Abstract
Honey from different botanical sources presents a great variability in chemical composition, physical properties, and sensorial attributes. The association between honey qualities and its geographical origin allows the recognition of honey, and beekeepers are economically benefited. The objective of this study was to evaluate the physicochemical characteristics of honey produced in Ortigueira, Brazil and to compare it with the honey produced in the neighboring localities for the request of geographical indication certification. In the 112 honey samples collected between 2010 and 2013 from Ortigueira and the neighboring localities, moisture acidity, pH, hydroxymethyl furfural (HMF), diastase activity (DA), sugars, proline (Pro), electrical conductivity (EC), color absorbance at 635 nm (C635), and Cielab color parameters were evaluated. HMF, reducing sugars (RS), total sugars (TS), proline, and color parameters (L*, a* and b*) were significantly different in Ortigueira 2010 honey seasons. Principal component analysis separated assapeixe (Vernonia sp) and capixingui (Croton floribundus) honeys from wild honeys (polyfloral). In addition, HMF, RS, L*, and lactonic acidity values promoted the separation between assapeixe and capixingui honeys. Ortigueira honey differs significantly from honeys from neighboring locality in pH, proline, HMF, DA, EC, RS, sucrose, TS, free and total acidity, and color parameters (C635, L*, a*, b*). The multivariate analysis applied to variables was efficient to discriminate honeys from different botanical sources and different locals, and is recommended for studies on the geographical indication of honey.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World consumption of honey since the earliest times is based on the recognition of its beneficial effects on human health. This consumption is associated to physicochemical, sensorial, and nutritional characteristics of honey. These characteristics are influenced by the predominant floral source at the production area (Baroni et al. 2009; Castro-Vázquez et al. 2008; Welke et al. 2008; Lacerda et al. 2010). Several studies have identified that the main physicochemical parameters of honey are correlated with its botanical origin (Terrab et al. 2004; Devillers et al. 2004). Color, pH, sugars, electrical conductivity, diastase activity, water content, ashes, proline, acidity (free, lactonic and total), and minerals are used to evaluate the chemical composition and quality of honey (Barth et al. 2005; Carvalho et al. 2009; Kaskoniene and Venskutonis 2010). Many of these compounds could be used as markers to define the geographical origin of honey (Acquarone et al. 2007; Baroni et al. 2009).
Plant species growth in a region depends on the soil and climatic conditions (humidity, wind, solar irradiation, rainfall regime), making unique the flora of each region. The bees feed on the nectar in the plants near the hives; therefore, the composition of honey reflects the nectar characteristics of the group of plants around a hive. Consequently, the association between honey composition and the region where it is produced is possible. This association allows for economical exploration and preserves the uniqueness of the product (Baroni et al. 2009; Iglesias et al. 2012) therefore the consumer has the guaranty of the origin of the honey.
The possibility of the association between the quality of a product and the place of production results in the creation of geographical indication (GI) certificates to guarantee it. These certificates are granted to those who prove this association. There are several forms of certification; protected Geographical Indication (IP), and Denomination of Origin (DO) certifications are the most common. The IP certification identifies the geographical name (country, city, or region) that has become known as a center for the extraction, production, or manufacture of a particular product or a center for service provision (Belleti et al. 2014). However, DO certification identifies the geographical name that provides the product or service, with qualities or characteristics related exclusively or essentially to the geographical environment, including natural and human factors. Therefore, products such as wine, coffee, and honey fit perfectly in this last category.
In Brazil, the Ortigueira region is recognized for a long time as the major producer of high quality honey, and according to general opinion, the quality of honey produced can be attributed to the characteristics of the flora in this territory. In this region, Asteraceae, Mimosaceae, Euphorbiaceae, and Myrtaceae are the main botanical families used as sources of nectar for local honey production. These botanical families also develop in regions close to Ortigueira; however, depending on edaphoclimatic adaptation to these local micro regions, different botanical groups can be found. Documents proving this connection are necessary through GI certification. Therefore, in case of honey production there must be confirmation of its origin through physicochemical and sensorial analyses (Viúda-Martos et al. 2010; Woodcock et al. 2007).
