in meat emulsions with vegetable oils, although values for TFR% in samples with fenugreek were lower in the present study, due to higher quantity of functional ingredients like fenugreek galactomannan or protein. Generally, water releases (WR%) followed the same trend with TFR%. Fat release (FR%), is a factor of great significance for manufacturing low fat meat emulsion meat products with vegetable oils, because production of these products is correlated with stability problems generating mainly from physical properties differences between vegetable oils and animal fats (Jimenez- Colmenero et al. 2010). In the present study, fat losses for samples containing fenugreek were significantly lower (P < 0.05) than samples containing starch. More specifically, the highest fat release was observed in Starch 2% sample (6.18%) and the lowest fat release was observed in Dtfg 4% sample (0.21%). This finding is in agreement with literature, where Choi et al. (2009) found 1.33% fat loss in meat emulsions with rice fiber. However, in the present study the lowest fat loss percentage of 0.21% for Dtfg 4%, is mainly due to the fat removal before inclusion which increases the ratio of functional ingredients, mainly hydrocolloid and protein, despite the fact that direct comparison between studies is not favorable due to composition differences. Increasing emulsion stability through lowering of TFR, WR and FR with increasing fenugreek seed powder, mainly defatted, can be attributed to two main factors. Firstly, the high galactomannan content, increases water retaining, through gel formation processes and through interaction with myofibrillar proteins, which can improve physicochemical characteristics, like emulsion stability (Morin et al. 2004). Secondly, the high percentage of protein (~ 30%, Table 2) induces emulsification of oil globules that leads to increasing of emulsion stability, which is in agreement with literature (Jimenez-Colmenero et al. 2010), especially in high inclusion levels (4%) where increasing of water content acts as plasticizer.
TPA analysis
Every change in fat level or additive with binding abilities in meat emulsion products, would affect texture properties, as these changes influencing directly physicochemical properties such as water holding capacity or meat emulsion stability. This interdependent system of physicochemical and texture properties is affecting consumer’s acceptability for a product, thus is very important to enlighten the kind and extent of changes in texture properties by changes in meat emulsion’s composition.
TPA parameters differed significantly (P < 0.05) among samples and treatments. Hardness, springiness, gumminess and chewiness values were significantly lower (P < 0.05) in Tfg and Dtfg samples than Starch samples (Table 3). Also, inclusion level had significant effect (P < 0.05) in hardness, springiness, gumminess and chewiness.
The significant (P < 0.05) lower hardness values for Tfg and Dtfg, which ranged between 14 and 26 N, than Starch samples ranged between 34 and 41 N, which was also observed in gumminess and chewiness indicates that fenugreek seed powder has a positive impact in texture properties of meat emulsions, especially defatted fenugreek seed powder. Also, Vasquez-Mejia et al. (2019), observed that addition of 1–3% of commercial beta glucan product in meat emulsion systems was correlated with improvement of texture characteristics related to starch. This effect is attributed to the binding properties of the functional ingredients like dietary fibers and more specifically fenugreek galactomannan through water retaining leading to more tender and juicy structure in final products. However, the categorization of dietary fibers to soluble and insoluble is an additional factor determining the extent of the positive effect. In this sense, in the study of Choi et al. (2009), addition of rice bran fiber 1–3% in meat emulsion systems led to increased hardness, which is attributed to the high percentage of insoluble fiber in rice bran fiber (53.25%), in the same time that fenugreek seed powder has 21.7% soluble and 26.8% insoluble fiber (Ahmad et al. 2016). Greater percentage of insoluble fiber is correlated with higher interaction between fiber and protein which increases forces in protein matrix, leading to enhanced resistance to suppression (Youssef and Barbut 2009). Thus, the enhanced ability of soluble dietary fibers, like fenugreek gum, to gel formation and water retaining, related to insoluble fibers, is observed in different studies (Ktari et al. 2014).
