Abstract
In this study, the occurrence of volatile nitrosamines were investigated in heat-treated sucuk, a kind of semi-dry fermented sausage. The pH, aw and residual nitrite of the samples were also determined. In addition, a principal component analysis (PCA) was also performed in order to elucidate the relationship between nitrosamine and these variables. Significant differences between brands were found in terms of NDMA (N- Nitrosodimethylamine), NPYR (N-Nitrosopyrolidine) and NPIP (N-Nitrosopiperidine) (p < 0.05). NDMA and NPYR varied from 1.71 to 3.57 µg/kg and 1.65 to 7.29 µg/kg, respectively. Higher levels were found for NPIP (5.19 to 16.40 µg/kg). NDEA (N-Nitrosodiethylamine) and NDBA (N- Nitrosodibutylamine) were not found in any of the heat-treated sucuk samples. The residual nitrite content was under 10 mg/kg in all samples. The aw and pH values varied between 0.913 and 0.940 and between 4.28 and 5.47, respectively. In PC1 explaining 72% of the variance, NDMA and NPYR were placed on the negative side, NPIP on the positive side. Residual nitrite and aw were more effective for NPIP, while pH was an important parameter for NDMA and NPYR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrate and nitrite used as curing agents in the meat industry, do not only act as preservatives but are also of crucial importance for color and flavor formation. Furthermore, these curing agents inhibit oxidative rancidity by delaying lipid oxidation (Drabik-Markiewicz et al. 2009; Ozel et al. 2010). In fermented sausages, nitrate and/or nitrite are used depending on the formulation and process conditions. However, nitrate should be reduced to nitrite to perform the same functions (Alahakoon et al. 2015). Nitrite plays an important role in the N-nitrosamine formation. N-nitrosamines are formed as a result of the reaction between the nitrosation agent and secondary amines (De Mey et al. 2017; Sallan et al. 2019).
The epidemiological studies showed that there is a potential relationship between nitrate, nitrite, and N-nitroso compounds and the risk of cancer (Alexander and Cushing 2011). In order to reduce nitrosamine formation in processed meat products, the use of nitrite/nitrate has been limited in many countries. However, many processed meat products still contain significant levels of nitrosamine as many factors influence the formation of nitrosamine (Molognoni et al. 2019). During the production of meat products, meat processing stages such as fermentation, drying and heat treatment can provide optimum conditions for the formation of nitrosamine (De Mey et al. 2017; Yurcenko and Mölder 2007). N-Nitrosamines are a subgroup of the N-nitroso compounds. These compounds can be divided into volatile nitrosamine and non-volatile nitrosamine (Herrman et al. 2015a). Most volatile nitrosamines are potential carcinogenic and mutagenic compounds that can induce to organ specific tumors (De Mey et al. 2017). Among N-nitrosamines, N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) are classified as probably carcinogenic. Other nitrosamines, such as N-nitrosodibutylamine (NDBA), N-nitrosopiperidine (NPIP), N-nitrosopyrrolidine (NPYR), and N-nitrosomorpholine (NMOR), which are commonly found in meat products, are possibly carcinogenic compounds (IARC, 2020).
Studies have been carried out on the formation and occurrence of nitrosamines in dry and semi-dry fermented sausages (Gavinelli et al. 1988; Yurchenko and Mölder 2007; De Mey et al. 2014a; Herrmann et al. 2015a,b). Studies on nitrosamines have also been carried out in sucuk, a kind of dry fermented sausage, produced in Turkey (Pirinçci et al. 1986; Ozel et al. 2010; Ata 2010; Sallan et al. 2019). Another type of fermented sausage produced in Turkey is heat-treated sucuk, a kind of semi-dry fermented sausage, and this product belongs to the group of the semi-dry fermented sausages. In the production of this product, there are three crucial main process steps, including short-term fermentation, heat treatment and drying. Heat-treated sausage production is preferred by the manufacturers due to its heat treatment application and short production time. The heat treatment application creates in the consumer the perception that the product is safer (Sallan et al. 2020). Although the degree of heat treatment applied varies among brands, the internal temperature in the heat treatment process is between 55 and 70 °C. However, the most common application is 60–68 °C (Armutcu et al. 2020). Although heat treatment is applied in the production of heat-treated sucuk, it is generally cooked before consumption (grilling or frying). This cooking treatment increases the nitrosamine content of heat-treated sausage depending on cooking degree (Sallan et al. 2020). Hence, occurrence of nitrosamines is an important issue in heat-treated sucuk. There is no study about the occurrence of nitrosamines in heat-treated sucuk available on Turkish market. The aim of the study was to determine the nitrosamine levels of the heat-treated sucuk samples available in the market, and to evaluate their relation with pH, aw and residual nitrite values.
