Abstract
This study was investigated to evaluate the phenolic acid composition, antioxidant potential and acceptance of rusk prepared by the progressive replacement of wheat flour with barley flour. The wheat-barley blends rusks were also evaluated for their pasting and sensorial properties. The pasting characteristics of wheat flour was influenced by barley flour incorporation with a increase in peak and final viscosity values with increasing amount of barley flour. The results revealed that incorporation of barley flour into wheat flour improved the nutritional and bioactive compounds profile. The free radical scavenging activities towards DPPH and ABTS+ was found to be higher for wheat-barley blends rusk in comparison with wheat rusk. As evident from total phenolic content, total flavonoids contents and antioxidant activities, barley flour was found to be rich in bioactive compounds in comparison with wheat flour. Supplementation of rusks with 30% barley flour were suggested to be nutritionally superior with an acceptable sensory score and is a successful approach to enrich rusks with nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Busy life style, hectic schedule and globalization have shifted consumer’s interest towards bakery products because of their ready-to-eat nature, convenience, and long shelf life. Biscuits, crackers, cakes, breads, rusks are most popular products of bakery industry. Wheat is the most versatile cereal and generally all the bakery foods are formulated from wheat. Although wheat provides nutrients and calories but it is still bio-actively and nutritionally poor. Wheat’s protein quality is inferior to when compared with other cereals because of low lysine, methionine and threonine content of wheat proteins (Yakoob et al. 2018). Traditionally neglected barley cereal used has been boosted either as whole grain or as an ingredient. Barley has an excellent nutritional profile and has increasingly become an active ingredient in healthy food because it has been known to have high amount of dietary fibres (β-glucan), phenolic compounds, arabinoxylans and bioactive polypeptide (Punia et al. 2017; Sandhu and Punia 2017; Alu’datt et al. 2012). Barley mainly contains phenolic such as ferulic acid, caffeic acid, p-coumaric acid, protocatechuic acid, flavonols and flavan-3-ols (Hernanz et al. 2001). Being a source of functional compounds, barley is reported to have potential of lowering blood cholesterol, glycemic index (GI) and reduce the risk of chronic diseases (Brennan and Cleary 2005). Barley is a healthier version of other cereals and addition of barley or barley constituents to foods is usually aimed at increasing the content of total and soluble fibre in foods, improving their physiological efficacy, and providing health benefits. Therefore, the supply of barley’s functional components through baked products may be an interesting opportunity to improve the nutritional behavior of bakery products (Dhull et al. 2019). By the consequences of modern life style and changing environment, development of such kind of functional food products increases nutritional as well as health status. Previous studies have reported effect of barley addition on the quality characteristics of different baked products cakes (Yakoob et al. 2018), cookies (Sharma and Gujral, 2014a), chapatti (Sharma and Gujral 2014b), chocolate chips cookies (Frost et al. 2011), bread (Holtekjolen et al. 2008) etc. The previously published studies have shown that addition of barley to the cereal-based products is a successful attempt of improving their phenolic level and antioxidant potential. To the best of our knowledge, studies regarding the incorporation of barley to wheat flour in rusks appear to be limited. This prompted us to investigate the effect of incorporation of barley at level of 10, 20, 30, 40 and 50% on the phenolic acid composition, antioxidant potential and sensorial properties of rusks.
Materials and methods
Materials
Wheat cultivar (cv.WH-1080) and barley cultivar (cv.BH-393) were procured from Chaudhary Charan Singh Haryana Agricultural University, Hisar for preparing rusks. The protein contents of wheat and barley were 12.33 and 13.3%, respectively. The grains were cleaned and milled into fine powder using grinder (Newport Super mill, Australia) to pass through 60 BSS (250 μm) sieve to obtain wheat and and stored in a refrigerator till further analysed.
Preparation of wheat flour and wheat–barley flour blends
Barley flour (BF) was incorporated to wheat flour (WF) and the blends were named as WF (100% wheat flour), BF-10% + WF-90%, BF-20% + WF-80%, BF-30% + WF-70%, BF 40% + WF-60%, and BF-50% + WF-50%.
