Abstract
Grain sorghum is a viable feedstock for lactic acid fermentation; however, tannins contained in some varieties affect the efficiency of hydrolysis and fermentation. This work objective was to assess the effect of pre-treatment of grain sorghum on the production of lactic acid and biomass after fermentation. Sorghum varieties with low, medium, and high tannins were pretreated, enzymatically hydrolyzed, and fermented with Lactobacillus casei. The pre-treatments consisted of cooking the grains in lime, cooking in plain water, and no treatment (control). Pretreated sorghum flours were hydrolyzed using thermostable α-amylase from Bacillus licheniformis and amyloglucosidase from Aspergillus niger. Lime pre-treatment showed a significant improvement in protein content, digestibility, and lactic acid production after fermentation, in relation to the non-treated samples. Although differences were not significant for low and medium tannins, lime treatment increase lactic acid production for the cooked-in-lime high-tannin sorghum in relation to the control. For this sorghum/treatment combination, the lactic acid production was 138 g/L, with a volumetric productivity of 1.57 g/L·h and 85/100 g yield based on initial starch, which is equivalent to 69 g of lactic acid per 100 g of sorghum d.b.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grain sorghum is an attractive agricultural feedstock for industrial fermentations and feed applications because of its high starch content and its agricultural advantages over other crops, such as the low requirement for water and fertilizer, wide adaptability for cultivation, high drought resistance, and high yields over other cereal crops. Sorghum’s ability to tolerate severe drought conditions and high-water use efficiency make a great choice for cultivation in arid regions. With a changing climate and increasing water scarcity, grain sorghum could be good substitute for others more nutrient and water demanding starch producing crops. On the downside, low protein content and quality and low bioavailability of micronutrients due to the presence of antinutritional factors such as polyphenols, and phytic acid restrict grain sorghum’s nutritional value (Egounlety and Aworh 2003; Mouquet et al. 2008).;
The antinutritional factors issue can be mitigated by either using low-tannin cultivars or by processing techniques. The so-called “white varieties” are low in polyphenols and phytic acid but, as a drawback, are more susceptible to birds and pests. These varieties are grown mainly in the USA, which is the largest producer and exporter of grain sorghum, accounting for 18% of world production and 79% of world sorghum exports in the last 5 years (USDA-FAS, 2020). Sorghum production is rising also in Australia and Europe in response to expanding market opportunities for industrial applications and in new food products, especially as an option for people with celiac disease (Tuinstra 2008). On the other hand, colored varieties of different polyphenols’ content are more resistant to pests, and these are the cultivars grown in Africa and South America (Elkhalifa et al. 2005).
Many different methods and processing techniques, such as cooking, germinating, milling, and fermenting, are used to improve cereal’s nutritional qualities. (Blandino et al. 2003; Pugalenthi and Vadivel 2005). Microbial fermentations have shown to be very effective at reducing antinutritional factors, improving mineral bioavailability, and enhancing the nutritional value of cereal substrates in general (Alka et al. 2012; Chen and Vadlani 2013; Hotz and Gibson 2007).
Condensed tannins in grain sorghum have been known to interact with proteins (Barros et al. 2012; Hagerman et al. 1992), digestive enzymes (Hargrove et al. 2011; Mkandawire et al. 2013), and starch (Díaz González et al. 2019). It is believed that condensed tannins may affect the in-vitro starch digestibility by inhibiting α-amylase during hydrolysis (Barros et al. 2012; Hargrove et al. 2011).
In previous research conducted in our lab, a chemical alkaline pre-treatment, also known as nixtamalization, was used to reduce the total polyphenols content in three types of grain sorghum. Three varieties containing low, medium, and high tannins concentrations were cooked with three different lime concentrations (0.0, 0.3, and 0.5%) for 35 min at 85 °C and steeped for 0, 8, 16, and 24 h at room temperature. The most successful pre-treatment (0.5% lime concentration and 8 h of steeping) reduced the polyphenol content by 79, 68, and 49% for high, medium, and low tannins varieties. Subsequent enzyme treatment with α‐amylase and amyloglucosidase showed that the reduction of polyphenols improved the conversion efficiency of starch into fermentable sugars. (Díaz González et al. 2019).