Materials and methods
Materials
To determine nitrosamine levels of heat-treated sucuk available on the Turkish market, 10 different brands (A to J) were selected. In order to evaluate the results more precisely, samples were purchased for each company at three different times according to the batch number. Thus, a total of 30 samples (3 samples per brand) were examined.
Determination of water activity, pH, and residual nitrite
The water activity (aw) value of the samples was determined using a water activity device (Novasina, TH-500 aw Sprint). The device was calibrated with six different salt solutions before use.
For pH analysis, 10 g sample was homogenized with 100 mL of distilled water using an Ultra-Turrax homogenizer (IKA Werk T25, Germany). The pH value of the homogenate was measured by a pH meter (ATI ORION 420, MA 02,129, USA).
For the residual nitrite analysis, the method suggested by Tauchmann (1987) was used. The residual nitrite content was detected based on the sample weight, dilution factor, the coefficient calculated using standard curve and absorbance value, and the results were given as mg/kg NaNO2.
Determination of N-nitrosamines
10 g of the homogenized sample was weighed into centrifuge tubes and homogenized after adding 10 mL of 0.1 M NaOH solution. Afterward, the methanol added (20 mL) homogenate was subjected to a centrifuge process at 10.000 rpm at 4 °C. After the sample taken from the centrifuge was filtered with glass microfiber (70 mm diameter, Whatman GF Healthcare Life Sciences, UK), the extract was transferred to the Chem Elut column (Agilent ChemElut, 20 mL, Unbuffered, USA) by adding 5 mL of 20% NaCl solution. Then, the dichloromethane-added (50 mL) mixture was connected to the Kuderna-Danish apparatus and concentrated to 1 mL. The concentrate was evaporated under nitrogen (N-EVAPT™ 111, Nitrogen Evaporator, Clarion Safety Systems) at 40 °C, and GC/MS (Agilent 6890 N/5973, Agilent, Santa Clara, CA) was used in the detection of nitrosamines. In the system, helium was used as a carrier gas, and DB-5MS (30 m × 0.25 mm × 0.25 μm, Agilent) was used as the column, and it was run in the selected ion monitoring (SIM) mode. N-Nitrosodipropylamine-d14 (N525482, TRC, Canada) was used as the internal standard. The oven temperature was kept at 50 °C for 2 min, then it was increased to 100 °C at a rate of 3 °C/min and kept at this temperature for 5 min, following which the temperature was increased to 250 °C at a rate of 20 °C/min. The nitrosamine standard (EPA 521 nitrosamine mix, Supelco, Bellefonte PA) was used for identification and quantification of nitrosamines (N-nitrosomethylethlamine, NMEA;N-nitrosodiethylamine, NDEA; N-nitrosopyrrolidine, NPYR; N-nitrosodimethylamine, NDMA; N-nitrosopiperidine, NPIP; N-nitrosodibutylamine, NDBA) were determined at the µg/kg level (Wang et al. 2015).