Water absorption capacity and water solubility index
Water absorption capacity and water solubility index of flour and blends were measured by the method described by Sosulski (1962).
Oil absorption capacity and foaming stability
For the determination of oil absorption capacity and foaming stability the method of Lin et al. (1974) was followed.
Emulsion stability
Emulsion stability was evaluated according to the method described by Naczk et al. (1985).
Pasting properties of blends
Pasting properties of flours were studied using in-build cell of Modular Compact Rheometer (MCR 52, Austria). Parameters recorded (Fig. 1) were pasting temperature, peak viscosity, trough viscosity, final viscosity, breakdown viscosity and setback viscosity. All the measurements were replicated thrice.
Color characteristics of blends and rusks
Hunter color measurement of flours was carried out using a Hunter Colorimeter fitted with optical sensor (Hunter Associates Laboratory Inc. Restan VA., USA) on the basis of L*, a*, b* color system.
Rusk preparation
Rusks were prepared by following the method described by Mallik and Kulkarni (2010). Dough was prepared by blending 500 g wheat flour/wheat-barley flour blends with 80 g fat, 80 g sugar, 4 g salt and 16 g yeast. In control samples, additionally 12 g skim milk powder and 220 g water were used. Wheat flour was replaced by barley flour at 10, 15, 20, 25 and 30% levels, respectively. The ingredients after mixing were kneaded to a smooth dough and allowed to stand for 20 min. Again the dough was kneaded and allowed to stand for further 20 min. The dough was then shaped and placed in greased baking tray and allowed to stand for 15 min, for final processing followed by baking at 225 °C for 15–20 min. After baking, the loaves were cooled for 1 h, and then placed in a refrigerator for about 2 h for the ease of slicing. The loaves were then mechanically sliced to pieces about 1 cm of thickness followed by roasting at 200 °C for 15 min in an oven.
Sensory evaluation
The rusks prepared from wheat flour and wheat-barley flour blends were subjected to sensory analysis by a semi trained panel comprising of 30 people. The panellists aged 20–35 years comprised of postgraduate students, research scholars, and faculty members of the department of Food Science and Technology, Chaudhary Devi lal University, Sirsa, Haryana. The sensory testing of the samples was done under ambient conditions in a comfortable and quite area without distractions under proper lighting and controlled temperature. The panellists evaluated the prepared rusks for the parameters crust color, crust hardness, crumb color, crumb chewiness, taste, aroma and overall acceptability.
The samples were served in porcelain dishes labelled randomly with three digit numbers. Each panellist received a rating form scored on a 9 point hedonic scale (9, ‘like extremely’; 8, ‘like very much’; 7, ‘like moderately’; 6, ‘like slightly’; 5, ‘neither like nor dislike’; 4, ‘dislike slightly’; 3, ‘dislike moderately’; 2, ‘dislike very much’; 1, ‘dislike extremely’) to rate the rusks for various parameters. Mineral water was provided as palate cleansers between each sample. After palate cleansing, a pause (15 s) was given before the next assessment. The sensory analysis of rusks was replicated three times with a fresh batch of rusks each time.
Total phenolic content of wheat-barley flour blends and rusks
Total phenolic content was determined by following the Folin–Ciocalteau method as described by Gao et al. (2002). Gallic acid was used as the standard, and results are expressed as μg gallic acid equivalents (GAE)/g of flour.