Color is the most important attributes for honey acceptability, and many factors interfere with this trait. Depending on the botanical origin, freshly harvested honey may have all the nuances of yellow color. Honey color is formed exclusively by the carotenoid, xanthophyll, and flavonoid colored pigments present in the nectar and the pollen collected by the bee (Pérez-Pérez et al. 2012). The color of the pollen can range from very pale yellow to very dark brown depending on the botanical species (Terrab et al. 2004; González-Miret et al. 2005), and this significantly interferes with honey color.
The color measurement using the CIELab system is a representation of the color quite close to that perceived by the human eye. The classification of honey based on visual evaluation of professional tasters established that the honey considered clear by the tasters has a value of L* greater than 50 units of CIELab (González-Miret et al. 2005).
Depending on the type of certification, additional documents of political and social relationship of the region with honey production are also necessary. The objective of this study was to evaluate the physicochemical characteristics of the honey produced in Ortigueira regions and neighboring municipalities, to provide data to request for GI certification of Ortigueira honey.
Material and methods
Samples
All the honey samples for this study were Apis mellifera honey. To establish the relationship between the chemical composition of honey and the producing region, 79 samples of honey were collected between 2010 and 2011 in Ortigueira bee farms. To establish the originality of Ortigueira honey composition, honey samples were also collected in bee farms on the south (E1) and the north (E2) of Ortigueira in 2010, 2012 and 2013 (Suppl Table 1).
In the region of Ortigueira, the humid subtropical mesothermic climate prevailed. In summer the temperature is moderate, and in winter there are frosts of different intensities. The annual average temperature in Ortigueira is 18.4 °C, and the annual rainfall is 1500 mm, being the rain concentration in summer. The mixed ombrophilous forest found in a large part of the Brazilian south region covers this territory predominantly. Based on the edaphoclimatic adaptation of each species in these micro-regions, different botanical family combinations can be found. The assapeixe (Vernonia sp), capixingui (Croton floribundus), eucalyptus (Eucalyptus sp), citrus (Citrus sp), and wild flora are the main plants cited by the beekeepers.
All honey samples (approximately 250 g of honey) collected in beekeeping properties were kept in a cool, ventilated place at a temperature below 20 °C until analyses. The indication of the botanical origin was given by the beekeepers, based on beekeepers’ experiences and the honey color (Tuberoso et al. 2014).
Physicochemical analyses
Saccharose and reducing sugar (RS) were separately determined. Initially, 0.7 g of honey was dissolved in 250 mL distilled water (S1) and 2 mL of this solution was added to 2 mL of 0.7 N HCl and 20 mL of distilled water. After a resting period of 24 h, this mixture was transferred to 100 mL volumetric flask and the volume was completed by adding distilled water. For RS determination, 2 mL of S1 was diluted in 100 mL of distilled water and left to stand for 24 h. Saccharose and RS were determined from these two-precedent preparations using the Somogyi and Nelson method. The total sugars (TS) are the sum of saccharose and reducing sugar.
Moisture and free, lactonic, and total acidity were determined by the method described in a previous study (AOAC 1990). Diastase activity (DA) was measured according to the method described by Bogdanov (2009). The buffered solution of honey and starch was incubated in a thermostatic water bath, and the activity of the enzyme was monitored by the decrease in the absorbance readings at 660 nm. The reaction was stopped when the absorbance reached 0.235 nm. A unit of DA is defined as the amount of enzyme, which converts 0.01 g of starch in one hour at 40 °C under the conditions of the test. Hydroxymethyl furfural (HMF) content was determined after the clarification of the honey with Carrez reagent, and the addition of sodium bisulfate. The absorbance was read at 284 nm and 336 nm (Bogdanov 2009). The electrical conductivity (EC) was determined at 20 °C in a solution of 20% honey and the result was expressed in micro Siemens. Proline content was determined using ninhydrin reagent and the absorbance was read at 517 nm (AOAC 1990) The pH of the honey (25 mL of honey solution in water in the proportion of 5 g of honey in 50 mL of water) was measured (Bogdanov 2009).
