Abstract
In this study probiotic microcapsules with three different shell compositions were produced through enzymatic gelation of skim milk powder by rennet, skim milk powder by transglutaminase and sodium caseinate by transglutaminase. Fabricated microcapsules and free Lactobacillus paracasei cells were incorporated into Iranian UF Feta cheese with different salt levels. Viability of L. paracasei (LAFTI L26), antioxidative capacity, ripening index, titrable acidity, salt content and texture profile analysis (TPA test) parameters including hardness, cohesiveness and stringiness were monitored during 45 days of storage time. Rennet based encapsulation was the most efficient method and could keep L. paracasei viable in all cheese samples (> 7 log10 CFU/g) at the end of storage time. Proteolysis pattern and acidification rate were strongly influenced by shell composition, salt level and storage time. Hardness and stringiness of probiotic cheese samples were influenced by shell composition of microcapsules and storage time but cohesiveness was only dependent on storage time. Therefore, storage time was the only effective factor on free radical scavenging activity of cheese samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are a group of live bacteria described by FAO and WHO (2001) as those ‘live microorganisms which confer a health benefit on the host as they are administered in adequate amounts (Poorbaghi et al. 2016). The most common and commercial probiotic bacteria are members of the genus Lactobacillus such as L. acidophilus, L. rhamnosus, L. brevis, L. plantarum and L. paracasei (Karimi et al. 2012). Among them, L. casei has the capacity to modulate the immune system and produce various bioactive compounds (Nag et al. 2011; Buriti et al. 2005). It has been suggested that probiotic foods should contain at least 106 live microorganisms per gram or milliliter at the time of consumption in order to be effective (FAO and WHO 2001; Ghasempour et al. 2012). Different environmental factors such as low pH of stomach and high bile salts concentration plus food related factors including salt content, acidity and Eh lead to a dramatic reduction in probiotics survival (De Prisco and Mauriello 2016). Microencapsulation is one of the applicable method for decreasing of this viability loss. Many encapsulation methods have been developed and generally involve spray drying and entrapment in gel particles of polysaccharides and proteins by emulsion or extrusion methods (Heidebach et al. 2009a, b). Proteins inverts to gels easily by heat treatment or enzymatic cross linkage. Also, milk proteins have excellent gelation properties (Garti and McClements 2012; Iravani et al. 2015) in addition to unique buffering capacity, which maintains good protection for probiotics against harsh acidic environment in gastrointestinal (GI) tract and intrinsic food conditions. Finally, their special functional properties help the feasibility control of the capsule size, which has important effect on the sensorial quality of microcapsules contained foods (Heidebach et al. 2009a, b).
Heidebach et al. (2009a, b) investigated two novel approaches based on rennet and transglutaminase (TGase) application for entrapment of Bifidobacterium bifidum and Lactobacillus paracasei in milk proteins. Different studies revealed that W/O emulsification enzyme-catalyzed gelation of milk proteins is a novel efficient method to encapsulate sensitive probiotic cells which can be incorporated in common dairy products such as yogurt or cheese (Iravani et al. 2015).
On the other hand, cheese seems to be the most suitable carrier for probiotics due to lower titrable acidity, good buffering capacity, dense matrix of the texture and greater protein as well as fat content (Karimi et al. 2012). The manufacture of UF-Feta cheese from pasteurized ultrafilterated milk is very popular in Iran. There are nearly 43 UF cheese plants in Iran with a capacity of 130,000 tons per year (Karami et al. 2009). Certain studies in white brined Feta cheese (Özer et al. 2009; Mirzaei et al. 2012) and cheddar cheese (Amine et al. 2014) about probiotic microencapsulation were performed, but the effect of shell material of microencapsulated cells on probiotic cheese characteristics has not been widely studied. Therefore, in the present study various types of microcapsules were made using rennet and TGase on the base of sodium caseinate (Na-Cs) and skim milk powder (SMP). The objective of the investigation was to evaluate the simultaneous effects of the incorporated microcapsules, salt levels and storage time on the proteolysis, salt content, acidity, antioxidative capacity, texture profile analysis (TPA) and probiotic viability in the probiotic UF cheese for the first time.