Lactic acid is one of the most widely used natural organic acids in pharmaceuticals, leather and textile industries, food manufacturing, and chemical feedstock. Production of lactic acid via fermentation is of interest because optically pure stereoisomers can be produced by using the right microbial strain (John et al. 2007).Specifically, L (+)-lactic acid is used as raw material for the synthesis of polylactic acid (PLA), an alternative to plastics derived from petrochemicals.
Raw materials used for the production of fermented lactic acid are very significant in the total production cost. Therefore, industrial production of lactic acid uses starchy materials as feedstock instead of refined sugars such as glucose, sucrose, or sucrose-containing molasses (Hofvendahl and Hahn–Hägerdal 2000).
In the last decade, an important body or research on sorghum fermentation has been published. However, the publications have been on fermented ethnic foods, fermented beverages, silage, sourdough bread, malting, bacterial isolation, and protein content and digestibility. No articles were found strictly on lactic acid production from grain sorghum.
In this research, we studied the production of lactic acid via fermentation with Lactobacillus casei NRRL B-441 using three varieties of lime-treated grain sorghum as substrates The objective was to investigate the viability of generating two products simultaneously: high purity L(+) lactic acid and a protein meal with enhanced digestibility.
The selection of L. casei NRRL B-441 was based on the work of Hujanen and Linko (1996) who screened seventeen homofermentative lactic acid bacterial strains for production of lactic acid efficiency and isomeric purity. Their results showed that L. casei NRRL B-441 was the best lactic-acid-producing strain. More recently John et al. (2007) reached a similar conclusion, and for these reasons we selected the NRRL B-441 strain for this research.
Materials and methods
Three sorghum varieties were chosen for this study based on their polyphenols content—low, medium, and high tannins. White low-tannin sorghum grain (hybrid 63WT6) was purchased from Blue Rivers Hybrid Organic Seeds Company (Kelley, Iowa USA). Medium-tannin (commercial black sorghum) and high tannins hybrid sorghum grain were purchased from Richardson Seeds, Ltd. (Vega, Texas USA). Food grade calcium hydroxide (Ca(OH)2), Mi Costeñita, was obtained from a local store. Enzymes used for liquefaction and saccharification were commercial α-amylase from B. licheniformis ≥ 500U/mg and amyloglucosidase from Aspergillus niger ≥ 300 U/mL from Sigma-Aldrich Co. (St. Louis, MO, USA). Homofermentative L. casei NRRL B-441 strain was obtained from the USDA microbial collection.
Experimental design
Each of the three sorghum varieties was pretreated, hydrolyzed with enzymes, and fermented with the L. casei. The pre-treatment consisted of cooking the grains in 0.5% lime, cooking in plain water, or performing no treatment at all (control). The pre-treatment followed a 3 × 3 factorial experimental design with two factors, Sorghum Variety and Pretreatment, and three levels. The levels for Sorghum Variety were white (low tannins), black (medium tannins), and high tannins (high tannins); and for the Pre-treatment were no treatment (control), cooked in plain water, and cooked in 0.5% lime. Three experiments were run in triplicates and the 27 experimental combinations were performed randomly.
Statistical difference was determine by calculating the standard error for a sample size of 3, and results were expressed as Mean ± Standard Error.
Pre-treatments of sorghum grains
The lime treatment followed the procedure described by Díaz-González et al. 2019. Briefly, 500 g of sorghum grains were incorporated into 1.5 L of a boiling 0.5% lime solution, and cooked for 35 min at 87 °C. Subsequently, the mix was let to cool down to ambient temperature, steeped for 8 h, and washed with DI water to remove leftover lime and loose pericarp. The grains were oven-dried at 60 °C for 2–4 h in a VWR gravity convection oven (Sheldon Manufacturing, Inc. Cornelius OR, USA) and ground. The second treatment, cooking in plain water, followed the same procedure, but deionized water was used instead of the lime solution. The third pre-treatment was the control, and therefore the grains were left untreated.
After treatment, all grains were ground in a Hamilton Beach 80,335 coffee grinder (Hamilton Beach Brands, Canada Inc. Ontario, Canada), and sieved through a 40-mesh screen to obtain the sorghum flours that would be used for the enzymatic hydrolysis. The flours were then placed in airtight containers and stored at 4 °C for few days until enzymatic hydrolysis.
