Abstract
The main objective of the study is to assess a comparative antibacterial potential of three new bacteriocins produced by Pediococcus sp. through partial characterization and mode of action against some food spoilage bacteria. The bacteriocins from three different Pediococcus sp. viz. Pediococcus sp. LAB 33 (HQ185406), Pediococcus sp. LAB 41 (HQ185407), and Pediococcus sp. LAB 51 (HQ184064) were partially purified by adsorption–desorption method and tested for autoclave heat, pH, detergent and enzymes stability. A comparative analysis by Tricin-SDS PAGE with MALDI-TOF MS was done to estimate their molecular weight. The mode of action studies were done by cell viability and lactate dehydrogenase assay against two food associated pathogens, viz. Listeria monocytogenes and Pseudomonas aeruginosa using standard protocols. The bacteriocins produced by the strains were resistant to autoclave heat, detergent, wide range of pH and were active against different food borne pathogens at a minimum dose of ~ 100 AU/ml. The mode of action studies showed bactericidal action with lysis of the targeted cells. Therefore, the selective low dose efficacy, heat and detergent stability of the bacteriocins produced by the three strains could be considered as potent bacteriocins for use as food preservatives.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The use of chemical preservatives or antibiotics in combating food spoilage raised the risk of environmental toxicity and health hazards, so people are trying to use natural antimicrobial compounds to protect the foods during preservation. Bacteriocins which are ribosomally synthesized proteinaceous antimicrobial compounds produced by a diverse group of microorganisms provide an alternative to this preservation process. Among the diverse group of bacteriocins produced by different microbes, bacteriocins produced by lactic acid bacteria (LAB) are most demanding especially in food preservation (De Vuyst and Leroy 2007; Silva et al. 2018) mainly for the virtue of their food-grade properties. Nisin is one such bacteriocin produced by different strains of Lactococcus lactis has GRAS (generally recognized as safe) status (21 CFR 184.1538) and is being used as food bio-preservative since 1950s (Federal Register 1988). Many more LAB bacteriocins are being trialed for GRAS status and also for global use in different industries (Chikindas et al. 2018).
Pediococcus spp. are one such lactic acid bacteria (LAB) known to produce different types of bacteriocins. They are stable in wide range of pH, temperature and show strong static or cidal effect against the closely related bacteria (De Vuyst and Vandamme 1994; Daw and Falkiner 1996). Pediococcal bacteriocins are generally cationic hydrophobic, or amphiphilic molecules composed of 12–60 amino acid residues and have large variations among the bacteriocin peptides, viz. in terms of length, amino acid sequence and composition, and antimicrobial activity (alone or in combination with other peptides). According to Klaenhammer’s classification and Heng and Tagg they belong to the class IIa, i.e. small heat stable peptides (< 10 kDa) that do not contain lanthionines (Klaenhammer 1988; Heng and Tagg 2006).
For commercialization and effective use of bacteriocins in food preservation, the product should have unique properties and also the purification strategies should be less cost and time demanding. The most widely used method of purification is based on the ionization behaviour of the molecules in certain pH values. As the cell wall of most lactic acid bacteria are mildly negatively charged therefore at neutral pH due to cationic nature of the bacteriocin molecules tend to adhere on the cell wall and at strong acid pH (~ 1.2–2.3) in saline solution they get released from the cell wall due to change in ionization. This purification strategy is very much supportive in bacteriocin purification and does not involve any addition of harsh chemical for precipitation and isolation. The partially purified product can effectively be used in further molecular characterization like molecular weight, pH and thermal stability to determine their uniqueness and potential applications. Mass spectrometry has been adapted for the rapid detection and molecular characterization of pediocin, nisin, brochocins A and B, and enterocins A and B from culture supernatants (García-Cayuela et al. 2017).
In the present communication the bacteriocins from three strains of Pediococcus sp. have been partially characterized and their potential antimicrobial spectrum and mode of action have been elucidated.