Materials and methods
Na-Cs containing 90% protein and SMP were obtained from Milad Khorasan Co. Ltd (Mashhad, Iran). Commercial strain of L. paracasei (LAFTI® L26) was obtained from DSM (Food Specialists, Moorebank, Australia). Microbial rennet powder (Meito, Sangyo Co. Tokyo, Japan) with milk clotting activity of 1 g per 100 kg milk was used for rennet based encapsulation. TGase Activa® MP (100 U/g) was obtained by great assistance of Ajinomoto Co. (Europe S.A.S, France). Extra virgin grape seed oil was purchased from a local market. All other chemicals were obtained from Merck (Germany) and they were of analytical grade.
Cheese making protocol
Cheese samples were manufactured at West Azerbaijan Pegah Co. (Urmia, Iran) according to a UF cheese making trend proposed by Tetra-Pak Processing Systems AB (Lund, Sweden). Firstly, milk bactofugation, pasteurization (75 °C for 15 s), ultrafiltration and homogenization steps were performed. Then, the retentate with a volume concentration factor of 4.2 was entered into the starter tank by adding the mesophilic starters (blend of R24/R26 choozit, Danisco, USA) and pH was reached to 6.2. In the aseptic filler chamber, rennet (Fromase, CHR. Hansen, Denmark), cultured retentate and probiotics (free or microencapsulated) were added simultaneously to each cheese container. Next, the cheese cups went through coagulation tunnel at 37 °C for 30 min and retentate was changed to pre-cheese form. In the sealing step, 2.5–4% (w/w) salt levels were incorporated into the parchment paper on the top of the cheese cups and then by using aluminum foil, all samples were fully sealed. In the maturing stage (28–30 °C), pH decreased to 4.6–4.7 and cheese samples were transferred to a cold-room (8 °C). At the time intervals of 1, 15, 30 and 45 days, cheese samples were subjected to microbiological, textural and chemical analysis.
Lactobacillus paracasei microencapsulation procedure
Probiotic microencapsulation was done using two methods described by Heidebach et al. (2009a, b) with some modifications. In rennet based microencapsulation, 28 g of 35% SMP was dissolved in distilled water with 0.7 g of L. paracasei DVS culture to create milk-cell concentrate of 108 CFU/ml of probiotic culture. The concentrate was cooled to 5 °C, incubated with 400 μl of 140 IMCU rennet stock solution and then kept at 5 °C to perform the cleavage of the κ-casein. After 1 h incubation, 180 μl of 10% (w/v) CaCl2 solution was added to the mixture and the encapsulation process was commenced. Cold-renneted mixture was added to 150 ml grapeseed oil and stirred at 500 rpm for 5 min to emulsify the mixture with oil. The temperature was slowly raised to 45 °C while the emulsion was further agitated for another 15 min. When the temperature increased over 18–20 °C, the emulsified droplets were turned into small gel particles instantly. In order to separate microcapsules from oil phase, centrifugation at 4000 rpm was applied for 3 min. The supernatant was discarded and the sediments comprising encapsulated probiotics were kept at − 20 °C prior to use.
In TGase triggered encapsulation, two suspensions of Na-Cs 12.5% and SMP 35% were prepared. The pH of the suspensions were adjusted to 7.0 with 5 M NaOH. To create a protein-cell concentrate with about 108 CFU/g, 0.7 g of freeze dried probiotic culture were mixed precisely with each of the suspensions. TGase was added at 45 °C to the protein-cell concentrates with an enzyme activity of 10 U per gram of substrate protein, and then encapsulation process was initiated. After TGase addition, the concentrates containing L. paracasei were added to tempered grapeseed oil and stirred with a magnetic stirrer for 3 h. Finally, the gelatinized microcapsules were separated from oil by gentle centrifugation (4000 rpm, 3 min). The supernatant was removed out and the microcapsules were stored at − 20 °C until use.