Enzymatic hydrolysis
Pretreated sorghum flours were enzymatically hydrolyzed in a two-step process using commercial α-amylase from B. licheniformis followed by amyloglucosidase from A. niger (Díaz González et al. 2019). Pretreated flours (30 g) were placed in 250‐ml Erlenmeyer flasks and suspended in 90 ml of distilled water. The pH was adjusted to 6.0 with 0.1 M HCl followed by the addition of 240 μl of the thermostable α‐amylase. The samples were incubated for 30 min in a VWR reciprocal shaking water bath, model 1227 (VWR Sheldon Manufacturing, Inc. Cornelius, OR, USA), set at 90 °C and 50 strokes/min. At the 30-min mark, the flasks were removed from the bath, and after cooling to 80 °C, a second dose of 360 μl of thermostable α‐amylase was added and returned to the water bath for 45 min. The flasks were then taken out of the water bath and cooled down to around 60 °C. The pH of the liquefied slurries was adjusted to 4.5–5, using 0.1 M HCl, followed by the addition of 1.2 mL of amyloglucosidase from A. niger ≥ 300 U/ml to each flask. The Erlenmeyer flasks were returned to the thermostatic bath and incubated for 1 h at 55–60 °C while agitating at a speed of 50 strokes per minute to complete the starch saccharification. Once the hydrolysis was complete, flasks were inoculated with L. casei to start the fermentation.
Fermentation process
Preparation of inoculum
Lactobacillus Casei (NRRL B-441) cultures arrived as lyophilized pellets in glass vials. The pellets were rehydrated in 0.6 mL of sterilized Lactobacilli MRS Broth (Difco, USA) and then aseptically transferred to a cryovial containing 6 mL of the same MRS broth. Two mL of this seed culture broth was transferred to an Erlenmeyer flask containing 25 ml of sterilized MRS media and incubated at 37 °C and 100 rpm for 24 h in a Thermo Scientific MaxQ 4450 (Thermo Fisher Scientific Waltham, MA, USA). After reanimating the bacteria, 1 mL stock cultures were prepared by mixing 850 µL of reanimated bacteria with 150 µL of sterile 80% glycerol solution and stored at –80 °C until needed for inoculum preparation. The inoculum for the fermentation was prepared by transferring the content of two stock culture vials into a sterile 250-mL flask containing 100 mL of MRS broth and incubating at 37 °C and 100 rpm for 24 h. After 24 h, 10 mL of the cell culture was transferred to a 250-mL Erlenmeyer flask containing 100 mL of new sterilized MRS broth and incubated for 24 h under the same conditions. This process was repeated one more time to produce the third generation of cells that were used for inoculation. Next, cells were counted with a hemocytometer on a microscope to ensure a viable population of 107–108 cells/ml. Before introducing the samples into the hemocytometer, the cell suspensions were diluted to approximately 10–2 CFU/mL with 0.1% peptone solution, gently vortexed to get a uniform suspension, and a 200-µl aliquot mixed with 200 µl of 0.4% trypan blue in a centrifuge tube. The tube content was gently vortexed one more time, and about 20 µl of the suspension was slowly pipetted into the counting chamber of the hemocytometer. An average of five cell counts (in one set of 16 squares of the hemocytometer) was used to calculate the number of viable cells/ml using the hemocytometer’s procedure.
Finally, the inoculum was centrifuged to remove the MRS medium, and the cell pellets were re-suspended in 5 mL sterile medium right before inoculation.