Cohesiveness was affected both by hydrocolloid type and inclusion level (P < 0.05). More specifically, Tfg and Dtfg samples had significantly lower values than starch samples. The results in the study of Vasquez-Mejia et al. (2019) did not follow same trend, leading in no significant differences for cohesiveness between samples with b-glucan and/or MCC and starch, which, however, is attributed to the low purity of commercial additives (b-glucan, Starch = 33,73%). Despite this, fall of cohesiveness values in meat emulsion systems with addition of different dietary fibers was observed in literature (Alvarez et al. 2013; Choi et al. 2009). Given the fact that cohesiveness and springiness express generally the integrity and stability of protein complex depends on interaction between myofibrillar proteins and free water (Vasquez-Mejia et al. 2019) from one side, and increasing fenugreek seed powder, which has the ability to form gels at low temperatures (Jiang et al. 2007), increases water retention from the other side, it can be assumed that in meat emulsions with fenugreek seed powder, protein complex is not fully developed leading to lower values for cohesiveness. This can also be explained by lower viscosity values for these samples (Data not shown). Conversely, starch, which gelatinizes in high temperatures (> 70 °C), permits higher protein complex development and finally higher cohesiveness values.
Color measurement
Instrumental color parameters (L*,a*,b*) of raw meat emulsions are presented in Table 5.L* values were not different (P < 0.05) among treatments on 1st day, but in the 5th day after manufacturing Tfg, Dtfg samples had higher (P < 0.05) L* values related to Starch samples. Generally, addition of dietary fibers combined also with vegetable oils induced lighter and brighter color to meat emulsion systems (Alvarez et al. 2013), something which is more correlated to the distinctive color properties of fiber used. In the present study, fenugreek seed powder has a bright yellow color. Thus, given the fact that color change of a product would affect consumer acceptability, lighter color additives are more wanted in food applications, because they are correlated with lower impact on general appearance of final product (Ahmad et al. 2010).
Redness (a*) values are of great significance for consumer acceptability, due to the correlation between increased a* values and impression of high meat percentage in meat emulsion systems. In the present study, it was observed that although samples with fenugreek and especially Tfg samples had significantly a* values than starch samples, ranging between 2 and 4 and 1–2 respectively, on the 1st day after manufacturing, the same values for a* were lower for Tfg and Dtfg than Starch samples on the 5th day after manufacturing, ranging between 2–4 and 5–6 respectively. Vasquez-Mejia et al. (2019) were not observed significant differences between samples with b-glucan and MCC related to starch for a* value throughout storage, while Eyiler-Yilmaz et al. (2016) suggested decrease on a* values for samples with xanthan gum and carrageenan in meat emulsions with vegetable oils and finally, also, increase on a* values was presented for 4% addition in meat emulsion products in literature (Alvarez et al. 2013). Although safe conclusions are not favorable, results of our study could be attributed to higher emulsion stability and lower cooking losses in samples with fenugreek than samples with starch, which is leading to generate of sticky and aqueous surface on the latter causing reflect of light during measurement and finally leading to higher a* values on these samples, taking into account also the inclusion level effect (P < 0.05) on a* values leading into higher a* values on 4% percentage of inclusion.
Analysis of variation (ANOVA) through the different days of measurement, revealed significant effect for L* and a* value for starch samples. More specifically the overall change in L*, a* and b* values was greater than that of Tfg, Dtfg samples. A significant increase on a* value in Starch samples, leading to more dark red products throughout storage, can be attributed to stronger oxidative phenomena that could affect the products color. For example, oxidation of oxymyoglobin (light red bright color) to metmyoglobin (dark red color) is a major factor of color change through storage. Although oxidative color degradation in meat emulsions is an inevitable phenomenon during storage, the degradation rate is an important factor that increases shelf life of products in a sensory point of view. Thus, the presence of various antioxidants in fenugreek seed powder (Ktari et al. 2014), could be a reason for color stability through the 5 days of storage for Tfg, Dtfg samples, related to samples with starch.