Physicochemical analysis
Cheese samples were analyzed for salt level by Volhard method (AOAC. 975.20), titrable acidity (lactic acid percent w/w%), and ripening index (water soluble nitrogen/total nitrogen × 100). Total nitrogen was determined by kjeldahl and water soluble nitrogen was measured based on the previous report (Alizadeh et al. 2006). Briefly, 20 g of grated cheese sample were mixed with 100 ml warm water and held at 40 °C for 1 h. After that insoluble solid was separated by centrifugation (3000 rpm) and supernatant was filtered. Nitrogen content was evaluated by automated kjeldahl unit (Behr, Inkjel S4, Germany).
Antioxidant activity
Water soluble extract of cheese was prepared according to Apostolidis et al. (2007) method. Briefly, 10 g of cheese samples were homogenized in a blender with equal volume of distilled water. Homogenized samples were centrifuged (23000 rpm, 10 min, 4 °C) and supernatant was clarified through a paper filter. Three ml of freshly prepared 60 µM 2, 2-diphenyl-1-picrylhydrazyl (DPPH) ethanolic solution was mixed with 250 μl of sample extract. The decrease in absorbance was recorded at 517 nm (A1) and compared with blank sample, which had just 250 μl of distillated water (A0). The scavenging activity was calculated using the following formula:
Microbiological analysis
To enumerate the microencapsulated bacteria incorporated to UF cheese samples, 10 g of each sample were suspended in 90 ml of warm sterile tri-sodium citrate solution and then microcapsules were disrupted mechanically by a stomacher device (Stomacher 400, Seward Medical, Sussex, UK) to release the entrapped cells completely (Nag et al. 2011; Özer et al. 2009). 0.1% peptone water solution (Oxide, Basingstoke, UK) was used for preparation of serial dilutions. Membrane sterilized (0.22 μm) vancomycin 0.5 g/l solution (Sigma Aldrich, UK) was added at the rate of 2 ml/l of molten MRS-Agar to count L. paracasei. Plates were incubated at 37 °C anaerobically (Gas pack Anaerobe, Merck, Germany) for 72 h (Ong and Shah 2009; Karimi et al. 2012).
Instrumental textural profile analysis (TPA)
Texture properties of cheeses were evaluated using a two-bite compression test by a Texture Analyzer equipped with a 5-kg load cell (TA-XT plus, Stable Micro Systems, UK). Identical cylindrical samples (20 mm diameter, 17 mm height) were cut carefully from each cheese and rested at room temperature 30 min before running test. The employed compression ratio was 50% from the initial height of the samples and a test speed of 4 mm/s was used. The trigger force was 3 g that was applied by a flat plate aluminum probe P/25. Measured parameters including hardness, cohesiveness and stringiness were obtained by the Exponent software (Stable Microsystems) version (6.0.6.0) from TPA curves.
Experimental design and statistical analysis
Three factors of storage time (0–45 days), salt level (2.5–4%) and shell composition of microcapsules (Free; RC: SMP by rennet; TGCC: SMP by TGase and TGSC: Na-Cs by TGase) were studied using Latin square experimental design (Table 1). General linear model was applied to each dependent Y variable and both main and interactive effects were evaluated at significance level of α = 0.05. SAS release 9 (SAS Institute Inc., Cary, NC, USA) was used for data analysis.