The limits of detection (LOD) and limit of quantification (LOQ) of the N-nitrosamines were determined by standard solutions (1, 5, 10, 20, 30 and 40 µg/L). The LOD and LOQ were calculated using formula: LOD = 3.3 × Sy/s and LO = 10 × Sy/s (Sy: the standard deviation of the response of the curve, s: the slope of the calibration curve). The regression equation was established between peak area (y) and the concentration (x); the values of peak area and the concentration were the average of five repeated measurements at each concentration level, respectively. Mean recoveries ranged from 92.4 to 106.7% with relative standard deviations (RSD) ranging from 1.43–11.94%. The coefficients of the regression line (R2) for nitrosamine standard curve were all > 0.999. In samples, NDMA (LOD = 0.95 µg/kg, LOQ = 2.89 µg/kg), NPYR (LOD = 0.94 µg/kg, LOQ = 2.84 µg/kg) and NPIP (LOD = 0.97 µg/kg, LOQ = 2.94 µg/kg) could be determined. The results were given as µg/kg.
Statistical analysis
In the study, the brand was taken as a factor and the sampling time was taken as replication. Data were analyzed by the two-way ANOVA using a general linear model. Brand (10 brands) was evaluated as main effect, and replications (3 sampling time) were evaluated as random effect. The individual standard deviations of the means are presented in the figures. Differences between the means were tested using Duncan's multiple range test at the p < 0.05 level. All statistical analyses were performed using the SPSS version 20 statistical program (2011). Principal component analysis (PCA) was performed with the aid of The Unscrambler software (CAMO software version 10.1).
Results and discussion
a w , pH and residual nitrite
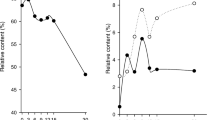
The mean aw and pH values and residual nitrite contents of the heat-treated sucuk samples obtained from different brands at different times are presented in Fig. 1 and Fig. 2, respectively. The highest mean aw value was determined in brand I as 0.940 ± 0.002, but there was no statistically significant difference between this mean value and the means of brands D, F, H and J (p > 0.05). Lower mean aw values were determined in the samples of three brands (A, G and C) (Fig. 1). Water activity varies between 0.900 and 0.950 in semi-dry fermented sausages (Caplice and Fitzgerald 1999) such as heat-treated sucuk (Armutcu et al. 2020). The pH value of the product can show a great variation. As can be seen from Fig. 1, the lowest mean pH value was determined in brand I as 4.28 ± 0.17, and the highest mean value was determined in brand A as 5.47 ± 0.29. In the Turkish Food Codex Meat and Meat Products Communiqué, pH value allowable is 5.6 in heat-treated sucuk (Anonymous 2019). In the present study, the pH value was below 5.0, except for A and G. It is thought that low pH values found are caused by starter cultures. Indeed, Honikel (2008) reported that low pH values promote nitrosamine formation. In addition, it was reported that N-nitrosamine can be formed more easily in dry fermented sausages, as the pH of the product comes closer to the optimum pH (pH 3.5) of the nitrosation reaction (De Mey et al. 2014a). However, it was emphasized that the reaction rate decreases tenfold for each unit increase in pH and there was also no sharp pH limit for nitrosation (Mirvish 1970).
Sodium nitrite is used as the curing agent in the production of heat-treated sucuk. However, it was reported that nitrite is the main nitrosation agent and directly leads to the formation of NDMA in dry-cured meat (Li et al. 2016). According to the Turkish Food Codex Food Additives Regulation (Anonymous 2013), the ingoing level for heat-treated sucuk is 150 mg/kg. The residual nitrite level was found to be under 10 mg/kg in all samples of the brands examined (Fig. 2). Since nitrite reacts with meat pigments and other compounds, the nitrite content decreases during the process and storage. Therefore, the residual nitrite content is quite low compared to the nitrite level added (Alahakoon et al. 2015).
N-nitrosamines
Nitrosodimethylamine (NDMA), Nitrosopyrolidine (NPYR) and Nitrosopiperidine (NPIP) were found in the all heat-treated sucuk samples, while Nitrosodiethylamine (NDEA), Nitrosomethylethlamine (NMEA) and Nitrosodibutylamine (NDBA) were not found in any of the samples.