Materials and methods
Chemicals, media and strains used in this study
Dithiothreitol, α-amylase, papain, proteinase K, RNase A and lysozyme, Coomassie brilliant blue R-250 were of AR grade and purchased from SRL, India; Tween 80, Citron X 100, 2-Mercaptoethanol, Hydrochloric acid, Sodium hydroxide, Sodium citrate, Sodium acetate, Dipotassium hydrogen phosphate, BSA, Phosphoric acid were of AR grade purchased from Merck, India. Lactobacillus MRS broth; Tryptone, glucose and yeast extract (TGE) were used as media constituents purchased from Hi Media, India; Gram stain kit, endospore staining kit, and carbohydrate fermentation, and biochemical tests kit were purchased from HiMedia, India; Genomic DNA purification kit was purchased from GeNei, India; dialysis kit (1 kDa cut off), primers, low MW markers, DNA ladder, α-Cyano-4-hydroxycinnamic acid, HPLC water, acetonitrile and trifluoroacetic acid, were purchased from Sigma, USA. Enterococcus faecalis MB1, Leuconostoc mesenteroides Ly and Pediococcus acidilactici LAB 5 were kindly provided by Professor Narayan C. Mandal, Department of Botany, Visva-Bharati, India; Bacillus cereus MTCC 1272, Bacillus megaterium MTCC 1684, Listeria monocytogenes MTCC 657, Pantoea ananetis MTCC 2307, Pseudomonas aeruginosa MTCC 741, Salmonella typhimurium MTCC 98, Staphylococcus aureus MTCC 96, Staphylococcus epidermidis MTCC 3086, and Streptococcus mutans MTCC 890 were purchased from Microbial Type Culture Collection (MTCC), Chandigarh, India.
Isolation of bacteriocin producing strains
Different traditionally fermented foods like salami and sausage of chicken, mutton, and pork, which are prepared as cottage preparation without any addition of any pre-added natural preservatives were procured from the local market and 1 g of the sample was added to lactobacillus MRS broth (Hi Media, India) and incubated at 30° C for 20 h. Dilution plates were prepared and white colonies were isolated randomly. Bacteriocin producing strains were selected on the basis of inhibition zone produced by the isolates in spot-on-lawn assay plates against E. faecalis Ly and L. mesenteroides MB1 which are the bacteriocin sensitive indicator strains following the method of Mandal et al. 2008. The bacteriocinogenic isolates were pure cultured and used for further studies.
Characterization of the isolates
Characterization of the isolates was done on the basis of Gram stain, morphology, motility test, endospore staining, and carbohydrate fermentation profile, biochemical tests like catalase activity, NaCl tolerance, pH tolerance, and starch hydrolysis following the standard protocol (Mandal et al. 2011). The genomic DNA of the isolates were extracted by using bacterial genomic DNA purification kit (GeNei, India) and 16S rDNA gene was amplified using DNA amplification kit (GeNei, India) by Bio-Rad MJ-Mini Thermal cycler system for 31 cycles using 27f (5′-AGAGTTTGATCMTGGCTCA) and 1525r (5′-AAGGAGGTGWTCCARCC) primers (Sigma Aldrich, USA). The amplified products were evaluated in 1% agarose gel in 1 × TAE buffer (pH 8.0) (Sambrook and Russell 2001). The amplified products were sequenced from Chromous Biotech Pvt. Ltd, Bangalore. 16r DNA gene sequences of the isolates were BLAST searched (Program BLASTN 2.2.24+) for strain identity and submitted in the GenBank for accession number. Phylogenetic tree was constructed using Clustal W2 software (version 1.83) for assembly and alignment using neighbour-joining method using Escherichia coli (KP941759) as outer group (Saitou and Nei 1987).
Production of bacteriocins
Bacteriocins quantification
Bacteriocin production was quantified by agar well diffusion method against the bacteriocin sensitive indicator strain, viz. E. faecalis MB1(Mandal et al. 2008). 30 µl of different dilution folds of heat killed cell free culture (CFC) aliquots of each of the three isolates were added to each well and were observed for clear zone of growth inhibition. The amount of bacteriocin production was calculated as arbitrary Activity Unit (AU). One AU is defined as the reciprocal of the highest serial two-fold dilution showing a clear zone of growth inhibition of the indicator strain (Mandal et al. 2008).
Selection of media, temperature, and pH for optimum growth and bacteriocin production and quantification
Different media like lactobacillus MRS, Tryptone-glucose-yeast extract (TGE), TGE amended with Tween 80 (0.05%, v/v), TGE amended with Tween 80 (0.05%, v/v) and buffer (containing sodium citrate, sodium acetate and dipotassium hydrogen phosphate, 0.2% each, w/v) broth were prepared following the methods of Mandal et al. (2008). Overnight grown active culture of the isolates were inoculated at 1% (v/v) and incubated at different temperatures like 30°, 37° and 45° C for 24 h. Optimal pH for growth and bacteriocin production was determined in MRS broth by adjusting at different pH (pH 5.5–7.5, with 0.2 variations) with 1 (N) HCl and 1 (N) NaOH. Growth (O.D.620nm) and final pH were measured. Bacteriocin production during growth was determined at 3 h intervals for 48 h at 37 °C. Bacteriocin production was quantified by agar well diffusion method as AU/ml against the two strains of L. monocytogenes MTCC 657 and Ps. aeruginosa MTCC 741 following the method of Mandal et al. (2008).