Results and discussion
Proteolysis
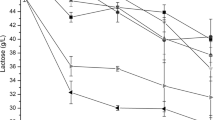
Proteolysis is the most influential phenomenon in cheese during storage time which alters flavor, texture and viability of adjunct cultures (Guinee 2004; Alizadeh et al. 2006). The percentage of WSN/TN which is characterized by proteolysis has considered as ripening index (RI). Our findings showed that linear effects of storage time and encapsulation type, interactive effect of time × shell composition and salt × shell composition and quadratic effect of storage time on primary proteolysis rate were significant (P < 0.05). Figure 1 shows that, the extent of proteolysis increased in all samples up to 23 days of storage time and decreased afterwards. This reduction could be related to further degradation of water soluble proteins and production of small sized peptides. Bergamini et al. (2006) reported that addition of probiotics into cheeses did not influence the production of medium and small-sized peptides which confirmed that RI is more related to proteinase activity compared to peptidase activity. Hesari et al. (2006) expressed that poor proteolytic defect of Iranian UF feta cheese is related to whey incorporation which restricts plasmin action. However, this defect would be compensated by use of L. paracasei. As shown in Fig. 1, at initial days of ripening, samples containing free probiotics had higher RI in comparison to encapsulated ones, while at the end of storage time, TGase encapsulated samples showed the lowest RI level. Ong et al. (2007) found that αs-CN fractions hydrolyzed faster in L. paracasei incorporated cheeses and resulted in more water soluble peptides which indicated the higher proteolytic activity of this strain.
Antioxidative capacity
DPPH has widely been used for estimation of the antioxidant capacity (Apostolidis et al. 2007). Statistical analyses revealed that the storage time was the only effective factor on the free radical scavenging activity (P < 0.05). The steady increase of antioxidant capacity until the end of ripening time resulted in 33% free radical inhibition rate (Fig. 2). In line with our results, Songisepp et al. (2004) also reported an increase in the ripening period for total antioxidant activity of probiotic cheese. Activity of adjunct cultures in ripening leads to release of numerous peptides, which may have antioxidant properties which was mentioned previously. For instance, Nishino et al. (2000) reported that lactic acid fermentation by L. casei increased the radical scavenging activity of the SMP due to the effects of peptidolytic enzymes. Although, Apostolidis et al. (2007) evaluated 25% DPPH radical inhibition for plain Feta cheese, Gupta et al. (2009) measured antioxidant capacity of cheddar cheese and found 30% scavenging activity after 40 days storage, which is in accordance with our results. Higher amount of DPPH scavenging activity in this study could be related to a greater degree of early proteolysis and L. paracasei extensive proteolytic and peptidolytic activity which produced functional small peptides (Singh et al. 1997). Several reports confirmed that production of antioxidant peptides in dairy products is related to proteolysis and is strain-specific. In addition, the sequence of these peptides is important in their antioxidant properties. Peptides with a Pro-His-His sequence showed the greatest antioxidant activity among all other peptides (Chen et al. 1996) which is due to their hydrogen-donating ability, lipid peroxy-radical trapping, or metal ion chelating ability of the imidazole group. We found no significant differences in DPPH scavenging activity between microencapsulated and free probiotics (P > 0.05). Therefore, it can be said that application of microencapsulation had no negative effects on antioxidant capacity of UF Feta cheese. The results also showed that salt levels did not influence DPPH inhibition significantly (P > 0.05).
Salt uptake trend
It is well known that salt influences the growth of starter and NLAB, the activity of various enzymes, the conformation of proteins and cheese texture (Guinee 2004; Özer et al. 2009). Furthermore, lower levels of salt are correlated with higher microbial contamination, excessive proteolysis, bitterness and loose texture (Farahnaky et al. 2013). Data analysis showed that linear effect of salt level and quadratic effect of time on salt content were significant (P < 0.05). A predictive model was obtained for salt content as a function of storage time and salt level (R2 = 0.85). As expected, the level of added salt had a positive effect on salt content of cheese samples. A quadratic effect of time on salt uptake was recorded which is in contrast with finding of Buriti et al. (2005), and Kasimoglu et al. (2004) who stated a constant rate of salt diffusion. Moreover, our findings were agreed with those of Ong et al. (2007), Buriti et al. (2005) and Souza and Saad (2009) indicated the independence of salt concentrations from probiotic incorporation.