Fermentation
Fermentations were conducted in 250-mL Erlenmeyer flasks containing the sorghum hydrolyzates and supplemented medium. Before inoculation, 30-g of hydrolyzed samples were supplemented with 90 mL of medium containing 0.2 g MgSO4·7 H2O, 0.05 g MnSO4·4 H2O, 0.5 g sodium acetate, 1.5 g KH2PO4, 1.5 g K2HPO4 and 10.0 g of yeast extract per liter of distilled water, plus 2% calcium carbonate (CaCO3) for buffering. The supplemented hydrolyzates were sterilized for 15 min at 121 °C. After cooling down to about 35 °C, the flasks were inoculated with the suspension of L. casei (108 cell/mL) and incubated in an orbital shaker Thermo Scientific MaxQ 4450 (Thermo Fisher Scientific Waltham, MA, USA), set at 37 °C and 150 rpm. Fermentations were monitored for up to 114 h to take periodic samples and adjust the pH. One-gram samples were taken every 3 h during the first 24 h and every 6 h subsequently until the end of the fermentation. Every time samples were taken, the pH was adjusted to 6.5 by the addition of ammonium hydroxide (NH4OH). Once the fermentations was complete, the remaining fermentation broth (~ 130 g) was centrifuged at 2360 × g for 15 min at 20 °C to separate the supernatant from the solids. A 5-mL aliquot of the supernatant was filtered with a 0.45 μm cellulose-acetate membrane syringe filter (VWR International LLC, Radnor, PA, USA) and frozen until analysis of lactic acid and sugar concentrations. The remaining liquid fraction was stored at 4 °C for later extraction and purification of lactic acid. The solid portion, which contained a thin layer of calcium carbonate on the surface, was frozen in the tubes for 24 h, so the calcium carbonate could be removed by scraping it off with a spatula. The remaining solids were oven-dried at 60 C for about 4–6 h and stored in sealed plastic containers for chemical analysis.
All fermentations were conducted in triplicate.
Analytical methods
Moisture and starch
Moisture content on the sorghum grains and flours was done in triplicates using a calibrated Omnimark uWave Microwave Moisture/Solid Analyzer (Omnimark Instrument Corporation, Long Island, NY USA). Total polyphenols content was determined according to the Folin‐Ciocalteu method (Singleton and Rossi 1965). Starch content was determined using the AACC Method 76‐13.01, Total Starch Assay Procedure (Megazyme Amyloglucosidase/alpha‐Amylase Method), using a Megazyme kit (Megazyme International Ltd. Bray, Ireland).
Fermentable sugars and lactic acid
The concentration of fermentable sugars in the hydrolyzates and lactic acid in fermented samples were determined by HPLC with Refractive Index detection using a Waters Chromatograph, consisting of a 1515 HPLC pump, a manual injector with a 50‐μl sample loop, a column heater, and a 2410 refractive index detector (Waters Corporation, Milford, MA, USA).
The column was a Phenomenex Rezex ROA‐Organic Acid H+ (8%) cation exchange column (300 × 7.8 mm) (Phenomenex Inc., Torrance, CA, USA), maintained at 60 °C. Compounds were eluted with a solution of 0.005 N sulfuric acid ran isocratically at a flow rate of 0.4 ml/min. Sugars and lactic acid concentrations were estimated using 5‐point calibration curves with glucose and lactic acid as the standards.
Protein content
Total crude protein on the sorghum flours was calculated by multiplying the difference between total nitrogen and ammonia nitrogen by 6.25. Crude nitrogen was obtained via total nitrogen combustion with an Elementar Variomax (Elementar Americas, Inc. Mt. Laurel, NJ, USA) by the Agricultural Diagnostic Laboratory, University of Arkansas (Fayetteville, AR USA). Ammonia nitrogen was assayed as follows: dry sample (1 g) and 30 ml of a 2 N solution of potassium chloride (KCl) were placed in sterile screw‐capped plastic centrifuge tubes. The tubes were shaken at room temperature for 15 min on an Eberbach reciprocal shaker (Eberbach Corporation, Ann Arbor, MI) set at high speed. Then, the extracts were filtered through Whatman #4 qualitative filter paper (GE Healthcare, Buckinghamshire, UK) into glass vials, and sent for ammonia nitrogen content analysis by Skalar automatic analyzer (Skalar Analytical, Breda, The Netherlands).
Protein digestibility
In vitro protein digestibility of the fermented and unfermented samples was determined following the method by Hsu et al. (1977). A multienzyme solution consisting of 1.6 mg trypsin (14,600 U/mg), 3.1 mg a-chymotrypsin (48 U/mg), and 1.3 mg peptidase (102 U/ml) (Sigma Aldrich Co. St. Louis, MO, USA)) was prepared fresh immediately before each test. The solid enzymes were dissolved in 1% sodium chloride and the pH adjusted to 8.0 with 1% sodium hydroxide. The multienzyme solution was kept in an ice bath until added to the slurries.