Partial purification of bacteriocins by adsorption–desorption method
Partial purification of bacteriocins was done following the adsorption–desorption method (Yang et al. 1992; Tulini and De Martinis 2010). For purification, the producer strains were grown as fed batch culture in MRS medium (pH 6.5) at 37 °C for 24 h in a rotary shaker at 100 rpm. The cultures were heat-killed and then cooled to room temperature. pH of the culture aliquots were adjusted to 5.2–5.3 (for LAB-33, and LAB-51) and 5.5–5.6 (for LAB-41) by 10 (N) NaOH and incubated at 37 °C for 6 h at a rotary shaker at 100 rpm to adsorb the bacteriocins to the producer cells. Cells were harvested by centrifugation at 10,000 rpm for 8 min at 4 °C and washed twice with sterile 5 mM sodium-phosphate buffer of pH 5.3 and 5.6 for respective strains and then centrifuged at 10,000 rpm for 8 min at 4 °C. The pellets were resuspended in 100 mM NaCl buffer (pH 1.5), pH of which was adjusted with 5% phosphoric acid, and stirred for 2 h at 4 °C. The cell free supernatant (CFS) was collected by centrifugation and dialyzed (Mol. Cut off 1.0 kDa) in a dialysis bag (Sigma, USA) and used as partially purified bacteriocins. The amount of proteins during every step of purification was done by Bradford method (Kruger 2002) using BSA as standard.
Characterization of bacteriocins
Stability of bacteriocin to different treatments like pH, temperature, detergents and enzymes
The partially purified bacteriocin was tested for sensitivity to different treatments like pH, temperature, detergents, viz. Citron X 100 (2%) and Tween 80 (20%), and treatments with reducing agents like 2-mercaptoethanol (2 M) and dithiothreitol (1 gm/ml). The stability of the bacteriocins to pH and temperature was tested according to the protocol of Mandal et al. (2008). For the analyses of detergents and enzymes stability, each treatment solution and partially purified bacteriocin were mixed vigorously in 1:1 ratio (v/v). Thereafter, 30 µl of each mixture was loaded in wells of indicator lawn of Enterococcus faecalis MB1 in MRS agar plates and incubated for 24 h at 28 °C. The residual activity was measured as the diameter of inhibition zones (mm) against the indicator strains. To determine the stability in different enzymes like α-amylase, papain, proteinase K, RNase A and lysozyme, 10 mg/ml of each enzyme was mixed with the partially purified bacteriocin as stated above, incubated at 37 °C (for α-amylase, Papain, RNase A and lysozyme) and 50 °C (for proteinase K) for 30 min, then kept in boiled water to inactivate the enzyme and the assay was carried as described previously.
Detection of bacteriocins in glycine SDS-PAGE and in-gel assay, and determination of molecular weight in Tricin SDS-PAGE
The partially purified bacteriocins were concentrated in evacuee to 1/10th volume and separated through 15% separating gel and 5% stacking gel with acrylamide and bis-acrylamide ratio 30:1 under denaturing condition (Laemmli 1970). 20 µl of each of the partially purified bacteriocin was mixed in 4:1 ratio with denaturing sample buffer and loaded in a well. The BSA (100 µg/ml) was used as marker protein. Electrophoresis was done at 20 mA for first 15 min and then next 1 h at 25 mA. Thereafter, the gel was removed and stained with Coomassie brilliant blue R-250 and de-stained to visualize the bands and the relative mobility was compared with molecular standard (BSA, Mw 66 kDa). To assure the protein bands as bacteriocins in the SDS-PAGE, activity gel study was done. The gel was tested for antimicrobial activity after washing repeatedly for 24 h in sterile double distilled water containing 0.1% Tween 80 (Keren et al. 2004; Mandal et al. 2011). The gel was then placed on a pre-poured MRS agar plate and overlaid with 10 ml melted MRS soft agar (0.6%, w/v) seeded with sensitive indicator L. monocytogenes MTCC 657. The plate was then incubated at 37 °C for next 24 h and observed for zone of growth inhibition. This was then matched with the protein bands of the stained gel to confirm the bacteriocin bands.
To determination the molecular weight of the bacteriocins the partially purified bacteriocins were run in 15% separating gel (with acrylamide and bis-acrylamide ratio 30:1, v/v) and 5% stacking gel under denaturing condition in Tricin SDS-PAGE (Schägger and von Jagow 1987). 20 µl of each of the partially purified bacteriocin and loaded in a well. The ultra-low range marker (Sigma) was used as marker protein. Electrophoresis was done at 20 mA for first 15 min and then next 1 h at 25 mA. Thereafter, the gel was removed and stained with Coomassie brilliant blue R-250 and de-stained to visualize the bands. The molecular weight was determined by comparing the relative mobility of standard molecular marker. The Rf values were calculated and the approximate molecular weight of the peptides were calculated by drawing a straight line curve of anti-log of molecular weight versus the Rf values of the standard peptides. The molecular weights of unknown peptides were calculated from the standard curve.