Hardness
Salt, fat contents, proteolysis, moisture of the curd, acidity, and process variables like scalding and whey off can change the casein network structure of cheese which is reflected on the cheese hardness (Gunasekaran and Ak 2003). Statistical analysis showed that storage time, encapsulation type and salt level influenced hardness of cheese samples (P < 0.05). Hardness in all cheese samples increased during the first month of ripening and then decreased (Fig. 3a). The increase in hardness may be due to syneresis of curd made by concurrent acidification of starter and L. paracasei. This improvement of cheese firmness by decreasing pH has been reported in different researches such as Farahnaky et al. (2013) in Iranian UF cheese, Madureira et al. (2011) in probiotic whey cheese, Souza and Saad (2009) in probiotic Minas Fresh cheese. Cleaved peptides become a part of the serum phase and cannot contribute to the structure of cheese resulting in lower hardness amounts (Lucey et al. 2003). Decreased pH results in solubilization of calcium and its dissociation from casein and migration to the serum phase. On the other hand, probiotic strain was also effective on hardness. Madureira et al. (2011) demonstrated that L. casei containing cheese was firmer than other samples. As shown in Fig. 3b, all of the encapsulated samples had higher hardness values compared to free samples. This may be related to the higher proteolysis and acidification in cheese samples containing free L. paracasei. Overall, hardness of probiotic contained UF cheese is related to the shell composition of microcapsules which plays an important role in proteolysis and acidification via cell release mechanism during storage time. Our results showed that enzymatically induced encapsulation in milk proteins led to improvement of texture in Iranian UF Feta cheese.
Cohesiveness
Cohesiveness is the strength of the internal bonds making up the body of the product (Bourne 2002). The cohesiveness of cheese samples during storage time showed an increasing trend but was not influenced by salt and microencapsulation type (P > 0.05). There are some controversies about the effects of probiotic on the cheese cohesiveness. Addition of probiotic cultures could made the cheese firmer and more cohesive. Also, Jooyandeh (2009) reported the reduction of cohesiveness during ripening for Iranian white brined cheese supplemented with fermented whey. Moreover, Bonczar et al. (2002) reported that UF cheese had higher cohesiveness that might be related to higher water bounding capacity of cheeses produced from retentate, which is similar to our investigation.
Stringiness
Stringiness, which defined as the distance food extends before it breaks away from the compression, is a textural characteristic (Steffe 1996). Although, Gunarsekan and Ak (2003) expressed that stringiness is characterized for stretchability of Mozzarella cheese, this property has not been widely studied in cheese. Our results showed that microencapsulated probiotic and salt could influence stringiness (P < 0.05). Also, the samples containing free probiotic had the highest stringiness value which could be related to high acid production rate. No significant difference was observed among shell composition agents. Lower stringiness might be related to slow release of microcapsules during storage time which is result of reinforced structure of enzyme based microencapsulation by milk proteins.
Titrable acidity profile
Titrable acidity, which is represented by lactic acid percent, is one of the crucial restricting factors for probiotics viability within cheese. The acidifying role of probiotic bacteria significantly affects qualitative properties such as textural and sensorial characteristics (Karimi et al. 2012). We found significant effects of salt, storage time, shell composition and interactive effects of salt- shell composition and time-salt on the acidification rate of probiotic UF-Feta cheese (P < 0.05) and our predictive model had R2 = 0.975. Figure 4 represents that salt level was inversely related to acidity content in all samples except in TGase induced samples. This is completely related to suppressing effect of salt in proliferation of L. casei (Guinee 2004). Free probiotic contained samples had the highest and therefore microencapsulation by milk proteins hindered acidification rate. This phenomenon might be related to the firmness of the capsule. Jalili et al. (2010) studied cell kinetic release of Bifidobacterium animalis through several coating materials. They indicated that the use of shell materials which had more protection against external components resulted in lower permeability. Therefore, it caused an inside inhibition factor that could lead to culture loss. Heidebach et al. (2009a, b) found that utilization of TGase resulted in firmer and more stable capsules because of the covalent cross linking of milk proteins. Furthermore, Jalili et al. (2010) reported that accumulation of acid inside the less diffusible capsules, made by covalent linkages, could also strengthen capsules structure.