The color parameters, luminosity (L*), a*, and b* were determined using the Minolta colorimeter model CR 400 (Viúda-Martos et al. 2010; Mondragón-Cortez et al. 2013). The a* value vary from red (positive) to green color (negative), and b* value ranged from yellow (positive) to blue color (negative) (Viúda-Martos et al. 2010; Mondragón-Cortez et al. 2013).
The color of honey was also determined through spectrophotometric measurement of 50% honey solution (m/v) with absorbance reading at 635 nm (Ferreira et al. 2009). The results of C635 were correlated with the Pfund scale (Table 1).
Statistical analyses
Variance analysis (ANOVA) was applied to determine the differences in data (p < 0.05), and mean values were contrasted by the Tukey test (p < 0.05). The principal component analysis (PCA) was used for physicochemical parameters data analysis. All statistical analyses were performed using XLstat software (Addinsoft 2010).
Results and discussion
Physicochemical analyses of honey produced in Ortigueira in 2010 and 2011
Honey is a solution of sugars with other dissolved chemical compounds. The category and quantity of the compounds present in honey confer specific characteristics to the honey. A great variability of the chemical composition of Ortigueira honey harvested in two years (2010 and 2011) was observed (Table 2). Studies have shown that variations in the physicochemical characteristics of honey can occur in different seasons due to the different botanical families at the time of honey production (Stolzenbach et al. 2011; Silva et al. 2016).
However, in the present study, the effect of season was not similar for all honey chemical compounds and properties. Significant differences in HMF, RS, TS and proline values, and color parameters (L*, a* and b*) were observed. However, moisture and saccharose contents, acidity (free, lactonic, and total), pH, EC, and C635 values were similar in the two seasons (Table 2).
The honey quality parameters such as TS sugar, saccharose, EC, DA and HMF of Ortigueira honey are low compare to honey from different geographic origin. These values suggest high control of production and good preservation state of the samples (Silva et al. 2016). Ortigueira honey shows relatively low RS content compared to honey from other Brazilian localities. In the Campos Gerais region, the mean value of RS was 72.02%, ranging from 69.97 to 74.63% (Borsato et al. 2014) while honey collected in the Paraná River Islands presented the average value of RS of 68.99%, ranging from 67.13 to 72.04% (Alves et al. 2011). However, the total acidicity values of Ortigueira honey are close to the values found in other Brazilian honeys which showed a variation from 8.75 to 37.92 meq Kg−1 (Borsato et al. 2014) and from 28.29 to 41.77 meq Kg−1 (Alves et al. 2011).
The color of honey varies from clear to amber tones, to almost black. The most common color of honey is bright yellow or reddish brown. According to Pfund scale (Table 1 and Fig. 1) most of Ortigueira honeys are classified as white (35%), extra light amber (32%), and light amber (21%). The color spectrum of honey of Ortigueira is different from that of other Brazilian localities (Fig. 1). The color of honey from several Brazilian localities is predominantly light amber and amber color (Borsato et al. 2014; Alves et al. 2011; Barros et al. 2010). Although the acceptance of the color of honey by consumers varies greatly, light-colored honey such as Ortigueira honey may have an additional economic value, since the light-colored honey is very appreciated and valorized by consumers of many countries.
The assapeixe (Vernonia sp) and capixingui(Croton floribundus) plants are widely found in Ortigueira, and they represent the main source of nectar for honey production. Nectar composition determines the color and the physicochemical characteristics of honey (Silva et al. 2016; Marchini et al. 2007).
The relationship between local flora and honey characteristics defines the honey identity and allows the establishment of its connection with the local production. Therefore, statistical analysis involving the greatest possible number of characteristics must be applied for identifies with greater efficiency the relationship between local flora and honey characteristics (Kadar et al. 2010).
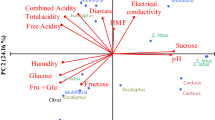
In the present study, the principal component analysis (PCA) was applied. In PCA, a better description of a sample set is achieved because most of the information about the variable is summarized in the new components used in the description of the samples. PCA results are presented in graphs and the samples are described as a function of the components.