The levels of nitrosamines can vary widely in cured meat products. Depending on the type of the product, volatile nitrosamines can occur at low levels (between < 5 µg/kg and 20 µg/kg), however, non-volatile nitrosamines occur higher levels (up to several thousand µg/kg) (Herrmann 2014). Among the volatile nitrosamines related to meat, NDEA is considered as the most potent carcinogen (Herrmann et al. 2015c). Unlike many fermented sausages, this nitrosamine was not found in any of the heat-treated sucuk samples. Ozel et al. (2010) determined NDEA (0.10–0.95 µg/kg) in 4 of 6 sucuk (dry-fermented sausage) samples. In a study conducted on salami samples taken from Italian markets, the NDEA content was determined to vary from nd to 4.04 µg/kg (Gavinelli et al. 1988). On the other hand, Yurchenko and Mölder (2007) investigated the occurrence of volatile N-nitrosamines in Estonian meat products and determined the mean level of 0.67 µg/kg for NDEA in salami samples. In the present study, NDBA was also not determined in heat-treated sucuk samples. In meat products, NDBA can generally be detected at very low levels or none (Yurchenko and Mölder 2007; Ozel et al. 2010; Sallan et al. 2020). However, Gavinelli et al. (1988) determined high NDBA (0.73–50.12 µg/kg) contents in salami samples. On the other hand, Yurchenko and Mölder (2007) reported the NDBA content for salami as 0.84 µg/kg.
NDMA is a volatile nitrosamine that is commonly found in processed meat products such as fermented sausages (Herrmann et al. 2015a). As is observed in Fig. 3, the lowest mean NDMA content in the heat-treated sucuk was determined in brand C as 1.71 ± 0.64 µg/kg. However, this mean value statistically differed only in brands H and I. The highest value was determined in brand I as 3.57 ± 0.57 µg/kg, and this value was found to be statistically different from the mean values of brands C, E, F and J. Ozel et al. (2010) detected nitrosamine in 5 of 6 sucuk (dry fermented sausage) samples and reported the highest NDMA content to be 0.78 µg/kg. On the other hand, Ata (2010) found a mean value of 2.21 ± 0.82 ng/g for NDMA in this type of fermented sausage. In contrast, Pirinçci et al. (1986) determined high levels for NDMA in sucuk samples (5.1 and 370 µg/kg). In commercial salami samples, researchers determined the maximum NDMA content to be 4.0 µg/kg and 7.2 µg/kg, respectively (Herrmann et al. 2015a). High nitrite levels can cause increase in the NDMA content (Drabik-Markiewicz et al. 2011). The use of nitrite up to 150 mg/kg is allowed in the production of heat-treated sucuk (Anonymous 2013). A study conducted on heat-treated sucuk reported that using 100 or 150 mg/kg nitrite increased the NDMA content. However, it was also stated that an additional cooking treatment (grilling for 1 min at 180 °C on hot plate) applied to the heat-treated sucuk did not increase the NDMA content (Sallan et al. 2020).
NPYR is another important nitrosamine found in cured meat products (Drabik-Markiewicz et al. 2010). The NPYR contents of the heat-treated sucuk samples obtained from different brands are presented in Fig. 3. Accordingly, the lowest mean NPYR content (1.65 ± 0.85 µg /kg) belongs to brand C, and this value did not differ statistically from the mean values determined in brands A, F, H and J. The highest mean value was determined to be 7.29 ± 0.80 µg/kg in brand B (Fig. 3). In another study conducted on sucuk, the NPYR content was 3.84 ± 0.88 ng/g (Ata 2010), and these results are in agreement with the present study results. On the other hand, it was reported that there was no relationship between nitrite and NPYR formation (Drabik-Markiewicz et al. 2009; Sallan et al. 2020). Proline is an important precursor, and it is even stated that the effect of proline on NPYR formation is higher than the ingoing nitrite level (Drabik-Markiewicz et al. 2009). On the other hand, the use of spices may pose a risk for the formation of nitrosamine due to nitrosamine precursors found in some spices (De Mey et al. 2017). Spices such as red pepper, black pepper, cumin and allspice are used in heat-treated sucuk production (Armutcu et al. 2020). For example, pyrrolidine found in black pepper is a good precursor for NPYR (De Mey et al. 2017). Therefore, the types and amounts of spices added to the formulation of heat-treated sucuk are thought to affect the nitrosamine content.