Probiotic viability
Data analysis showed that storage time, shell composition and salt level were effective on the probiotic viability (Table 2). Viability of free cells and skim milk-based microcapsules made by rennet or TGase was not statistically different (P = 0.05). Capsules made through TGase gelation of Na-Cs showed the lowest viability. Indeed, low-density gel network which provided sufficient space for the production of concentrated biomass would be preferred to dense matrix like TGase induced gels. This dense matrix which is the result of strong covalent bondages, led to lower diffusibility of the capsules. Most of the previous reports indicated an increased protection of probiotics by microencapsulation (Özer et al. 2009). However, some researchers exhibited that higher probiotic cell count was found in cheeses containing free cells at the end of storage, compared to those containing encapsulated cells. Totally, microencapsulation technique (emulsification, extrusion or coacervation), hydrocolloid matrix (κ-carrageenan or alginate), poor disposal of accumulated metabolites (lactic acid and acetic acid), different methods of capsules disruption and salt penetration into beads may influence microcapsules leakage efficiency (Garti and McClements 2012). Abd El-Salam and El-shibiny (2015) defined that dense gel matrix, the surface charge and hydrophobicity of the bacteria could be involved in the interaction between bacteria and milk proteins and affect cell release kinetics. However, there is still a high interest for application of microencapsulation in fermented dairy products because of its helps to avoid great loss of viable probiotic cells during gastrointestinal transition.
Conclusion
Lactobacillus paracasei entrapped by different milk proteins through enzymatic gelation was successfully incorporated to Iranian UF Feta cheese. Samples supplemented with rennet based microencapsulated probiotic remained alive with population above 8 log CFU/g during storage time. However, entrapment by use of TGase showed lower viability (> 7 log CFU/g) because of strong covalent bounds of microcapsules. On the other hand, primary proteolysis and titrable acidity rate were hindered by use of microcapsules in comparison to free samples. Also, incorporation of L. paracasei resulted in significantly increment of antioxidative capacity of UF Feta cheese. Our findings indicated that shell composition of microcapsules influenced cheese textural characteristics such as hardness and stringiness. These findings remark the possibility of probiotic encapsulation using proteins originated from milk as an applicable technique in dairy industries.
References
Abd El-Salam MH, El-Shibiny S (2015) Preparation and properties of milk proteins-based encapsulated probiotics: a review. Dairy Sci Technol 95:393–412. https://doi.org/10.1007/s13594-015-0223-8
Alizadeh M, Hamedi M, Khosroshahi A (2006) Modeling of proteolysis and lipolysis in Iranian white brine cheese. Food Chem 97:294–301. https://doi.org/10.1016/j.foodchem.2005.05.009
Amine KM, Champagne CP, Raymond Y, St-Gelais D, Britten M, Fustier P, Salmieri S, Lacroix M (2014) Survival of microencapsulated Bifidobacterium longum in Cheddar cheese during production and storage. Food Control 37:193–199. https://doi.org/10.1016/j.foodcont.2013.09.030
AOAC (Association of Official Analytical Chemists) (1997) Official methods of analysis (16th edn, 3rd rev. method 920.123), Arlington, VA, USA
Apostolidis E, Kwon YI, Shetty K (2007) Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg 8:46–54. https://doi.org/10.1016/j.ifset.2006.06.001