MALDI TOF–MS analysis of the partially purified bacteriocin
The exact molecular weight was determined by MALDI-TOF MS. For Mass spectrometry the partially purified bacteriocins was used following the standard procedure of MALDI-TOF MS analysis. This was done using a saturated matrix solution mixed with the analyte solution (α-Cyano-4-hydroxycinnamic acid in 50:50 H2O: acetonitrile with 0.1% trifluoroacetic acid, giving a matrix-to-sample ratio of about 5000:1. An aliquot (0.5–2.0 ml) was applied to the sample target and allowed to dry. The sample was analysed in a MALDI TOF–MS equipment (Model: Bruker Daltonics, Inc., Billerica, MA) using Daltonics flex Analysis software (Department of Biochemistry, University of Calcutta, Kolkata, W.B.)
Antimicrobial spectrum of the bacteriocins
Antimicrobial efficacy of the bacteriocins produced by the three strains was determined by spot-on-lawn method and confirmed by agar well diffusion method (Mandal et al. 2008) against a number of human pathogenic and food-spoilage bacteria as listed in Table 3. The bacteriocin sensitivity was determined by measuring the diameter (mm) of inhibition zone.
Effect of bacteriocins on sensitive indicator bacteria
The bacteriocins effect on growth and viability was studied against L. monocytogenes MTCC 657 and Ps. aeruginosa MTCC 741. The treated and untreated cells were measured for lactate dehydrogenase (LDH) content (UV 1700, Shimadzu, Japan). For LDH activity study, one part of the indicator cell suspensions was sonicated by 100% amplitude, at 0.9 cycles for 5 min (Labsonic M, Sartorius, Germany) and the other part of the suspension was treated with bacteriocins and incubated at 37 °C for 5 h. Cell free supernatants were collected at 1 h, 3 h and 5 h of incubation time and the amount of LDH released was measured following the method of Stockland and San Clemente (1968). The reaction mixture was prepared by sodium lactate (1 ml of 10 mM), NAD (150 µl of 20 mM), Tris–HCl (1.75 ml of 50 mM, pH 7.5) and 100 µl enzyme (the CFSs were used as enzyme source). The reaction mixtures were incubated for 5 min and absorbance was measured at 340 nm. One unit of LDH was calculated as 1 µM of NAD reduction per min.
Results and discussion
Characterization of the strains
Under microscope LAB 33 showed paired cocci, while LAB 41 was in cluster and LAB 51 was in small chain (Figure not shown here). None of the isolates were motile. NaCl tolerance study showed that the isolates could resist up to 6.5% (wt/vol) of NaCl concentration which indicates that high salt concentration is detrimental for their growth. Furthermore, all three isolates could grow in acidic pH (4.5) as well as in alkaline pH (9.6). LAB 33 showed relatively higher growth at pH 7.0, LAB 41 showed relatively higher growth at pH 4.5 while LAB 51 showed higher growth between pH 4.5 and 7.0. At pH 9.6 all strains, however, showed relatively lesser growth than pH 7. The isolates were also able to grow in anaerobic condition. From these above observations it could be assumed that the isolates are different in their physio-biochemical properties and can be assigned as independent strain. The16S rDNA sequencing and subsequent BLAST search showed that all the isolates are the strains of Pediococcus acidilactici. The GenBank with accession numbers for the strains were as Pediococcus sp. LAB 33 (HQ185406), Pediococcus sp. LAB 41 (HQ185407), and Pediococcus sp. LAB 51 (HQ184064). Phylogenetic relation of the strains was shown in Fig. 1.