Slurries were prepared by adding enough dry sample to 50 mL of 1% sodium chloride solution to obtain a concentration of 6.25% protein. The amount of sample needed to prepare the slurries was based on the crude protein content of each sample. Next, the slurries were equilibrated at 37 °C and the pH adjusted to 8.0 with 0.1 M sodium hydroxide. Once the desired pH for each of the samples was stabilized, 5 mL of the multienzyme solution was added, and the pH followed for 10 min using a calibrated pH meter. Casein was used as a standard in the assay, and the percent in vitro protein digestibility was calculated according to the formula:
Lactic acid recovery and analysis
For the lactic acid recovery, the remaining liquid portion (~ 100 mL) was filtrated through a Whatman #1 filter paper, the filtrate mixed with activated carbon, and stirred for 3 h to eliminate color impurities. The activated carbon was then removed with a Whatman #1 filter paper under vacuum. The clear solution was then concentrated in a Buchi R-114 Rotary evaporator (Marshall Scientific, Hampton, NH, USA) at 60 °C under reduced pressure (2.5 kPa). The concentrate was then acidified to pH 2 with a 50% sulfuric acid solution. The lactic acid was then extracted from the concentrate into ethyl acetate using ultrasonic-aided solvent extraction, according to Hu et al (2017). Briefly, the concentrate was mixed with ethyl acetate (1:2 v/v) and extracted by sonication for 15 min at room temperature. The ethyl acetate containing the lactic acid was separated from the aqueous layer (raffinate) using a separatory funnel. A second extraction with ethyl acetate was performed on the raffinate under the same conditions to improve the final recovery. The lactic acid was separated from the ethyl acetate by evaporating the ethyl acetate at 40 °C and 2.5 kPa in the Buchi rotary evaporator. The lactic acid was further purified by running through an HPLC Bio-Rad Aminex HPX-87H organic acid column with 0.005 N H2SO4 as the mobile phase at 0.4 mL/min. Fractions corresponding to the lactic acid peak were collected immediately after the detector. The HPLC used was a Shimadzu with two LC-20AB pumps, a SIL-10AF autosampler equipped with a 50-µL sample loop, a DGV-20A3degasser, a CTO-20A column oven set at 65 °C, and an SPD-20AV UV–Vis detector set at 200 nm (Shimadzu Corporation, Kyoto, Japan). Lactic acid was diluted with deionized water, filtered through a 0.45-µm syringe filter, and a 50 µL aliquot injected. Multiple injections were performed to collect enough volume to confirm the isomeric purity.
The isomeric purity of the purified lactic acid was determined by measuring the optical activity with an automatic Rudolph AUTOPOL III Polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA) at a wavelength of 589 nm and 25 °C. A water solution of enantiomerically pure L (+)-lactic acid at a concentration of 0.1 g /mL was used as a standard.
Results and discussion
Pre-treatment and enzymatic hydrolysis
The untreated sorghum flours’ protein content was 10.74 ± 0.05, 12.77 ± 0.07, and 11.84 ± 0.04% (mean ± Standard Error), for low, medium, and high tannins varieties. The protein and starch contents of the lime-treated flours in this study (Table 1) were very similar to those obtained previously in our lab (Diaz González et al. 2019). After lime treatment, the sorghum flours from the three varieties showed just a slight increase in crude protein content compared to the untreated samples. On the other hand, the starch content of the lime-treated sorghum varieties revealed a significant increase of 13.42, 10.24, and 9.74%, for low, medium, and high tannins varieties respectively. This increase in starch content is due to the concentration of the grain endosperm after eliminating the pericarp and germ from kernels during the lime treatment.
After the enzymatic hydrolysis, the conversion efficiency of sorghum starch to fermentable sugars was estimated as reported in a previous work—Conversion efficiency (%) = Mass of sugars (g)/Mass of starch (g) × 1.11), where the factor 1.11 is used to adjust for the mass gain during the hydrolysis process (Diaz González et al. 2019). The hydrolysis conversion efficiency was higher in the lime treated sorghum varieties with levels of 95, 83, and 88% for low, medium, and high tannins sorghum varieties.
The low tannins variety showed the higher percent conversion yield, since they had the lowest tannins content, to begin with, and it had shown the higher increment in starch content upon lime treatment. These results reinforce the hypothesis that condensed tannins interfere with the enzymatic hydrolysis of starch, and lime treatment is a viable method to improve starch availability for the subsequent enzymatic hydrolysis (Diaz González et al. 2019).