Bergamini CV, Hynes ER, Zalazar CA (2006) Influence of probiotic bacteria on the proteolysis profile of a semi-hard cheese. Int Dairy J 16:856–866. https://doi.org/10.1016/j.idairyj.2005.09.004
Bonczar G, Domagala J, Walczycka M (2002) The influence of ultrafiltration of ewe’s milk on soft cheeses properties. Electr J Pol Agric Univ 5(1)
Bourne M (2002) Food texture and viscosity: concept and measurement. Elsevier, Amsterdam. https://doi.org/10.1016/b978-0-12-119062-0.x5000-1
Buriti FC, da Rocha JS, Assis EG, Saad SM (2005) Probiotic potential of Minas fresh cheese prepared with the addition of Lactobacillus paracasei. LWT Food Sci Technol 38:173–180. https://doi.org/10.1016/j.lwt.2004.05.012
Chen HM, Muramoto K, Yamauchi F, Nokihara K (1996) Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agric Food Chem 44:2619–2623. https://doi.org/10.1021/jf950833m
De Prisco A, Mauriello G (2016) Probiotication of foods: a focus on microencapsulation tool. Trends Food Sci Technol 48:27–39. https://doi.org/10.1016/j.tifs.2015.11.009
FAO/WHO (2001) Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina
Farahnaky A, Mousavi SH, Nasiri M (2013) Role of salt in Iranian ultrafiltered Feta cheese: some textural and physicochemical changes during ripening. Int J Dairy Technol 66:359–365. https://doi.org/10.1111/1471-0307.12051
Garti N, McClements DJ (2012) Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Elsevier, Amsterdam
Ghasempour Z, Alizadeh M, Bari MR (2012) Optimization of probiotic yoghurt production containing Zedo gum. Int J Dairy Technol 65:118–125. https://doi.org/10.1111/j.1471-0307.2011.00740.x
Guinee TP (2004) Salting and the role of salt in cheese. Int J Dairy Technol 57:99–109. https://doi.org/10.1111/j.1471-0307.2004.00145.x
Gunasekaran S, Ak MM (2003) Cheese rheology, chapters 5, 7 and 10. CRC Press, Boca Raton
Gupta A, Mann B, Kumar R, Sangwan RB (2009) Antioxidant activity of Cheddar cheeses at different stages of ripening. Int J Dairy Technol 62:339–347. https://doi.org/10.1111/j.1471-0307.2009.00509.x
Heidebach T, Först P, Kulozik U (2009a) Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll 23:1670–1677. https://doi.org/10.1016/j.foodhyd.2009.01.006
Heidebach T, Först P, Kulozik U (2009b) Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int Dairy J 19:77–84. https://doi.org/10.1016/j.idairyj.2008.08.003
Hesari J, Ehsani MR, Khosroshahi A, McSweeney PL (2006) Contribution of rennet and starter to proteolysis in Iranian UF white cheese. Le Lait 86:291–302. https://doi.org/10.1051/lait:2006011
Iravani S, Korbekandi H, Mirmohammadi SV (2015) Technology and potential applications of probiotic encapsulation in fermented milk products. J Food Sci Technol Mys 52:4679–4696. https://doi.org/10.1007/s13197-014-1516-2
Jalili H, Razavi H, Safari M, Amrane A (2010) Kinetic analysis and effect of culture medium and coating materials during free and immobilized cell cultures of Bifidobacterium animalis subsp. lactis Bb 12. Electron J Biotechnol 13:2–3. https://doi.org/10.2225/vol13-issue3-fulltext-4
Jooyandeh H (2009) Effect of fermented whey protein concentrate on texture of iranian white cheese. J Texture Stud 40:497–510. https://doi.org/10.1111/j.1745-4603.2009.00194.x
Karami M, Ehsani MR, Mousavi SM, Rezaei K (2009) Changes in the rheological properties Iranian UF-Feta cheese during ripening. Food Chem 112:539–544. https://doi.org/10.1016/j.foodchem.2008.06.003
Karimi R, Mortazavian AM, Amiri-Rigi A (2012) Selective enumeration of probiotic microorganisms in cheese. Food Microbiol 29:1–9. https://doi.org/10.1016/j.fm.2011.08.008
Kasımoglu A, Goncuoglu M, Akgun M (2004) Probiotic white cheese with Lactobacillus acidophilus. Int Dairy J 14:1067–1073. https://doi.org/10.1016/j.idairyj.2004.04.006