Phylogenetic tree showing the relative position of the three strains as inferred by the neighbor-joining method of partial 16S rDNA sequences. References of the type strains used for comparison are given, as well as the accession numbers for all 16S rDNA sequences (between brackets). E. coli was used as an out-group
Optimization of media, pH and temperature for growth and bacteriocin production by the strains during growth
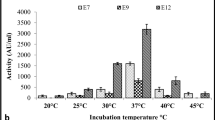
The maximum bacteriocin production of 300 AU/ml for LAB 33, 475 AU/ml for LAB 41 and 425 AU/ml for LAB 51 were found at optimum pH of 6.5 in MRS medium at 37 °C for 24 h. (Figure not shown here). In this medium bacteriocin production was started at early exponential phase and quickly maximized at late exponential phase. Cell growth was also gradually maximized in this medium. There was very insignificant bacteriocin production as well as growth of all the isolates in other media like TGE, TGE amended with Tween 80 (0.05%, vol/vol), and TGE amended with Tween 80 (0.05%, vol/vol) and buffer in comparison to MRS medium. Though the strains of Pediococcus sp. were found to produce more bacteriocins in TGE amended with Tween 80 (0.05%, vol/vol) and buffer medium at 37 °C but the present report was contradictory to the earlier observation of Mandal et al. (2008). Guerra and Pastrana (2003) reported that Lactococcus lactis subsp. lactis CECT 539 and Pediococcus acidilactici NRRL B-5627 were able to produce bacteriocins in whey buffered medium with an initial pH of 6.3 with different concentrations of buffering agent. The suitability of pH for better bacteriocins production in MRS was varied greatly among the strains. The LAB 33 strain produces maximum bacteriocin in the pH range of 6.5–7.3 and beyond this range the production was significantly less, LAB 41 strain produces maximum and equal amount of bacteriocin between pH range of 5.5–7.3 indicating its wide range of pH suitability for bacteriocin production, while the strain LAB 51 found to produce maximum bacteriocin in the pH range of 5.7–6.7 (Figure not shown here). The production of bacteriocin by these strains showed primary metabolic kinetics, as evidenced by the higher bacteriocin production with the increase of cell density in the logarithmic phase of growth curve (Mandal et al. 2008). It was reported that both nisin and pediocin production were typified as primary metabolites in each culture (Mandal et al. 2008; Suda et al. 2017). It is also observed that higher pH drop enhances both nisin and pediocin production by Lactococcus lactis and Pediococcus acidilactici, respectively (van Niel and Hahn-Hägerdal 1999). Many lactic acid bacteria viz., Lactococcus lactis, Lactobacillus sakei and Carnobacterium piscicola produce class I and class II bacteriocin, respectively, in a cell density dependent manner. It was promoted by auto-induction of extracellular pheromone like peptides by a quorum sensing mechanism (Kleerebezem et al. 1997; Diep et al. 2000; 2003). The amount of free bacteriocin in the medium was found to decrease with prolonged incubation time (Figure not shown here). This might be due to self-proteolytic enzymes which were synthesized in greater amount in the late stationary phase as reported earlier (Ikram-ul-Haq and Mukhtar 2006; Mandal et al. 2008).
Partial purification of bacteriocins by adsorption–desorption method
The bacteriocins produced by these strains showed maximum adsorption on to the cells at pH in between 5.1 and 5.3 (LAB 33, and LAB 51) and pH 5.5 and 5.7 (for LAB 41) and desorption at pH between 1.5 and 1.8. Many bacteriocin producer strains, Pediococcus, Lactobacillus, Lactococcus, and Leuconostoc, are known to adsorp bacteriocin molecules by their own cells (Yang et al. 1992; Tulini and De Martinis 2010). The desorption of bacteriocins is also influenced by some non-ionic detergents like Tween 80. Partial purification of the bacteriocins following the adsorption–desorption method showed 5 times, 2.5 times and 9 times of bacteriocin recovery from the strains LAB 33, LAB 41 and LAB 51, respectively, which was equivalent to 900 AU/ml, as compared to the initial heat-killed culture aliquot activity of 100 AU/ml against L. monocytogenes.
Characterization of the bacteriocins
Chemical stability of bacteriocins to different treatments
Chemical stability of the bacteriocins was tested to different treatments like pH, temperatures, detergents, and enzymes showed that the bacteriocins were stable to a wide range of acidic pH (2–7.3) as well as in the high alkaline (pH 11.0) condition as shown in the Table 1 (Figure not shown here). The loss of bacteriocin activity at alkaline pH (i.e. pH 12) might be due to alkali lysis of the bacteriocin molecules as observed for nisin (Gough et al. 2017) and pediocin AcH (Bhunia et al. 1991; Mandal et al. 2008). The bacteriocins were resistant to the high temperature treatments (viz., 100 °C, 121 °C for 20 min) and produced the inhibition zone of the same diameter as untreated one (Table 1). However, bacteriocin of LAB 33 showed lower stability when treated at 121 °C for 20 min (Table 1). Functional impairment of bacteriocins in reducing agent like β-mercaptoethanol and dithiothreitol indicates that the bacteriocin molecules might have essential cystine disulphide bridge(s). The sensitivity to Proteinase K of all the three bacteriocins indicates their absolute proteinaceous nature.
Molecular characterization of the bacteriocins
-
1.