The NPIP contents of the heat-treated sucuk samples obtained from different brands are presented in Fig. 3. The lowest mean value was determined in brand A as 5.19 ± 3.13 µg/kg, but this value did not differ statistically from the values determined in brands B, D, and I. On the other hand, the highest mean value was determined in brand F as 16.40 ± 3.44 µg/kg, and this value also differed statistically from the mean NPIP values of other brands. In a study, a lower mean NPIP value (3.07 ± 0.89 ng/g) was found in Turkish dry-fermented sausage (Ata 2010). In a study conducted on heat-treated sucuk, it was reported that the nitrite level increased the NPIP level (Sallan et al. 2020). In addition, Herrmann et al. (2015a) reported that the NPIP level increased with increasing black pepper level in the formulation of fermented sausage. Another study also emphasized that black pepper could be a good source in the formation of NPIP (Yurchenko and Mölder 2007). In contrast, Sallan et al. (2019) stated that the NPIP content decreased depending on the black pepper amount. Antioxidant compounds found at high levels in black pepper inhibit the formation of NPIP by displaying an inhibitory effect on nitrosamine formation (Sallan et al. 2019).
Principal component analysis (PCA)
In this study, eigenvalues, variances and their cumulative proportions are shown in Table 1 and coefficients of first three principal components are given in Table 2. The first three principal components were analysed having eigenvalues greater than 1. By the first principal component 71.75% of the total variation was explained, 88.97% by the first two components and 95.73% by three ones. The first three principal components were plotted to show and highlight related data in a scatter plot with three axes (Fig. 4a, b and Fig. 5a, b).
In PC1, NDMA showed a negative correlation with aw and residual nitrite values, while NDMA in PC2 displayed a negative correlation with pH value. NPYR had a negative correlation aw and residual nitrite values except pH in PC1. In PC2, there was a negative correlation between NPYR and pH value. NPIP had a negative correlation with pH value both PC1 and PC2. In contrast, it showed a positive correlation aw and residual nitrite values in PC1.
Sallan et al. (2020) found that fermented sausage with starter culture showed higher NPIP and NPYR levels than samples without starter culture due to the lower pH value. The same study also reported that NDMA was not affected by pH. On the other hand, De Mey et al. (2014b) reported that a significant increase in NPIP levels was observed at lower pH. In the same study, the reduction of water activity also inhibited the NPIP formation. The samples exhibited an inhomogeneous distribution as can be seen Fig. 4b and samples obtained from the same brands were not separated with PC1 or PC2. However, samples taken from the same brands placed usually same side of PC1 or PC2 (Fig. 4b). In PC3, residual nitrite placed on positive side and this variable was related positively with NDMA, NPIP, NPYR and aw value (Fig. 5a). On the other hand, samples obtained from the same brands were also not separated with PC1 or PC3 (Fig. 5b). NDMA and NPYR placed on the negative side in PC1, NPIP on positive side in PC1. However, NDMA, NPYR and NPIP had a positive correlation in PC2 (Fig. 4a and Fig. 5a). According to the results of PC1 explaining 72% of the variance, aw and residual nitrite are more effective on NPIP, while pH is an important property for NDMA and NPYR. Sallan et al. (2020) reported that a combination of starter culture (low pH) and high nitrite content (150 mg/kg) was effective in NPYR formation.
Conclusion
In this study, NDMA, NPYR and NPIP were found in heat-treated sucuk samples obtained from different brands available in markets. However, higher NPIP levels are noticeable in heat treated sucuk. According to PCA results, NDMA and NPYR correlated with pH, while NPIP correlated with aw and residual nitrite. pH, aw and nitrite play a considerable role in the formation of nitrosamine. However, these factors can have different effects depending on the type of nitrosamine in heat-treated sucuk.