Honey physicochemical data from the two seasons were subjected to PCA (Fig. 2). The first component (F1) accounts for 34.82% of the variance and the second component (F2) accounts for 18.09% of the variance, making it 52.95% of the initial variability. The variables presented different contribution levels in the formation of F1 and F2. The free acidity, C635, DA, EC, pH, proline, moisture, and a* value had high contribution in the formation of F1, while RS, HMF, lactonic acidity, L*, and b* values had high contribution in the formation of F2.
PCA described the predominant characteristics of the local honey. The separation of the honey samples according to botanical origin was observed. Assapeixe and capixingui honey samples were separated from wild and eucalyptus honey samples according to F1-forming variables (Fig. 2). Consequently, assapeixe and capixingui honeys had lower free and lactonic acidity, EC, RS, and proline values than the wild and eucalyptus honeys (Fig. 2). In addition, assapeixe and capixingui honeys had high saccharose and L* values compared to the wild and eucalyptus honeys, as seen in Fig. 2. Besides the high L* values, the low color a*, b*, and C635 values result to the classification of assapeixe and capixingui honeys as a light colored honey (González-Miret et al., 2005). The acacia, sunflower and tilia honeys, which are recognized as light-colored honeys, presented L* values of 53.95, 45.17, and 47.73 respectively (Kadar et al. 2010).
The relationship between a* and b* showed the presence of pigments in the honey. The negative values of a* and b* indicate low presence of reddish pigments in the honey; therefore the lower the value of a* and b*, the less red the honey is (Terrab et al. 2004; González-Miret et al. 2005). Most assapeixe and capixingui honeys had negative values of a* and b*, suggesting light-colored honey.
Comparison between honey from Ortigueira and honey and honey from neighboring locality
To identify the differences between Ortigueira honey and honey from neighboring locality, honey samples from Ortigueira, E1, and E2, locally harvested in 2010, 2012, and 2013 seasons were evaluated (Suppl Table 1). This comparison is necessary to attribute the characteristics of a honey should be attributed to the place of production.
All the samples evaluated (Table 3) presented values of moisture within the international limits, indicating good conditions of production, and good conservation of the samples (Bogdanov 2009). The sugars (TS, saccharose, and RS) values of the honeys were similar for the three localities evaluated. Mean EC values were significantly different and ranged from 331.02 to 480.40 μS cm−1. The RS content ranged from 63.92 to 67.40%, and saccharose values were always less than 3% (Table 3), and are inferior to limite (8%) of international legislation (Bogdanov 2009). The TS contents ranged from 66.25 to 69.42%, and were similar to other Brazilian honeys (Welke et al. 2008; Alves et al. 2011). The chemical variations in honey samples are attributed mainly to the variability of botanical nectar source (Mato et al. 2003). Honey characteristics associated with nectar composition, such as acidity and proline, showed significant differences among the three localities (Table 3).
The acidity of the honey confers chemical and sensorial characteristics (Mondragón-Cortez et al. 2013; Mato et al. 2003; Barra et al. 2010), and contributes to stability during storage (Ananias et al. 2013). More than 30 organic acids are responsible for the acidity of honey. These compounds are formed when the nectar is transformed into honey or they are obtained directly from nectar (Silva et al. 2016; Mato et al. 2003). Gluconic acid is present in all honey at higher concentrations and it is formed by the activity of glucose oxidase during maturation (Silva et al. 2016; Mato et al. 2003).
In the present study, no significant difference was found in lactonic acidity values in the honey from the three localities, suggesting similar level of maturation among the honeys (Silva et al. 2016; Barra et al. 2010).
However, a significant difference in the free and total acidity was found among honeys from the three localities, and this indicates variability of botanical origin of the honey (Mato et al. 2003).
Proline is the main free amino acid in honey, and proline value is closely linked to the origin of the nectar (Hermosin et al. 2003; Carratù et al. 2011). In the present study, proline values were significantly different among localities (Table 3). The lowest proline value was observed in Ortigueira honey and it was lower than honey from E1 and the E2 localities (Table 3). Studies conducted in different countries recommend that the values can be used as marker of botanical origin of honey (Hermosin et al. 2003; Carratù et al. 2011; Janiszewska et al. 2012). As low values of this amino acid are characteristic of assapeixe and capixingui honeys, it could be used as marker of the botanical origin of Ortigueira honey.