High performance liquid chromatography (HPLC) analysis of phenolic acids of wheat-barley flour blends and rusks
The prepared methanolic extracts were analyzed for identification and quantification of gallic, ferulic, chlorogenic acid, p-hydroxybenzoic, syringic, p-coumaric, and ferulic acid. HPLC analysis was carried out using Agilent 1260 Infinity series HPLC (Agilent Technologies, Santa Clara, CA) equipped with Quaternary Pump VL (G1311C) and degasser, 1260 ALS auto-sampler (G1329B) and 1260 DAD VL detector (G1315D). Separations were achieved using ZORBAX Eclipse C18 column (Agilent Technologies) under the gradient flow of 0.1% acetic acid in water (v/v) as solvent A and methanol as solvent B. The gradient flow was run as described by Kumar et al. (2014) (0–1 min, 30% B; 1–10 min, 65% B; 10–14 min, 80% B; 14–16 min, 80% A; 16–17 min, 40% B; 17–20 min, 35% B and 20–21 min, 30% B) and the detection wavelength was set as 280 nm. The flow rate was adjusted as 1 ml min−1 and the column temperature was set as 30 °C. The injection volume was set as 5 µl. For the purpose of quantification, the calibration curve was prepared using a mixture of purified standards such as gallic, ferulic, chlorogenic acid, p-hydroxybenzoic, syringic, p-coumaric, and ferulic acid. The results were expressed as µg/g DW of the sample.
Antioxidant activity (AOA) of wheat-barley flour blends and rusks
AOA was measured using a modified version of the method described by Brand-Williams et al. (1995). Methanol was used as a blank, and antioxidant activity was calculated as percent discoloration:
Total flavonoids content of wheat–barley flour blends and rusks
Total flavonoids content was determined by following the method described by Jia et al. (1998). Catechin was used as standard and the results were reported as μg catechin equivalents (CE)/g of sample.
Metal chelating activity of wheat–barley flour blends and rusks
Metal chelating activity was measured by following the method described by Dinis et al. (1994). The chelating activity of the extract for Fe2+was calculated as follows:
ABTS+ scavenging capacity of wheat–barley flour blends and rusks
ABTS+ scavenging activity was measured by following the method described by Re et al. (1999). A standard curve was prepared by using different concentrations of vitamin C similar to DPPH assay. ABTS+ scavenging property was expressed as vitamin C in μmol/g of wheat.
Statistical analysis
The data reported in all the tables are expressed as mean ± standard deviation of three independent replications. The data were subjected to one-way analysis of variance using Minitab statistical software version 15 (Minitab, Inc., State College, PA).
Results and discussion
Physico–hemical properties of wheat flour and wheat–barley flour blends
Physico-chemical properties of wheat flour and wheat-barley flour blends differed significantly (p < 0.05). The water absorption capacity, water solubility index, oil absorption capacity, foaming stability and emulsion stability of wheat flour were 135%, 8.12, 135%, 73.2%, and 44.5%, respectively. The incorporation of barley flour to wheat flour in the blends significantly (p < 0.05) increased the measured functional properties of flours. Both water and oil absorption capacity increased significantly (p < 0.05) and the values of blends ranged between 141 and 173% and 137–151%, respectively. Sharma and Gujral (2014a) reported that an increase in water absorption capacity after incorporating barley flour into wheat flour may be due to increased levels of dietary fibres including β-glucan which hold more water. An increase in oil absorption capacity may be attributed to the higher fiber content of the blends as the surface of fiber has a high capacity to hold oil by a mechanical process. Wheat-barley flour blends showed increased foaming stability and emulsion stability in comparison to wheat flour; values ranged between 75.3–78.9% and 45.6–49.2%, respectively. Alu’datt et al. (2012) reported that enhanced solubility of wheat flour by incorporating barley flour may be responsible for formulating bakery products which require good emulsifying and foaming properties.