Conclusion
It has been established in the present study that Trigonella foenum-graecum seed powder is a multifunctional plant material that can successfully improve physicochemical, texture and color properties of meat emulsion products with olive oil, mainly due to the distinctive combination of high galactomannan and protein percentages. More specifically, fenugreek seed powder lowers cooking losses and increases emulsion stability with increasing of inclusion level, related to control samples with starch. Especially defatted fenugreek seed powder fat release levels were below 1% for 4% inclusion level. Also, incorporation of defatted fenugreek seed powder has the advantage of low lipid oxidation levels during storage related to normal fenugreek seed powder. This could be linked with the high TPC and AC values for meat emulsion samples with defatted fenugreek seed powder. Additionally, fenugreek seed powder improved texture properties of meat emulsion with decreasing of hardness and chewiness in all levels, but a decrease in cohesiveness in samples with fenugreek was observed related to meat emulsions with starch, which is mainly attributed to the great retention of moisture which leads to the reduction of forces inside protein complex and a more flexible structure. In terms of color properties, fenugreek seed powder seems to improve color stability lowering the ratio of L* and a* degradation during storage. To our knowledge, this marks is the first and extensive study upon incorporation of Trigonella foenum-graecum seed powder in meat emulsion systems with olive oil related to starch. In this context, the results indicate that fenugreek and especially defatted fenugreek seed powder incorporation in meat emulsions with total replacement of animal fat with olive oil has a positive impact on physicochemical, texture and color properties. These are important findings in search for functional ingredients with applications on meat products with high olive oil content with extended shelf life from a physicochemical and sensory point of view.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aburezq H, Mansour M, Safer A, Afzal M (2008) Cyto-protective and immunomodulating effect of Curcuma longa in Wistar rats subjected to carbon tetrachloride-induced oxidative stress. Inflammopharmacology 16:87–95. https://doi.org/10.1007/s10787-007-1621-1
Ahmad A, Anjum FM, Zahoor T, Nawaz H, Ahmed Z (2010) Extraction and characterization of beta-D-glucan from oat for industrial utilization. Int J Biol Macromol 46(3):304–309. https://doi.org/10.1016/j.ijbiomac.2010.01.002
Ahmad A, Alghamdi SS, Mahmood K, Afzal M (2016) Fenugreek a multipurpose crop: potentialities and improvements. Saudi J Biol Sci 23(2):300–310. https://doi.org/10.1016/j.sjbs.2015.09.015
Álvarez D, Barbut S (2013) Effect of inulin, β-Glucan and their mixtures on emulsion stability, color and textural parameters of cooked meat batters. Meat Sci 94(3):320–327. https://doi.org/10.1016/j.meatsci.2013.02.011
AMSA (2012) Meat color measurement guidelines. American Meat Science Association. Illinois USA
AOAC (2016a) Official methods of analysis. Method 991.36. Fat (Crude) in meat and meat products (20th ed.). Gaithersburg, MD: Assoc Anal Chem
AOAC (2016b) Official methods of analysis. Method 990.03. Protein (crude) in animal feed, combustion method (18th ed.). Arlington, VA: Assoc Anal Chem
Arnous A, Makris DP, Kefalas P (2002) Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J Food Compos Anal 15(6):655–665. https://doi.org/10.1006/jfca.2002.1070
Bourne M (1978) Texture profile analysis. Food Technol 32:62–67
Bulotta S, Celano M, Lepore SM, Montalcini T, Pujia AD (2014) Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J Transl Med 12:219. https://doi.org/10.1186/s12967-014-0219-9
Choi Y, Choi J, Han D, Kim H, Lee M, Kim H, Jeong J, Kim C (2009) Characteristics of low-fat meat emulsion systems with pork fat replaced by vegetable oils and rice bran fiber. Meat Sci 82(2):266–271. https://doi.org/10.1016/j.meatsci.2009.01.019
Eyiler-Yilmaz E, Vural H, Yadigari RJ (2016) Thermal, microscopic, and quality properties of low-fat frankfurters and emulsions produced by addition of different hydrocolloids. Int J Food Prop 20(9):1987–2002. https://doi.org/10.1080/10942912.2016.1230743
Hernández-Hernández E, Ponce-Alquicira E, Jaramillo-Flores ME, Guerrero Legarreta I (2008) Antioxidant effect rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and colour of model raw pork batters. Meat Sci 81(2):410–417. https://doi.org/10.1016/j.meatsci.2008.09.004