The HMF and DA values are indicators of hive management practices applied by beekeepers in honey production. The HMF and DA values of honey in all localities were within the limits proposed for good quality honey (Bogdanov 2009); however, they were significantly different among the localities (Table 3), suggesting that different beekeeping practices are performed at these sites.
The quality of honey is associated with its color and the consumer acceptance and preference depends on its color (Terrab et al. 2004; Kadar et al. 2010). The mean values of L* in Ortigueira honey was 52.65 (Table 3), which corresponds to lighted honey (Terrab et al. 2002). In contrast, honeys from E1 and E2 localities had a darker color, presenting mean values of 44.98 and 44.89, respectively (Table 3). Furthermore, these honeys showed significant differences in a* value (green–red) and high C635 value (Table 3).
Ortigueira honey is also classified as clear honey by the Pfund scale presenting lower C635 value than honey from E1 and E2 localities. Most Ortigueira honey belongs to the light amber category (Fig. 3), whereas in the other locality, the amount of dark colored honey (such as amber and dark) was relatively high.
Color of honey and correlation with chemical composition
The color of honey is strictly associated with nectar composition, and many chemical compounds of honey can be correlated. In the preset study, significant correlations between chemical compounds and color parameters were found (Suppl Table 3). The C635 was positively correlated with pH, free and total acidity, proline, HMF, DA, and EC. In the same way, the luminosity of the honey (L*) correlated negative and significantly with all chemical parameters, except with saccharose content (Suppl Table 3).
Chemical parameters like DA, EC, free and total acidity, pH, and saccharose correlated significantly with a* and b* chromatic components (Suppl Table 3). EC represented indirectly the mineral contents of the honey (Terrab et al. 2004; Kaskoniene and Venskutonis, 2010). In the present study a significant and negative correlation between EC and L*was observed and then the higher the value of EC the darker the honey. This correlation sustained the correlation between color of honey and EC found in the present study.
Correlations between sugars in honey and pollen origin are also reported Kaskoniene and Venskutonis (2010), which determine the color of honey as was demonstrated by the correlation between parameters of color (a* and b*) and saccharose (Suppl Table 3). Therefore light honey as assapeixe and capixingui honeys present lower content of sucrose.
Acidity parameters such as free and total acidity, and pH were correlated to nectar composition (Silva et al. 2016; Marchini et al. 2007), which determined the correlation between these compounds and color of honey. In the present study, honey lighter as assapeixe and capixingui honey have lower acidity due to the nectar composition of these plants.
However, no significant correlation between lactonic acidity and color parameters was observed in the honey evaluated (Suppl Table 3), probably due to the association between lactonic acidity and maturation of honey (Silva et al. 2016; Mato et al. 2003).
These correlation data showed narrow relationship between the color of honey and the chemical composition of nectar, and allowed the determination of the association of botanical and local origin with the honey quality.
Conclusion
Significant differences in HMF, DA, RS, TS, proline, and L*, a* and b* were observed between Ortigueira honeys harvested in 2010 and 2011, suggesting the influence of the flora on the chemical composition of honey. From the PCA including all physicochemical parameters, honey separation was observed according to botanical origin. Assapeixe and capixingui honeys were separated from wild and eucalyptus honeys. The main differences in the honeys from different botanical origin were seen in luminosity (L*), HFM, and saccharose.
Comparing Ortigueira honey and honeys from neighboring locality, significant difference in C635, L*, a*, proline, HMF, DA, EC, saccharose, and free and total acidity values were found.
Ortigueira honey were significantly clearer than honeys from other neighboring localities, since they had the lowest mean value of C635, highest value of L*, and low proline value. Significant correlations among the physicochemical characteristics of honey showed the association of honey color with honey composition.
The application of a multivariate analysis in the group of variables was efficient to discriminate honeys from different botanical sources and is recommended for studies on geographical indication of honey.