Pasting properties of wheat flour and wheat–barley flour blends
The results regarding pasting properties of wheat flour and wheat-barley flour blends are presented in Table 1. Paste viscosity and final viscosity of wheat flour was observed to be 330 mPa s and 827 mPa s, respectively. Peak viscosity is an indicator of ease with which the starch granules are disintegrated and often correlated with final product quality (Yaqoob et al. 2018). Incorporation of 10–50% barley flour in wheat flour increased peak and final viscosity with values ranging between 363–741 mPa s and 859–1349 mPa s, respectively. Setback viscosity also increased as the incorporation of barley flour increased in wheat-barley flour blends and the values ranged between 549 and 757 mPa s. As proportion of barley flour in wheat flour increased, a progressive increase in peak viscosity, final viscosity and breakdown viscosity was observed, which may be due to the soluble fibers in the barley flour. It is reported that dilution of wheat starch with barley constituents such as β-glucan also contributes to such a trend in pasting properties (Yaqoob et al. 2018). Lazaridou et al. (2003) also reported that a solution containing β-glucan is more viscous than starch solution of same concentration. Hence, an increase in β-glucan content in flour blends with increase in barley fraction will result in increase in the pasting properties. Pasting temperature of wheat flour was 64.8 °C and at increased level of incorporation of barley flour no significant increase was observed.
Gravimetric characteristics
Gravimetric properties (water absorption capacity, loaf weight and loaf height) are shown in Table 2. Water absorption capacity of dough varied significantly (p < 0.05) and ranged between 81 and 151 ml. Water absorption capacity of dough increased as the level of barley flour in wheat flour was increased. Increase in water absorption capacity may be due to presence of dietry fibres (β-glucan) which holds more water. The loaf weight for wheat flour was 225 g and with increase the level of barley flour in wheat flour, the loaf weight was increased (244–275 g). Before baking, the height of dough ranged from 4.7 to 5.5 cm. Higher water absorption capacity is responsible for higher loaf weight. The height of dough after baking increased; values ranged from 5.5 to 8.7 cm. wheat flour dough showed the maximum loaf height after baking (8.7 cm) whereas blend with 50% barley flour showed the lowest loaf height. The decrease in loaf height with the increase in level of barley may be owing to less gas retention power of the wheat-barley blends. A dilution effect on gluten is observed with the addition of non-wheat flour to wheat flour that leads to less retention of carbon dioxide gas causing a depression in loaf height.
Hunter color characteristics of wheat flour and wheat–barley flour blends and rusks
Hunter color characteristics of wheat flour and wheat-barley flour blends and rusks are summarized in Table 2. The L* value indicates the lightness, 0–100 representing dark to light, a* value, indicator of degree of the red-green color and b* value indicates the degree of yellow-blue color, with higher positive b* value indicating more yellow. The hunter color values of L*, a* and b* of wheat flour were 85.3, 2.01, and 13.2, respectively. L* value of wheat-barley flour blends ranged from 86.1 to 87.6. Incorporation of barley flour to wheat flour significantly (p < 0.05) increased L* value, however, substitution at higher levels showed no significant effect. Values of a* and b* was significantly (p < 0.05) lowered and the values ranged between 1.53–1.83 and 8.1–12.9, respectively among wheat-barley flour blends. Baking led to a significant (p < 0.05) decrease in L* value whereas a* and b* values were significantly (p < 0.05) increased as compared to their corresponding counterparts flours. The L* value of flours from rusk (control) prepared from wheat flour was lowered to 73.1 while those prepared by incorporating barley flour to wheat flour exhibited L* value in the range from 60.3 to 72.3. Decrease in L* can be attributed to the heat employed during the baking that leads to Maillard reactions and compounds formed contribute to the aroma, taste and color of foods and are influenced by many factors, including temperature, reactant concentration, reaction time and water activity (Manzocco et al. 2000). The rusk prepared from wheat flour showed an increase in a* value (4.11) while those prepared by incorporating barley flour to wheat flour also showed increase in a* and the values ranged between 1.66 and 3.94. Similarly, b* value of rusk prepared by wheat flour exhibited an increase (24.5) while those prepared from blends exhibited an increase in the values ranging from 19.4 to 23.6. During baking process, some maillard reactions are responsible to produce brown pigments and temperature, water activity, pH, types of sugars are the major factors, which are responsible for such changes (Stojceska et al. 2009).