Fermentation
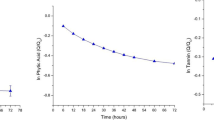
The duration of the fermentation runs with L. casei varied greatly depending on the pre-treatment and the sorghum variety, which dictated the amount of fermentable sugars available in the hydrolyzates prior fermentation. For sorghum varieties subjected to lime pre-treatment, with low, medium, and high tannins concentrations, the average fermentation time of three replicates was 106, 100, and 114 h. For the varieties cooked in plain water, the average times ranged from 94, 82, and 104 h for low, medium, and high tannins. Moreover, for the untreated low, medium, and high tannins lines, the times were 91, 77, and 96 h. As anticipated, the lengthier fermentations were those which substrates that underwent lime treatment, since they have shown the higher concentration of fermentable sugars compared to the samples that underwent different pre-treatments (Fig. 1).
Protein content and digestibility after fermentation
Overall, all fermented samples independently of the sorghum variety showed a significant rise in crude protein content after fermentation (Table 2). In terms of the type of pre-treatment applied before saccharification and fermentation, cooking in lime solution showed the highest protein content increment compared to those cooked in plain water or untreated for the three sorghum varieties. The increment in crude protein content from the naturally occurring 10.74 ± 0.05, 12.77 ± 0.07, and 11.84 ± 0.04% (mean ± Standard Error)—for low, medium and high tannins varieties respectively—to more than 18% for the untreated samples and 24% for the lime-treated ones after fermentation was the result of biomass production during the fermentation process (Table 2). Previous research attributes the protein increase in fermented cereals to the microbial synthesis of proteins from metabolic intermediates during bacterial growth cycles (Zamora and Fields 1979).
The effect of treatment on in vitro protein digestibility varied with the cultivar (Fig. 2). In the case of the low-tannin variety, there was not significant difference among the control and both treatments. The medium-tannin variety showed no difference between the control and cooking in water; however, protein digestibility was greatly benefited by the lime treatment. The high-tannin variety was favored by both cooking in plain water and cooking in lime. These observations reinforce the hypothesis that condensed tannins interfere with protein digestibility and are degraded during fermentation. Condensed tannins and phytic acid are known to bind to proteins and affect their availability (Hagerman et al. 1992). However, the detrimental effect of phytic acid and even condensed tannins can be diminished during microbial fermentation. Ali et al. (2003) reported that fermentation improves cereals’ nutritional value by triggering essential changes in their chemical composition while reducing antinutritional factors. Moreover, bacterial fermentation has also shown to decrease polyphenols and phytic acid content in pearl millet, probably due to the activation or production of enzymes.
Alternatively, improvement of protein digestibility of medium and high tannins varieties after processing could be have been resulted from the considerable reduction of the condensed tannins in the substrates during the pre-treatments. In general, the improvement in protein content and in vitro protein digestibility after fermentation may also be due to the partial degradation of complex storage proteins to simpler and more soluble molecules (Chavan et al. 1988). Another factor that might be contributing to this positive outcome is the further degradation of polyphenols and phytic acid by microbial enzymes produced during the fermentation process. The increase in protein digestibility could be the result of the production of proteolytic enzymes during fermentation and the reduction of antinutritional factors, which are known to form complexes with proteins diminishing their digestibility. These results are consistent with other reports that endogenous phytase of pearl millet or that produced by the fermenting microbes contribute significantly to the reduction of the phytate content of fermented pearl millet flour (Mahajan and Chauhan 1987).
Lactic acid production
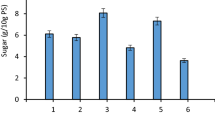
The effect of pre-treatments—cooking in water and in lime— on lactic acid production (Fig. 3) is not as straightforward as in the case of protein digestibility. There is an upward trend from the untreated (control) towards the treated samples within each variety. However, lactic acid production levels are not significantly different, so it is fair to say that the pre-treatments did not affect lactic acid production. When comparing the cooked-in-water treatments, lactic acid production is better for low-tannins than for medium tannins, and the output for high-tannins is not statistically different from the other two varieties.
The specific rotation of the obtained lactic acid was confirmed by comparison with the specific rotation of the enantiomerically pure L (+) lactic acid standard. The specific rotational value [α] of the obtained lactic acid was = + 2.54°, which is relatively close to +2.67° for pure lactic acid.