The relationship between the honey color and the chemical composition of the nectar was shown in the correlation between the physicochemical variables and resulted in the association of botanical and local origin with the honey quality.
Considering the characteristics and the history of local beekeeping, the Ortigueira honey was certified denomination of origin in May 2015 by the National Institute of Industrial Property—INPI.
References
Acquarone C, Buera P, Elizalde B (2007) Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem 101(2):695–703
Addinsoft (2010) XLStat: software for statistical analysis. Version 2010. Paris. 1 CD-ROM
Alves EM, Sereia MJ, Toledo VAA, Marchini LM, Neves CA, Toledo TCSOA, Almeida-Anacleto D (2011) Physicochemical characteristics of organic honey samples of africanized honeybees from Parana River islands. Food Sci Technol 31(3):635–639
Ananias KR, Melo AAM, Moura CJ (2013) Analysis of moisture content, acidity and contamination by yeast and molds in Apis mellifera L. honey from central Brazil. Braz J Microbiol 44(3):679–683.
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington
Baroni MV, Arrua C, Nores ML, Fayé P, Díaz MP, Chiabrando GA, Wunderlin DA (2009) Composition of honey from Córdoba (Argentina): assessment of north/south provenance by chemometrics. Food Chem 114(2):727–733
Barra MPG, Ponce-Díaz MC, Venegas-Gallegos C (2010) Volatile compounds in honey produced in the central valley of Ñuble province Chile. Chilean J Agric Res 70(1):75–84
Barros LB, Torres FR, Azeredo LC, Barth MO, Freitas MQ (2010) Physicochemical characterization of honey produced by Apis mellifera in the state of Rio de Janeiro. Rev Bras Ci Vet 17(3/4):117–120
Barth MO, Maiorino C, Benatti APT, Bastos DHM (2005) Determinação de parâmetros físico-químicos e da origem botânica de méis indicados monoflorais do sudeste do Brasil Cienc Tecnol Alime 25(2):229–233
Belleti G, Marescotti A, Paus M, Reviron S, Deppeler A, Stamm H, Thévenod-Mottet E The effects of protecting geographical indications-Ways and means of their evaluation. Swiss Federal Institute of Intellectual Property, Publication No 7; 2 revised edition, 09.11 https://www.ige.ch. Accessed 4 Apr 2014
Bogdanov S (2009) Harmonised Methods of the International Honey Commission. https://www.bee-hexagon.net/en/network.htm. Accessed 05/05/2010
Borsato DM, Farago PV, Fernandes CPL, Alencar MS, Mendes MA (2014) Physicochemical quality, botanical origin and antioxidant properties of floral honeys from Campos Gerais region Brazil. Interciencia 39(4):249–254
Carratù B, Ciarrocchi M, Mosca M, Sanzini E (2011) Free amino acids, oxalate and sulphate for honey characterization. J ApiProd ApiMed Sci 3(2):81–88
Carvalho CAL, Sodré GS, Fonseca AAO, Alves RMO, Souza BA, Clarton L (2009) Physicochemical characteristics and sensory profile of honey samples from stingless bees (Apidae: Melliponinae) submitted to dehumidification process. An Acad Bras Cienc 81(1):143–149
Castro-Vázquez L, Díaz-Maroto MC, González-Viñas M, De La Fuente AE, Pérez-Coello MS (2008) Influence of storage conditions on chemical composition and sensory properties of citrus honey. J Agric Food Chem 56(6):1999–2006
Devillers J, Morlot M, Pham-Delègue MH, Dore JC (2004) Classification of monofloral honeys based on their quality control data. Food Chem 86(2):305–312
Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM (2009) Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem 114(4):1438–1443
González-Miret ML, Terrab A, Hernanz D, Fernández-Recamales MA, Heredia FJ (2005) Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J Agric Food Chem 53(7):2574–2580
Hermosin I, Chicon RM, Cabezudo MD (2003) Free amino acid composition and botanical origin of honey. Food Chem 83(2):263–268