Total phenolic content of wheat and wheat–barley flour blends and rusks
Phenolic compounds are antioxidants and help in protecting body tissues from oxidative damage, delay food deterioration and maintain its nutritional profile. Total phenolic content of wheat flour was 885 µg (GAE)/g, it increased significantly (p < 0.05) as the proportion of barley flour in the blends increased and the values ranged between 1004–2090 µg (GAE)/g (Table 3). Liyana-Pathirana and Shahidi (2007) reported total phenolic content of 769 mg FAE/g in defatted wheat flour. Wheat is reported to have less phenolic compounds than barley. Holtekjolen et al. (2008) reported that replacement of wheat flour with barley flour led to a significant (p < 0.05) increase in total phenolic content. Baking led to a significant (p < 0.05) decrease in total phenolic content and the values ranged between 434 to 764 µg (GAE)/g in blends with a percentage decrease of 56.7–63.4%. Sharma and Gujral (2014b) reported upto 17% reduction in total phenolic content in chapattis upon baking. Same results of decrease in total phenolic content were also reported by Holtekjolenet al. (2008) during bread making. Leenhardt et al. (2006) reported that decrease in total phenolic content may be due to damage or degradation of antioxidant active compounds present in flours as a result of the thermal process upon baking. Molecular structure of phenolic compounds changes as a result of heating which leads to either reduced chemical reactivity or decreases their extractability due to certain degree of polymerization (Altan et al. 2009).
Quantification of phenolic compounds of wheat and wheat–barley flour blends and rusks
The content of phenolic acids of wheat and wheat-barley flour blends and rusks are summarized in Table 4. The phenolic acids detected in control and blended rusks were gallic acid, ferulic acid, chlorogenic acid, p-hydroxybenzoic, syringic acid, and p-coumaric acid. The gallic, ferulic acid, chlorogenic acid, p-hydroxybenzoic, syringic and p-coumaric of 41.4, 7.2, 0.41, 0.8, 2.1 and 0.5 µg/g was observed for control wheat flour. As the concentration of barley flour in wheat is increased, the phenolic acid content were also increased. Gallic acid was observed to be highest in 30% barley blends (acceptable by sensory) (82.2 µg/g) followed by ferulic > syringic acid > p-coumaric acid > 4-hydroxybenzoic acid > chlorogenic, respectively with values of 8.64, 3.68, 1.67, 1.30 and 1.15 µg/g.). Phenolic acid very are heat sensitive phenolic acids and baking process led to decrease in phenolic acids. The highest decrease in 30% barley incorporated rusks was found in gallic acid (86.4%) followed by p-coumaric acid (63.4%), chlorogenic acid (40.8%), syringic acid (39.6%), ferulic acid (33.1%) and 4-hydroxy benzoic acid (22.3%). Fares and Menga (2010) also reported decrease of phenolic acids during thermal thermal which may be due to presence oxygen, water and heat which induce oxidative degradation of phenolics. Abdel-Aal and Rabalski (2013) reported that phenolics are dependent of formulation process, heating conditions and phenolic compounds.
Antioxidant activity of wheat and wheat–barley flour blends and rusks
The DPPH assay is an electron transfer-based assay, which can measure the capacity of an antioxidant in the reduction of organic nitrogen radicals. The antioxidant activity of wheat flour was observed to be 11.8%. Increasing barley flour proportion in the wheat-barley flour blends progressively increased the antioxidant activity from 15 to 31% which is due to the higher antioxidant activity of the barley flour as compared to wheat flour (Table 3). Rusks supplemented with barley are more nutritious and provide higher consumption intake of fibres and contain significant amount of antioxidants. Baking decreased antioxidant activity in wheat flour and wheat-barley flour blends and the values ranged between 5.6 and 12.7% with a percentage decrease by 40.4 to 59%. The decrease may be due that phenolics are reactive compounds which easily degraded due to heat during baking. The phenolic compounds are heat labile and less resistant to the heat, and heating over 70–80 °C may destroy or alter their nature. Therefore, upon baking, decrease in AOA may be due to thermal destruction of phenolic compounds. Holtekjolen et al. (2008) also reported that the antioxidants, which are present in cereals, can be modified during thermal processes and due to this levels of DPPH decreased in breads in comparison to the flours. Structural deformation of phenolic compounds during thermal treatment leads to reduction in extraction of antioxidants (Altan et al. 2009).