These findings demonstrate that high tannin grain varieties treated with lime can be used as feedstock for the production of L (+) lactic acid with high optical activity, and comparable yields to those produced by other cereals (Hujanen et al. 2001).
The conversion efficiency of fermentable sugars into lactic acid was over 90% for all sorghum varieties, regardless of the treatment. After hydrolysis, glucose concentration in the hydrolyzates of lime-treated low and high tannins variety was of 79.1 g and 73.2/100 g of sorghum d.b. Sugar consumption with the concurrent lactic acid production started within the first 3 h of fermentation. The process was stopped when the glucose concentration was almost zero at 81 and 92 h for the low and high tannins varieties respectively (Fig. 4).
The optimal pH for L. casei is between 6.3 and 6.5; therefore, pH control was used during the fermentation process. Thus, as could be expected, adjusting the pH to about 6.5 with ammonium hydroxide (15%) proved relevant in lactic acid production. The results demonstrated that the maximum lactic acid production (138 g/L) was accomplished with the lime-treated high tannin flour with a productivity of 1.57 g/L·h and an 85/100 g yield based on the initial starch. The lactic acid yields from high-tannin flours cooked in water and untreated were calculated to be 66/100 and 74/100 g based on initial starch, respectively, with a productivity of 1.07 and 1.26 g/L·h. Evidently, the lactic acid yield was lower when based on initial grain sorghum (Fig. 3).
The results of volumetric lactic acid productivity and yields presented in this research are similar or better in some cases to other lactic acid fermentations reported in the literature. Using liquefied barley starch and L. casei, Hujanen et al. (2001) obtained 44–68 g of lactic acid per 100 g of starch. Also using L. casei NRRL B-441, Linko and Javanainen (1996) and Hujanen and Linko (1996) obtained 98.0 and 91.0 g of lactic acid per 100 g of hydrolyzed barley starch or glucose supplemented with barley malt sprouts respectively.
Sorghum fermented with other bacterial strains such as L. plantarum, and L. delbrueckii sp. bulgaricus gave volumetric productivities of 4.5 and 2.8 g/L·h from sorghum extracts and sorghum extracts supplemented with vetch juice respectively (Samuel et al. 1980; Hofvendahl and Hahn–Hägerdal 2000).
Conclusion
Sorghum grains with low or no tannin content without any treatment had a better protein digestibility than high-tannin sorghum varieties. However, lime pre-treatment on high tannins- varieties have shown to be very effective in generating a protein-enriched product. For the low-tannin and medium-tannin varieties there is a clear trend on the improvement of lactic acid production after pre-treatment. However, the results are not statistically different; therefore, it cannot be interpreted as a real improvement. For the high-tannin grain sorghum, the lime treatment clearly improves lactic acid production in relation to the untreated control.
Availability of data and material
Data available within the article.
References
Ali MA, El Tinay AH, Abdalla AH (2003) Effect of fermentation on the in vitro protein digestibility of pearl millet. Food Chem 80(1):51–54
Alka S, Neelam Y, Shruti S (2012) Effect of fermentation on physicochemical properties and in vitro starch and protein digestibility of selected cereals. Int J Agric Food Sci 2(3):66–70
Barros F, Awika JM, Rooney LW (2012) Interaction of tannins and other sorghum phenolic compounds with starch and effects of in vitro starch digestibility. J Agric Food Chem 60:11609–11617
Blandino A, Al-Aseeria ME, Pandiella SS, Cantero D, Webb C (2003) Cereal-based fermented foods and beverages. Food Res Int 36:527–543
Chavan UD, Chavan JK, Kadam SS (1988) Effect of fermentation on soluble proteins and in vitro protein digestibility of sorghum, green grain and sorghum-green grain blends. J Food Sci 53(5):1573–1575
Chen L, Madl RL, Vadlani PV (2013) Nutritional enhancement of soy meal via Aspergillus oryzae solid-state fermentation. Cereal Chem 90(6):529–534
Díaz González D, Morawicki R, Mauromoustakos A (2019) Effect of nixtamalization treatment of three varieties of grain sorghum on the reduction of total phenolics and their subsequent enzymatic hydrolysis. J Food Process Preserv 43(9):14067. https://doi.org/10.1111/jfpp.14067