Detection bacteriocins in glycine SDS-PAGE and in-gel assay
From Rf values comparison with standard protein (BSA, 66 kDa) it assumed that the molecular weight of partially purified bacteriocins was much lower than 66 kDa and they might be near to 5–10 kDa as evidenced in Glycine-SDS PAGE (Figure not shown here). The peptides produced prominent inhibition zones in the activity gel against L. monocytogenes MTCC 657 (Figure not shown here) indicating that the Coomassie Brilliant Blue R-250 stained bands in the gel were bacteriocins and that are stable in SDS and electrical field applications and in their peptide form.
-
2.
Determination of molecular weight in Tricin SDS-PAGE
The Tricine SDS-PAGE analysis of the partially purified bacteriocin showed presence of two to three bands in each lane. From Rf values comparison with standard molecular weight marker indicate that the partially purified bacteriocins of LAB 51 contains 03 bands with approximate molecular weight of 4265.79, 4466.83 and 7585.77 Da; of LAB 41 contains 03 bands with MW of 10,592.537, 6210.00 and 3480.00 Da; and of LAB 33 contains one band with MW of 4466.83 Da as shown in the Fig. 2a, b and Table 2.
Determination of molecular weight in Tricine SDS-PAGE and MALDI TOF–MS analysis of the partially purified bacteriocins. a 15% Tricine SDS-PAGE of the partially purified bacteriocins. Here, lane M indicates marker proteins (Da); Lane 2: bacteriocin of Pediococcus sp. LAB 33; Lane 3 and 4: bactiocins of Pediococcus sp. LAB 41 and LAB 51. b Molecular weight estimation of the partially purified bacteriocins of Pediococcus sp. LAB 51. c MALDITOF-MS of the partially purified bacteriocin of Pediococcus sp. LAB 51
-
3.
MALDI TOF–MS analysis of the partially purified bacteriocin
The partially purified bacteriocins of LAB 51 showed four major peaks at 4648.483 Da, 5625.950 Da, 6535.623 Da and 7869.389 Da as shown in Fig. 2c. It is very much surprising to note that our Rf values comparison with the peaks of MALDI TOF–MS were approximately same. Though the recent discovery of MALDI TOF–MS brought some accuracy in biomolecular characterization but the old age methodology of gel banding based molecular weight determination could not be ignored. Therefore, the developing laboratories can still now depend on such molecular weight estimation without any doubt. The missing of peptide bands of MW 6536.623 Da in the gel might be due to poor resolution of peptide bands or bands overlap. Further sample purification and better adoption of methodologies in separating very close peptide molecules need to be developed in the bacteriocin research. The MALDI-TOF MS has also been applied to detect pediocin PA-1 (4629 Da), enterocin A (4829 Da) in cell free supernatants of P. acidilactici PAC-1.0 and E. faecium CTC 492, respectively (Vera Pingitore et al. 2012; García-Cayuela et al. 2017; Phumisantiphong et al. 2017).
Antimicrobial spectrum of the bacteriocins
Antimicrobial efficacy of the bacteriocins produced by the strains was determined against some human pathogenic and food spoilage bacteria as shown in Table 3. Bacteriocins were found to inhibit the growth of E. faecalis MB1, L. mesenteroides Ly, L. monocytogenes MTCC 657, and Ps. aeruginosa MTCC 741 in spot on lawn method at a minimum concentration of ~ 100 AU/ml. It have also been reported that a few bacteriocins such as pediocin produced by non-human strain of Pediococci have been shown to inhibit the growth of Gram-negative bacteria (Kalchayanand et al. 1992). It is well known that nisin can also inhibit the growth of Listeria spp.(Cai et al. 1997).
Mode of action of bacteriocins on sensitive bacterial cells
The effect of partially purified bacteriocins was evaluated on growth and viability of the indicator strains.
Effect of bacteriocins on growth and viability
Effect of bacteriocins on growth and viability was evaluated against the two pathogenic strains, L. monocytogenes MTCC 657 and Ps. aeruginosa MTCC 741 as shown in Fig. 3a, b. The effect of bacteriocins on growth parameters showed a rapid decrease in cell density and loss of viability in bacteriocin treated cells. The bacteriocins caused very rapid loss of cell viability in L. monocytogenes MTCC 657 from an initial 12.38 log cfu/ml (untreated, at zero h of treatment) to log 6.09 cfu/ml (for LAB 33), log 7.29 cfu/ml (for LAB 41), and log 6.49 cfu/ml (for LAB 51) after 3 h of treatment. Whereas in case of Ps. aeruginosa MTCC 741 the loss of viability was estimated to log 6.09 cfu/ml (LAB 51), log 6.49 cfu/ml (for LAB 41), and log 6.74 cfu/ml (for LAB 33) from an initial log 12.05 cfu/ml (untreated, at 0 h of treatment) after 3 h of treatment. A reduction in colony forming units (CFU) per milliliter following treatment indicated that the bacteriocins were bactericidal in nature (Bhunia et al. 1991; Mandal et al. 2011). These findings suggested that LAB 33 had a much significant cidal effect on growth and viability on L. monocytogenes MTCC 657 in comparison to other two strains while LAB 51 could cause more loss of viability of Ps. aeruginosa MTCC 741 than other two strains. Therefore, it is assumed that the degree of bacteriocin sensitivity is bacteriocin specific.