Total flavonoids content of wheat and wheat-barley flour blends and rusks
Total flavonoids content of wheat flour was 112 μg CE/g whereas wheat flour replaced by barley flour at levels of 10, 20, 30, 40 and 50% exhibited total flavonoids content of 141, 169, 211, 252, 319 μg CE/g, respectively. Incorporation of barley flour to wheat flour led to a significant (p < 0.05) increase in total flavonoids content (Table 3). Barley is a rich source of flavonoids as compared to wheat therefore, increasing its proportion in wheat flour increased the total flavonoids content significantly (p < 0.05). Baking led to a significant (p < 0.05) decrease in total flavonoids content. The rusk prepared by wheat flour exhibited total flavonoids content of 71.8 μg CE/g whereas those prepared by incorporating 10, 20, 30, 40 and 50% barley flour to wheat flour showed total flavonoids content values of 94.8, 87.3, 125.5, 151.6 and 182.2 μg CE/g, respectively. The percentage decrease in total flavonoids content upon baking ranged from 32.7 to 48.3%. Sharma and Gujral (2014b) reported decrease in total flavonoids content upto 30.7% upon chapatti baking. A reduction in flavonoids compounds upon thermal processing has also been reported by Angioloni and Collar (2011) upon bread making from multigrain cereals and such changes might be due to flavonoids breakdown during heating. The flavonoids are thermally unstable and the heat treatment during processing destroys the flavonoids compounds (Xu and Chang 2008), however, destruction of these compounds are dependent on duration of heating during processing (Sharma and Gujral 2014b).
Metal chelating activity of wheat–barley flour blends and rusks
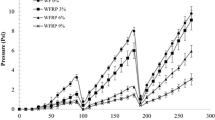
For wheat flour, metal chelating activity was observed to be 26.1%. A significant increase in metal chelating activity was observed upon incorporation of barley flour in wheat flour and the values ranged between 29.4 and 45.5% (Fig. 1) which is attributed to the higher metal chelating activity and antioxidant activity of barley flour as compared to wheat flour. Baking of the dough into rusk led to a significant (p < 0.05) decrease in metal chelating activity in rusk made from wheat flour (14.6%) and wheat-barley flour blends (16.6–22.1%). Fang et al. (2008) reported that the antioxidant activity is mainly due to the total phenolic content and total phenolic acids. Therefore, a decrease upto 51.4% in metal chelating activity is due to decrease in amount of phenols. Stevenson et al. (2008) reported that antioxidant compounds are very heat sensitive and during processing some compounds like maillard reaction compounds are formed (Gawlik-Dziki et al. 2009) which may mask decrease in total phenolic content as well as loss of antioxidant activity in products during the heat treatment.
ABTS+ Scavenging activity of wheat–barley flour blends and rusks
ABTS+ scavenging activity of wheat flour was 6.32%. Increasing the level of barley flour in the wheat-barley flour blends progressively increased the scavenging activity from 7.8 to 11.3 μmol/g (Fig. 2) which could be due to the higher scavenging activity of barley as compared to wheat. Baking led to a significant (p < 0.05) decrease of scavenging activity in rusk made from wheat flour with 3.3 μmol/g and in wheat-barley flour blends (4.2–6.1 μmol/g). Moore et al. (2009) reported a decrease in antioxidant activity of wheat pizza upon baking. Chlopicka et al. (2012) also observed decrease in activity of breads in comparison to the flours and reported that the antioxidants, which were present in cereals, can be modified during thermal processes.