Egounlety M, Aworh OC (2003) Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and ground bean (Macrotyloma geocarpa Harms). J Food Eng 56:249–254
Elkhalifa AO, Schiffler B, Bernhardt R (2005) Effect of fermentation on the functional properties of sorghum flour. Food Chem 92:1–5
Hagerman AE, Robbins CT, Weerasuriya Y, Wilson TC, McArthur C (1992) Tannin chemistry in relation to digestion. Rangeland ecology & management. J Range Manag 45(1):57–62
Hargrove JL, Greenspan P, Hartle DK, Dowd C (2011) Inhibition of aromatase and α-Amylase by flavonoids and proanthocyanidins from sorghum bicolor bran extracts. J Med Food 14:799–807. https://doi.org/10.1089/jmf.2010.0143
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol 26(2–4):87–107
Hotz C, Gibson RS (2007) Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J Nutr 137(4):1097–1100
Hsu HW, Vavak Dl, Satterlee LD, Miller GA (1977) A multienzyme technique for estimating protein digestibility. J. Food Sci 42:1269–1273
Hu Y, Kwan TH, Daoud WA, Lin CSK (2017) Continuous ultrasonic-mediated solvent extraction of lactic acid from fermentation broths. J Clean Prod 145:142–150
Hujanen MLYY, Linko YY (1996) Effect of temperature and various nitrogen sources on L (+)-lactic acid production by Lactobacillus casei. Appl Microbiol Biotechnol 45(3):307–313
Hujanen M, Linko S, Linko YY, Leisola M (2001) Optimization of media and cultivation conditions for L (+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Appl Microbiol Biotechnol 56(1):126–130
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74(3):524–534
Linko YY, Javanainen P (1996) Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb Technol 19(2):118–123
Mahajan S, Chauhan BM (1987) Phytic acid and extractable phosphorus of pearl millet flour as affected by natural lactic acid fermentation. J Sci Food Agric 41(4):381–386
Mkandawire NL, Kaufman RC, Bean SR, Weller CL, Jackson DS, Rose DJ (2013) Effects of sorghum [Sorghum bicolor (L.) Moench] tannins on α-amylase activity and in vitro digestibility of starch in raw and processed flours. J Agric Food Chem 61:4448–4454. https://doi.org/10.1021/jf400464j
Mouquet-Rivier C, Icard-Vernière C, Guyot JP, Hassane Tou E, Rochette I, Trêche S (2008) Consumption pattern, biochemical composition, and nutritional value of fermented pearl millet gruels in Burkina Faso. Int J Food Sci Nutr 59(7–8):716–729
Pugalenthi M, Vadivel V (2005) Nutritional evaluation and the effect of processing methods on antinutritional factors of sword bean (Canavalia gladiata (Jacq.) DC). J Food Sci Technol 42(6):510–516
Samuel WA, Lee YY, Anthony WB (1980) Lactic acid fermentation of crude sorghum extract. Biotechnol Bioeng 22(4):757–777
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Tuinstra MR (2008) Food-grade sorghum varieties and production considerations: a review. J Plant Interact 3(1):69–72
USDA Foreign Agricultural Service (2020). USDA foreign agricultural service production, Supply, and distribution database. Foreign agricultural service, department of agriculture: https://data.nal.usda.gov/dataset/usda-foreign-agricultural-service-production-supply-and-distribution-database. Accessed 16 April 2021
Zamora AF, Fields ML (1979) Microbiological and toxicological evaluation of fermented cowpeas (Vigna sinensis) and chickpeas (Cicer arietinum). J Food Sci 44(3):928–929
Acknowledgements
To the United Sorghum Checkoff Program for the financial support to conduct this research.
Funding
The United Sorghum Checkoff Program and the United States Department of Agriculture.
Author information
Authors and Affiliations
Contributions
Author DG was responsible for the data acquisition, interpretation of data, and manuscript preparation. The corresponding author, Morawicki, contributed to the experimental design, data analysis, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Consent for publication
Both authors, Diaz Gonzalez and Morawicki, agreed to submit this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diaz Gonzalez, D., Morawicki, R. Lactic fermentation of grain sorghum: effect of variety and pretreatment on the production of lactic acid and biomass. J Food Sci Technol 59, 1221–1229 (2022). https://doi.org/10.1007/s13197-021-05132-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05132-2