Effect of bacteriocins treatments on the growth, viability and morphology of the indicator strains at 37 °C. a, b Effect on Listeria monocytogenes MTCC 657 and Pseudomonas aeruginosa MTCC 741, respectively. Here, solid lines indicate optical density and dotted lines indicate CFU counts; ‘opened square’ indicates treatment with bacteriocin of Pediococcus sp. LAB 33; ‘opened triangle’ of Pediococcus sp. LAB 41, and ‘opened circle’of Pediococcus sp. LAB 51. Results shown here were the average of triplicate trials. c, d Effect of bacteriocins on cell morphology as measured as LDH activity assay of L. monocytogenes MTCC 657 and Ps. aeruginosa MTCC 741, respectively. Results shown here were the average of triplicate trials
Effect of bacteriocins on cellular integrity
The LDH assay against L. monocytogenes MTCC 657 and Ps. aeruginosa MTCC 741 was shown in Fig. 3c, d. It was found that all the bacteriocins at 400 AU/ml and 800 AU/ml concentrations had caused lysis on both the strains. The rate of lytic action had increased in course of time. This lytic efficiency was quite higher than the sonication procedure. This lytic activity is comparable to other bacteriocins like pediocin AcH/PA-1 and pediocin DT 10 (Bhunia et al. 1991; Chikindas et al. 1993; Elegado et al. 1997), Pediocin NV-5 (Mandal et al. 2010). The efficiency of antibacterial activity of these bacteriocins against the food spoilage bacteria at a very low dose (400 AU/ml) shows their applicability in controlling food spoilage organisms in the processed food preservation.
Conclusion
From the present study it can be concluded that the bacteriocins from the three strains of Pediococcus sp. viz. LAB 33, LAB 41 and LAB 51, have high temperature (autoclaving temperature, 121 °C for 20 min) and low pH stability. Moreover, these bacteriocins have substantial antimicrobial activity against pathogenic and food spoilage bacteria like Listeria monocytogenes, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus mutans at a minimum dose of ~ 400 AU/ml with bactericidal effect. All these properties together would make them to consider as potent bacteriocins for use as food preservatives. Therefore, detailed molecular characterization, mode of action and also development of very low cost purification strategy from these strains could provide a big support in food bio-preservation.
References
Bhunia AK, Johnson MC, Ray B, Kalchayanand N (1991) Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J Appl Bacteriol 70:25–33
Cai Y, Ng LK, Farber JM (1997) Isolation and characterization of nisin-producing Lactococcus lactis subsp. lactis from bean-sprouts. J Appl Microbiol 83:499–507
Chikindas ML, García-Garcerá MJ, Driessen AJ et al (1993) Pediocin PA1, a bacteriocin from Pediococcus acidilactici PAC l.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol 59(11):3577–3584
Chikindas ML, Weeks R, Drider D et al (2018) Functions and emerging applications of bacteriocins. Curr Opin Biotechnol 49:23–28. https://doi.org/10.1016/j.copbio.2017.07.011
Daw MA, Falkiner FR (1996) Bacteriocins: nature, function and structure. Micron 27:467–479. https://doi.org/10.1016/S0968-4328(96)00028-5
De Vuyst L, Leroy F (2007) Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Mol Microbiol Biotechnol 13:194–199
De Vuyst L, Vandamme EJ (1994) Antimicrobial potential of lactic acid bacteria. In: Bacteriocins of lactic acid bacteria. Springer, pp 91–142
Diep DB, Axelsson L, Grefsli C, Nes IF (2000) The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiol Read Engl 146(Pt 9):2155–2160. https://doi.org/10.1099/00221287-146-9-2155
Elegado FB, Kim WJ, Kwon DY (1997) Rapid purification, partial characterization, and antimicrobial spectrum of the bacteriocin, Pediocin AcM, from Pediococcus acidilactici M. Int J Food Microbiol 37:1–11
García-Cayuela T, Requena T, Martínez-Cuesta MC, Peláez C (2017) Rapid detection of Lactococcus lactis isolates producing the lantibiotics nisin, lacticin 481 and lacticin 3147 using MALDI-TOF MS. J Microbiol Methods 139:138–142. https://doi.org/10.1016/j.mimet.2017.06.002