Sensory characteristics of rusk
The sensory aspects and consumer acceptance have to be considered during the developing new products. With the addition of barley flour, the appearance became more grained (Table 5). The sensory results showed that the crust and crumb properties, chewing properties, taste, aroma and overall acceptability of rusks were best for rusks made from only from wheat flour (control). Color of the rusk surface was similar to the control rusk up to 30% barley flour addition; then it became more intense when higher levels of barley flour (40–50%) was incorporated. The same tendency was observed for flavor when higher levels of barley was substituted which gave stronger intensity of this attribute. Effort of chewing was affected after 40% barley flour was incorporated, the rusk became hard to chew. The incorporation of barley flour to wheat flour at levels of 10–30% did not affect the sensory attributes significantly. Rusk prepared from wheat-barley flour blends (40%) were moderately acceptable due to its gummy taste. Further incorporation of barley flour to wheat flour at levels of 50% decreased the sensory score drastically and rusk were unacceptable due to its irregular shape, gluten dilution, gummy mouth feel and characteristic aroma.
According to sensory analysis, overall acceptance of rusks were found the best for control sample. Rusks prepared with barley flour addition to wheat flour were liked moderately and like slightly by panelists. Although, as the incorporation of barley flour to wheat flour increased the bioactive potential and antioxidants content of rusks, but addition of upto 30% barley flour gave satisfactory sensorial results in terms of overall acceptability score.
Conclusion
The results of present study revealed that barley incorporated rusks were nutritionally superior that wheat rusks. Addition of increasing amount of barley flour into wheat flour significantly affected the pasting properties of wheat flour. As the concentration of barley flour in wheat is increased, total phenolic content, phenolic acid composition and antioxidants properties were also increased. Among the phenolics, gallic acid was observed to be highest in the highest concentration followed by ferulic, syringic acid, p-coumaric acid, 4-hydroxybenzoic acid, chlorogenic, respectively. Barley flour may be added to wheat flour upto a level of 30% without any detrimental effect on rusks sensory properties. Therefore, supplementation of barley flour into wheat flour may be useful strategy for the consumption of foods rich in antioxidants which have health benefits.
References
Abdel-Aal ESM, Rabalski I (2013) Effect of baking on free and bound phenolic acids in wholegrain bakery products. J Cereal Sci 57(3):312–318
Altan A, McCarthy KL, Maskan M (2009) Effect of extrusion process on antioxidant activity, total phenolics, and β-glucan content of extrudates developed from barley-fruit and vegetable by products. Int J Food Sci Technol 44:1263–1271
Aludatt MH, Rababah T, Ereifej K, Alli I, Alrababah MA, Almajwal A, Masadeh N, Alhamad MN (2012) Effects of barley flour and barley protein isolate on chemical, functional, nutritional and biological properties of Pita bread. Food Hydrocoll 26:135–143
Angioloni A, Collar C (2011) Polyphenol composition and “in vitro” antiradical activity of single and multigrain breads. J Cereal Sci 53:90–96
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30
Brennan CS, Cleary LJ (2005) The potential use of cereal (1/3), (1/4) β-D-glucans as functional food ingredients. J Cereal Sci 42:1–13
Chlopicka J, Pasko P, Gorinstein S, Jedryas A, Zagrodzki P (2012) Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci Technol 46:548–555
Dhull SB, Punia S, Sandhu KS, Chawla P, Singh A (2019) Effect of debittered fenugreek (Trigonella foenum graecum L.) flour addition on physical, nutritional, antioxidant and sensory properties of wheat flour rusk. Legume Sci Wiley. https://doi.org/10.1002/leg3.21
Dinis TCP, Madeira VMC, Almeidam LM (1994) Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and peroxylradicals scavengers. Arch Biochem Biophys 315:161–169
Fang Z, Hu Y, Liu D, Chen J, Ye X (2008) Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem 108:811–817