Lucey JA, Johnson ME, Horne DS (2003) Perspectives on the basis of the rheology and texture properties of cheese. J Dairy Sci 86:2725–2743
Madureira AR, Pintado AI, Gomes AM, Pintado ME, Malcata FX (2011) Rheological, textural and microstructural features of probiotic whey cheeses. LWT Food Sci Technol 44:75–81. https://doi.org/10.1016/j.lwt.2010.06.030
Mirzaei H, Pourjafar H, Homayouni A (2012) Effect of calcium alginate and resistant starch microencapsulation on the survival rate of Lactobacillus acidophilus La5 and sensory properties in Iranian white brined cheese. Food Chem 132:1966–1970. https://doi.org/10.1016/j.foodchem.2011.12.033
Nag A, Han KS, Singh H (2011) Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int Dairy J 21:247–253. https://doi.org/10.1016/j.idairyj.2010.11.0
Nishino T, Shibahara-Sone H, Kikuchi-Hayakawa H, Ishikawa F (2000) Transit of radical scavenging activity of milk products prepared by Maillard reaction and Lactobacillus casei strain Shirota fermentation through the hamster intestine. J Dairy Sci 83:915–922. https://doi.org/10.3168/jds.s0022-0302(00)74954-x
Ong L, Shah NP (2009) Probiotic Cheddar cheese: influence of ripening temperatures on survival of probiotic microorganisms, cheese composition and organic acid profiles. LWT Food Sci Technol 42:1260–1268. https://doi.org/10.1016/j.lwt.2009.01.011
Ong L, Henriksson A, Shah NP (2007) Chemical analysis and sensory evaluation of Cheddar cheese produced with Lactobacillus acidophilus, Lb. casei, Lb. paracasei or Bifidobacterium sp. Int Dairy J 17:937–945. https://doi.org/10.1016/j.idairyj.2007.01.002
Özer B, Kirmaci HA, Şenel E, Atamer M, Hayaloğlu A (2009) Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int Dairy J 19:22–29. https://doi.org/10.1016/j.idairyj.2008.07.001
Poorbaghi SL, Gheisari H, Dadras H, Sepehrimanesh M, Zolfaghari A (2016) Effects of simple and microencapsulated Lactobacillus acidophilus with or without inulin on the broiler meat quality infected by avian influenza virus (H9N2). Probiot Antimicrob Protein 8:221–228. https://doi.org/10.1007/s12602-016-9224-z
Singh TK, Fox PF, Healy Á (1997) Isolation and identification of further peptides in the diafiltration retentate of the water-soluble fraction of Cheddar cheese. J Dairy Res 64:433–443. https://doi.org/10.1017/s0022029997002227
Songisepp E, Kullisaar T, Hutt P, Brilene T, Zilmer M, Mikelsaar MA (2004) A new probiotic cheese with antioxidative and antimicrobial activity. J Dairy Sci 87:2017–2023. https://doi.org/10.3168/jds.s0022-0302(04)70019-3
Souza CH, Saad SM (2009) Viability of Lactobacillus acidophilus La-5 added solely or in co-culture with a yoghurt starter culture and implications on physico-chemical and related properties of Minas fresh cheese during storage. LWT Food Sci Technol 42:633–640. https://doi.org/10.1016/j.lwt.2008.07.015
Steffe JF (1996) Rheological methods in food process engineering. Freeman Press, East Lansing. https://doi.org/10.1177/108201329700300108
Acknowledgements
The authors are grateful to Ajinomoto Co. Ltd (France) for their kind assistance in the preparation of ACTIVA MP (100 U/g). Also we would like to extend our great appreciation to Pegah Dairy Company (Urmia, Iran) for their cooperation in production of UF cheese samples at industrial scale. Finally, the authors are thankful to Dr. Z. Ghasempour for her truly encouragement and cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghaddas Kia, E., Alizadeh, M. & Esmaiili, M. Development and characterization of probiotic UF Feta cheese containing Lactobacillus paracasei microencapsulated by enzyme based gelation method. J Food Sci Technol 55, 3657–3664 (2018). https://doi.org/10.1007/s13197-018-3294-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3294-8