Iglesias A, Feás X, Rodrigues S, Seijas JA, Vázquez-Tato MP, Dias LG, Estevinho ML (2012) Comprehensive study of honey with protected denomination of origin and contribution to the enhancement of legal specifications. Molecules 17:8561–8577
Janiszewska K, Aniołowska M, Nowakowski P (2012) Free amino acids content of honeys from Poland. Pol J Food Nutr Sci 62(1):85–89
Kadar M, Juan-Borrás M, Hellebrandova M, Domenech E, Escriche I (2010) Differentiation of acacia, sunflower and tilia honeys from different countries based on sugar composition, physicochemical and color parameters. Bull USAMV Agric. 67(2):252–258
Kaskoniene V, Venskutonis PR (2010) Floral markers in honey of various botanical and geographical origins: a review. Compr Rev Food Sci F 9:620–634
Lacerda JJJ, Santos JS, Santos SA, Rodrigues GB, Santos MLP (2010) Influência das características físico-químicas e composição elementar nas cores de méis produzidos por Apis mellifera no sudoeste da Bahia utilizando análise multivariada. Quim Nova 33(5):1022–1026
Marchini LC, Moreti ACCC, Otsuk IP, Sodré GS (2007) Physicochemical composition of Apis mellifera honey samples from São Paulo State Brazil. Quim Nova 30(7):1653–1657
Mato I, Huidobro JF, Simal-Lozano J, Sancho MT (2003) Significance of nonaromatic organic acids in honey. Food Protec 66(12):2371–2376
Mondragón-Cortez P, Ulloa JA, Rosas-Ulloa P, Rodríguez-Rodríguez R, Resendiz-Vázquez JA (2013) Physicochemical characterization of honey from the West region of México. CyTA-J Food 11(1):7–13
Pérez-Pérez E, Vit P, Rivas E, Sciortino R, Sosa A, Tejada D, Rodríguez-Malaver AJ (2012) Antioxidant activity of four color fractions of bee pollen from Mérida Venezuela. Arch Latinoam Nutr 62(4):375–380
Silva PM, Gouche C, Gonzaga LV, Costa ACO, Feet R (2016) Honey: chemical composition, stability and authenticity. Food Chem 196(4):309–323
Stolzenbach S, Byrne VB, Bredie LP (2011) Sensory local uniqueness of Danish honeys. Food Res Int 44(9):2766–2774
Terrab A, Dıez MJ, Heredia FJ (2002) Chromatic characterisation of Moroc can honeys by diffuse reflectance and tristimulus colorimetry—non-uniform and uniform colour spaces. Food Sci Tech Int 8(4):189–195
Terrab A, Recamale AF, Hernanz D, Heredia FJ (2004) Characterization of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem 88(4):537–542
Tuberoso CIG, Jerkovic I, Sarais G, Congiu F, Marijanovic Z, Kus PM (2014) Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined L*C*abH°ab chromaticity coordinates. Food Chem 145(2):284–291
Viúda-Martos M, Ruiz-Navajas Y, Zaldivar-Cruz JM, Kuri V, Fernández-López J, Carbonell-Barrachina AA, Pérez-Álvarez JA (2010) Aroma profile and physico-chemical properties of artisanal honey from Tabasco Mexico. Int J Food Sci Tech 45(6):1111–1118
Welke JE, Reginatto S, Ferreira D, Vicenzi R, Soares JM (2008) Caracterização físico-química de méis de Apis mellifera L. da região Noroeste do estado do Rio Grande do Sul. Ciên Rur 36(6):1737–1741
Woodcock T, Downey G, Kelly JD, O’Donnell C (2007) Geographical classification of honey samples by near-infrared spectroscopy: a feasibility study. J Agric Food Chem 55(22):9128–9134
Acknowledgements
The authors would like to thank the Secretaria de Estado de Ciência, Tecnologia e Ensino Superior—SETI (TC 30/10), and Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico for financial suportes. We also thank beekeepers who kindly provide the honey samples for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that do not have any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scholz, M.B.S., Quinhone Júnior, A., Delamuta, B.H. et al. Indication of the geographical origin of honey using its physicochemical characteristics and multivariate analysis. J Food Sci Technol 57, 1896–1903 (2020). https://doi.org/10.1007/s13197-019-04225-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04225-3