Frost DJ, Adhikari K, Lewis DS (2011) Effect of barley flour on the physical and sensory characteristics of chocolate chip cookies. J Food Sci Technol 48:569–576
Gao L, Wang S, Oomah BD, Mazza G (2002) Wheat quality: antioxidant activity of wheat millstreams. In: Ng P, Wrigley CW (eds) Wheat quality elucidation. American Association of Cereal Chemists, St Paul, pp 219–233
Gawlik-Dziki U, Dziki D, Baraniak B, Lin R (2009) The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci Technol 42:137–143
Hernanz D, Nuñez V, Sancho AI, Faulds CB, Williamson G, Bartolomé B, Gómez-Cordovés C (2001) Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J Agric Food Chem 49(10):4884–4888
Holtekjolen AK, Baevere AB, Rodbotten M, Berg H, Knutsen SH (2008) Antioxidant properties and sensory profiles of breads containing barley flour. Food Chem 110:414–421
Jia Z, Tang M, Wu J (1998) The determination of flavonoids content in mulberry and their scavenging effects on superoxides radicals. Food Chem 64:555–559
Lazaridou A, Biliaderis CG, Izydorczyk MS (2003) Molecular size effects on rheological properties of oat β-glucans in solution and gels. Food Hydrocolloid 17(5):693–712
Leenhardt F, Lyan B, Rock E, Boussard A, Potus J, Chanliaud E (2006) Wheat lipoxygenase activity induces greater loss of carotenoids than vitamin E during breadmaking. J Agric Food Chem 54:1710–1715
Lin MJY, Humbert ES, Sosulski FW (1974) Certain functional properties of sunflower meal products. J Food Sci 39:368–370
Liyana-Pathirana CM, Shahidi F (2007) Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem 101:1151–1157
Mallik J, Kulkarni S (2010) Quality of rusks prepared by incorporation of concentrated whey. J Food Sci Technol 47:339–342
Manzocco LS, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR (2000) Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol 11:340–346
Moore J, Luther M, Cheng Z, Yu L (2009) Effects of baking conditions, dough fermentation, and bran particle size on antioxidant properties of whole wheat pizza crusts. J Agric Food Chem 57:832–839
Naczk M, Diosady LL, Rubin LJ (1985) Functional properties of colona meals produced by a two phase solvent extraction system. J Food Sci 50:1685–1688
Punia S, Sandhu KS, Siroha AK (2017) Difference in protein content of wheat (Triticumaestivum L.): effect on functional, pasting, color and antioxidant properties. J Saudi Soc Agric Sci. https://doi.org/10.1016/j.jssas.2017.12.005
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Sandhu KS, Punia S (2017) Enhancement of bioactive compounds in barley cultivars by solid substrate fermentation. J Food Meas Charact 11(3):1355–1361
Sharma P, Gujral HS (2014a) Antioxidant potential of wheat flour chapattis as affected by incorporating barley flour. LWT-Food Sci Technol 56:118–123
Sharma P, Gujral HS (2014b) Cookie making behavior of wheat-barley flour blends and effects on antioxidant properties. LWT-Food Sci Technol 55:301–307
Sosulski FW (1962) The centrifuge method for determining flour absorption in hard red spring wheats. Cereal Chem 39:344–349
Stevenson DG, Inglett GE, Chen D, Biswas A, Eller FJ, Evangelista RL (2008) Phenolic content and antioxidant capacity of supercritical carbon dioxide-treated and air-classified oat bran concentrate microwave-irradiated in water or ethanol at varying temperatures. Food Chem 108:23–30
Stojceska V, Ainsworth P, Plunkett A, Ibanoglu S (2009) The effect of extrusion cooking using different water feed rates on the quality of ready-to-eat snacks made from food byproducts. Food Chem 114:226–232
Xu B, Chang SKC (2008) Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J Agric Food Chem 56:7165–7175
Yaqoob S, Baba WN, Masoodi FA, Shafi M, Bazaz R (2018) Effect of sprouting on cake quality from wheat–barley flour blends. J Food Meas Charact 12(2):1253–1265
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Punia, S., Sandhu, K.S. & Kaur, M. Quantification of phenolic acids and antioxidant potential of wheat rusks as influenced by partial replacement with barley flour. J Food Sci Technol 57, 3782–3791 (2020). https://doi.org/10.1007/s13197-020-04410-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04410-9