References
Alahakoon AU, Jayasena DD, Ramachandra S, Jo C (2015) Alternatives to nitrite in processed meat: Up to date. Trends Food Sci Tech 45:37–49. https://doi.org/10.1016/j.tifs.2015.05.008
Alexander DD, Cushing CA (2011) Red meat and colorectal cancer: a critical summary of prospective epidemiologic studies. Obes Rev 12(5):e472–e493. https://doi.org/10.1111/j.1467-789X.2010.00785.x
Anonymous (2013) Turkish Food Codex Food Additives Regulation, 2013/28693. Republic of Turkey Ministry of Agriculture and Forestry. Official Gazette Date and Issue: 30.06.2013–28693, Ankara, Turkey
Anonymous (2019) Turkish Food Codex Communiqué on Meat and Meat Products 2018/52. Republic of Turkey Ministry of Agriculture and Forestry. Official Gazette Date and Issue: 29.01.2019–30670, Ankara, Turkey.
Armutcu Ü, Hazar FY, Yılmaz Oral ZF, Kaban G, Kaya M (2020) Effects of different internal temperature applications on quality properties of heat-treated sucuk during production. J Food Process Pres 44(6):e14455. https://doi.org/10.1111/jfpp.14455
Ata Ş (2010) Nitrite, nitrate, secondary amines and nitrosoamines in biological, food and enviromental samples. Zonguldak Karaelmas University, Graduate School of Natural and Applied Sciences Department of Chemistry, Ph.D. Thesis, Turkey.
Caplice E, Fitzgerald GF (1999) Food fermentations: Role of microorganisms in food production and preservation. Int J Food Microbiol 50:131–149. https://doi.org/10.1016/S0168-1605(99)00082-3
De Mey E, De Maere H, Goemaere O, Steen L, Peeters MC, Derdelinckx G, Paelinck H, Fraeye I (2014a) Evaluation of N-Nitrosopiperidine formation from biogenic amines during the production of dry fermented sausages. Food Biopro Tech 7:1269–1280. https://doi.org/10.1007/s11947-013-1125-5
De Mey E, Viaene J, Dejaegher B, De Maere H, Dewulf L, Paelinck H, Vander Heyden Y, Fraeye I (2014b) A study of the effects of pH and water activity on the N-nitrosopiperidine formation in a protein-based liquid system. Food Bioprocess Technol 7:2978–2985. https://doi.org/10.1007/s11947-013-1249-7
De Mey E, De Maere H, Paelinck H (2017) Volatile N-nitrosamines in meat products: Potential precursors, influence of processing and mitigation strategies. Crit Rev Food Sci Nut 57(13):2909–2923. https://doi.org/10.1080/10408398.2015.1078769
Drabik-Markiewicz G, Maagdenberg KV, De Mey E, Deprez S, Kowalska T (2009) Role of proline and hydroxyproline in N-nitrosamine formation during heating in cured meat. Meat Sci 81:479–486. https://doi.org/10.1016/j.meatsci.2008.10.002
Drabik-Markiewicz G, Dejaegher B, De Mey E, Impens S, Kowalska T, Paelinck H, Vander Heyden Y (2010) Evaluation of the influence of proline, hydroxyproline or pyrolidine in the presence of sodium nitrite on N-nitrosamine formation when heating cured meat. Anal Chim Acta 657:123–130. https://doi.org/10.1016/j.aca.2009.10.028
Drabik-Markiewicz G, Dejaegher B, De Mey E, Impens S, Kowalska T, Paelinck H, Vander Heyden Y (2011) Influence of putrescine, cadaverine, spermidine or spermine on the formation of N-nitrosamine in heated cured pork meat. Food Chem 126:1539–1545. https://doi.org/10.1016/j.foodchem.2010.11.149
Gavinelli M, Fanelli R, Bonfanti M, Davoli E, Airoldi L (1988) Volatile nitrosamines in foods and beverages: Preliminary survey of the Italian market. Bull Environ Contam Toxicol 40(1):41–46
Herrmann SS (2014) N-nitrosamines in processed meat products-analysis, occurance, formation, mitigation and exposure, PhD Thesis, Techinal University of Denmak, National Food Intitute, Division of Food Chemistry.