Gough R, Gómez-Sala B, O’Connor PM et al (2017) A simple method for the purification of Nisin. Probiotics Antimicrob Proteins 9:363–369. https://doi.org/10.1007/s12602-017-9287-5
Guerra NP, Pastrana L (2003) Influence of pH drop on both nisin and pediocin production by Lactococcus lactis and Pediococcus acidilactici. Lett Appl Microbiol 37:51–55
Heng NCK, Tagg JR (2006) What’s in a name? Class distinction for bacteriocins. Nat Rev Microbiol 4:160. https://doi.org/10.1038/nrmicro1273-c1
Ikram-ul-Haq null, Mukhtar H (2006) Biosynthesis of protease from Lactobacillus paracasei: kinetic analysis of fermentation parameters. Indian J Biochem Biophys 43:377–381
Kalchayanand N, Hanlin MB, Ray B (1992) Sublethal injury makes Gram-negative and resistant Gram-positive bacteria sensitive to the bacteriocins, pediocin AcH and nisin. Lett Appl Microbiol 15:239–243
Keren T, Yarmus M, Halevy G, Shapira R (2004) Immunodetection of the bacteriocin lacticin RM: analysis of the influence of temperature and Tween 80 on its expression and activity. Appl Environ Microbiol 70:2098–2104
Klaenhammer TR (1988) Bacteriocins of lactic acid bacteria. Biochimie 70:337–349
Kleerebezem M, Quadri LEN, Kuipers OP, de Vos WM (1997) Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol Microbiol 24:895–904. https://doi.org/10.1046/j.1365-2958.1997.4251782.x
Kruger NJ (2009) Detection of polypeptides on immunoblots using enzyme- conjugated or radiolabeled secondary ligands. In: Walker JM (ed) Protein protocols handbook. Humana Press, New Jersey, pp 405–414
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mandal V, Sen SK, Mandal NC (2008) Optimized culture conditions for bacteriocin production by Pediococcus acidilactici LAB 5 and its characterization. Indian J Biochem Biophys 45:106–110
Mandal V, Sen SK, Mandal NC (2010) Assessment of antibacterial activities of Pediocin produced by Pediococcus acidilactici LAB 5. J Food Saf. https://doi.org/10.1111/j.1745-4565.2010.00230.x
Mandal V, Sen SK, Mandal NC (2011) Isolation and characterization of Pediocin NV 5 producing Pediococcus acidilactici LAB 5 from vacuum-packed fermented meat product. Indian J Microbiol 51:22–29. https://doi.org/10.1007/s12088-011-0070-0
Phumisantiphong U, Siripanichgon K, Reamtong O, Diraphat P (2017) A novel bacteriocin from Enterococcus faecalis 478 exhibits a potent activity against vancomycin-resistant enterococci. PLoS ONE 12:e0186415. https://doi.org/10.1371/journal.pone.0186415
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Silva CCG, Silva SPM, Ribeiro SC (2018) Application of bacteriocins and protective cultures in dairy food preservation. Front Microbiol 9:594. https://doi.org/10.3389/fmicb.2018.00594
Stockland AE, San Clemente CL (1968) Lactate dehydrogenase activity in certain strains of Staphylococcus aureus. J Bacteriol 95:74–80
Suda S, Field D, Barron N (2017) Antimicrobial peptide production and purification. Methods Mol Biol (Clifton, NJ) 1485:401–410. https://doi.org/10.1007/978-1-4939-6412-3_22
Tulini FL, De Martinis ECP (2010) Improved adsorption-desorption extraction applied to the partial characterization of the antilisterial bacteriocin produced by Carnobacterium maltaromaticum C2. Braz J Microbiol Publ Braz Soc Microbiol 41:493–496. https://doi.org/10.1590/S1517-838220100002000032
van Niel EWJ, Hahn-Hägerdal B (1999) Nutrient requirements of lactococci in defined growth media. Appl Microbiol Biotechnol 52:617–627. https://doi.org/10.1007/s002530051569
Vera Pingitore E, Todorov SD, Sesma F, de Melo Gombossy, Franco BD (2012) Application of bacteriocinogenic Enterococcus mundtii CRL35 and Enterococcus faecium ST88Ch in the control of Listeria monocytogenes in fresh Minas cheese. Food Microbiol 32:38–47. https://doi.org/10.1016/j.fm.2012.04.005
Yang R, Johnson MC, Ray B (1992) Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol 58:3355–3359
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dey, B.C., Rai, N., Das, S. et al. Partial purification, characterization and mode of action of bacteriocins produced by three strains of Pediococcus sp.. J Food Sci Technol 56, 2594–2604 (2019). https://doi.org/10.1007/s13197-019-03744-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03744-3