Horita CN, Morgano MA, Celeghini RMS, Pollonio MAR (2011) Chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci 89(4):426–433. https://doi.org/10.1016/j.meatsci.2011.05.010
Hozzein WM, Saleh AM, Habeeb TH, Wadaan MAM, AbdElgawad H (2019) CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem 308:125661. https://doi.org/10.1016/j.foodchem.2019.125661
Hur SJ, Jin SK, Kim IS (2008) Effect of extra virgin olive oil substitution for fat on quality of pork patty. J Sci Food Agric 88(7):1231–1237. https://doi.org/10.1002/jsfa.3211
Isikli ND, Karababa E (2005) Rheological characterization of fenugreek paste (cemen). J Food Eng 69:185–190. https://doi.org/10.1016/j.foodeng.2004.08.013
Jiang JX, Zhu LW, Zhang WM, Sun RC (2007) Characterization of galactomannan gum from fenugreek (Trigonella foenum-graecum) seeds and its rheological properties. Int J Polym Mater 56(12):1145–1154. https://doi.org/10.1080/00914030701323745
Jiménez-Colmenero F, Herrero A, Pintado T, Solas MT, Ruiz-Capillas C (2010) Influence of emulsified olive oil stabilizing system used for pork backfat replacement in frankfurters. Food Res Int 43:2068–2076. https://doi.org/10.1016/j.foodres.2010.06.010
Jo S, Lee S (2020) Evaluation of the effects of aldehydes on association colloid properties and oxidative stability in bulk oils. Food Chem 338:127–778. https://doi.org/10.1016/j.foodchem.2020.127778
Kaltsa O, Spiliopoulou S, Yanniotis S, Mandala I (2016) Stability and physical properties of model macro- and nano/submicron emulsions containing fenugreek gum. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2016.06.025
Kasran M, Cui SW, Goff HD (2013) Emulsifying properties of soy whey protein isolate fenugreek gum conjugates in oil-in-water emulsion model system. Food Hydrocoll 30:691–697. https://doi.org/10.1016/j.foodhyd.2012.09.002
Kouzounis D, Lazaridou A, Katsanidis E (2017) Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurter sausages. Meat Sci 130:38–46. https://doi.org/10.1016/j.meatsci.2017.04.004
Ktari N, Smaoui S, Trabelsi I, Nasri M, Ben Salah R (2014) Chemical composition, techno-functional and sensory properties and effects of three dietary fibers on the quality characteristics of Tunisian beef sausage. Meat Sci 96(1):521–525. https://doi.org/10.1016/j.meatsci.2013.07.038
Morin L, Temelli F, McMullen L (2004) Interactions between meat proteins and barley (Hordeum spp.) β-glucan within a reduced-fat breakfast sausage system. Meat Sci 68:419–430. https://doi.org/10.1016/j.meatsci.2004.04.009
Okarini IA, Purnomo H, Radiati LE (2013) Proximate, total phenolic, antioxidant activity and amino acids profile of bali indigenous chicken, spent laying hen and broiler breast fillet. Int J Poult Sci 12(7):415–420. https://doi.org/10.3923/ijps.2013.415.420
Roberts RT, Cui SW, Wu Y, Williams SA, Wang C, Graham T (2014) Physicochemical evaluation of fenugreek gum and extrusion modified fenugreek gum and effects on starch degradation in bread. Bioact Carbohydr Diet Fibre 4(2):176–183. https://doi.org/10.1016/j.bcdf.2014.09.006
USDA (2016). National nutrient database for standard reference release 27, basic report: 07950, frankfurter, meat
Vasquez Mejia SM, de Francisco A, Bohrer BM (2019) Replacing starch in beef emulsion models with β-glucan, microcrystalline cellulose, or a combination of β-glucan and microcrystalline cellulose. Meat Sci 153:58–65. https://doi.org/10.1016/j.meatsci.2019.03.012
Wani SA, Kumar P (2016) Fenugreek: a review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci 17(2):97–106. https://doi.org/10.1016/j.jssas.2016.01.007
Youssef MK, Barbut S (2011) Fat reduction in comminuted meat products-effects of beef fat, regular and pre-emulsified canola oil. Meat Sci 87(4):356–360. https://doi.org/10.1016/j.meatsci.2010.11.011
Acknowledgements
The author expresses his honest gratefulness to professor Mourtzinos Ioannis and Katsanidis Eugenios of the Department of Food Science and Technology of Aristotle University of Thessaloniki, for their advice throughout the experiments of this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frangopoulos, T. Incorporation of Trigonella Foenum-Graecum seed powder in meat emulsion systems with olive oil: effects on physicochemical, texture, and color characteristics. J Food Sci Technol 59, 2060–2070 (2022). https://doi.org/10.1007/s13197-021-05220-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05220-3