Herrmann SS, Granby K, Duedahl-Olesen L (2015a) Formation and mitigation of N-nitrosamines in nitrite preserved cooked sausages. Food Chem 174:516–526. https://doi.org/10.1016/j.foodchem.2014.11.101
Herrmann SS, Granby K, Duedahl-Olesen L (2015b) Occurance of volatile and non-volatile N-nitrosamines in processed meat products and role of heat treatment. Food Cont 48:163–169. https://doi.org/10.1016/j.foodcont.2014.05.030
Herrmann SS, Duedahl-Olesen L, Christensen T, Olesen PT, Granby K (2015c) Dietary exposure to volatile and non-volatile N-nitrosamines from processed meat products in Denmark. Food Chem Toxicol 80:137–143. https://doi.org/10.1016/j.fct.2015.03.008
Honikel KO (2008) The use and control of nitrate and nitrite for the processing of meat products. Meat Sci 78:68–76. https://doi.org/10.1016/j.meatsci.2007.05.030
IARC, International Agency for Research on Cancer (2020) Agents Classifed by the IARC Monographs, Volumes 1–128, Last updated: 02.12.2020, https://monographs.iarc.fr/agents-classified-by-the-iarc/
Li F, Zhuang H, Qiao W, Zhang J, Wang Y (2016) Effect of partial substitution of NaCl by KCl on physicochemical properties, biogenic amines and N-nitrosamines during ripening and storage of dry-cured bacon. J Food Sci Technol 53:3795–3805
Mirvish SS (1970) Kinetics of dimethylamine nitrosation in relation to nitrosamine carcinogenesis. J Natl Cancer Inst 44(3):633–639
Molognoni L, Dauger H, Motta GE, Merlo TC, De Dea LJ (2019) Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res Int 125:108608. https://doi.org/10.1016/j.foodres.2019.108608
Ozel MZ, Gogus F, Yagcı S, Hamilton JF, Lewis AC (2010) Determination of volatile nitrosamines in various meat products using comprehensive gas chromatography–nitrogen chemiluminescence detection. Food Chem Toxicol 48:3268–3273. https://doi.org/10.1016/j.fct.2010.08.036
Pirinçci İ, Acet HA, Batı B (1986) Detection of N-nitrosamine compounds by gas chromatography in sausages. J Fac Vet Medic Selçuk Univ 2(1):75–89
Sallan S, Kaban G, Kaya M (2019) Nitrosamines in Sucuk: Effects of black pepper, sodium ascorbate and cooking level. Food Chem 288:341–346. https://doi.org/10.1016/j.foodchem.2019.02.129
Sallan S, Kaban G, OğraşŞişik Ş, Çelik M, Kaya M (2020) Nitrosamine formation in a semi-dry fermented sausage: Effects of nitrite, ascorbate and starter culture and role of cooking. Meat Sci 159:107917. https://doi.org/10.1016/j.meatsci.2019.107917
Tauchmann F (1987) Methoden der chemisschen Analytik von Fleisch und Fleischwaren. Kulmbach: Bundensanstalt für Fleischforschung, 80.
Wang Y, Li F, Zhuang H, Chen X, Li L, Qiao W, Zhang J (2015) Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT- Food Sci Tech 60:199–206. https://doi.org/10.1016/j.lwt.2014.09.022
Yurchenko S, Mölder U (2007) The occurance of volatile N-nitrosamines in Eastonian meat products. Food Chem 100:1713–1721. https://doi.org/10.1016/j.foodchem.2005.10.017
Funding
There is no funding in the study.
Author information
Authors and Affiliations
Contributions
GK: Supervision, validation, methodology, writing-review and editing. ZP: Formal analysis, investigation, writing-orginal draft preparation. SS: Formal analysis, methodology. MK: Writing-review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaban, G., Polat, Z., Sallan, S. et al. The occurrence of volatile N-nitrosamines in heat-treated sucuk in relation to pH, aw and residual nitrite. J Food Sci Technol 59, 1748–1755 (2022). https://doi.org/10.1007/s13197-021-05186-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05186-2