Abstract
Protein hydrolysates from white shrimp (Litopenaeus vannamei) with different degrees of hydrolysis (DH—10 and 20%) were prepared using the enzymes Alcalase 2.4 L and Protamex. The hydrolysates were evaluated for amino acid composition, solubility, foaming properties, emulsifying and antioxidant activity. All the hydrolysates showed high concentrations of Glutamic Acid, Aspartic acid, Arginine, Glycine, Lysine, Proline. It was found that the increase in the production of negatively charged amino acids was related to increase in DH. The hydrophobic amino acids were higher for hydrolysates obtained with Alcalase (10% DH) and Protamex (20% DH). The results indicated that higher degree of hydrolysis showed positive relation with the protein solubility of the hydrolysates, while negatively influenced foam and emulsification properties. The antioxidant properties presented by the white shrimp protein hydrolysates were influenced by the composition and peptides size. Hydrolysates with higher peptide chain showed the highest antioxidant power for the 2,2-Diphenyl-1-picrylhydrazyl radical scavenging and reducing power, while hydrolysates with lower peptide chain showed higher antioxidant power for 2,2′-azinobis (3-ethylbenzothiazoline sulfonic acid) radical scavenging. All hydrolysates showed dose-dependent antioxidant activities. Therefore, the results of the present study suggest that white shrimp is a potential source of protein hydrolysates as bioactive ingredients for the use in the formulation of functional foods as well as natural antioxidants in lipid food systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture has been highlighted as a sustainable alternative capable of supplying 50% of the global needs of fish and seafood, given that the demands for marine proteins surpass the sustainable yield of the oceans (FAO 2014). Among the aquaculture practices, white shrimp (Litopenaeus vannamei) is the most cultivated species. The cultivation of the species has intensified through the use of systems without water changes, known as biofloc system. This system contains microbial flakes, and results in improved water quality since it reduces nitrogen concentration and converts ammonia to cellular proteins, as well as serving as an additional source of nutrition for the cultivated species (Souza et al. 2016).
White shrimp is one of the most popular and low calorie crustaceans, with a high content of iron, calcium, vitamins and minerals (Gunasekaran et al. 2015). When cultivated by system, it presents a differentiated composition, in protein contents (Martini et al. 2015). Several researchers have focused their efforts on the use of white shrimp protein byproducts to obtain protein hydrolysates (Cheung and Li-Chan 2014; Dey and Dora 2014; Gunasekaran et al. 2015).
Protein hydrolysates have a variety of applications, whether in the food or pharmaceutical industry (Villamil et al. 2017). Protein hydrolysis consists of the cleavage of protein molecules into peptide units of different sizes. The breakdown of the protein structure enables the modification or improvement of functional properties (Liu et al. 2014). In addition to the functionalities, the hydrolysates obtained through the enzymatic hydrolysis process of proteins of marine origin are highlighted as important natural antioxidants (Alemán et al. 2011; Ngo et al. 2014; Shavandi et al. 2017). Studies indicate that the degree of hydrolysis influences the functionality and antioxidant activity of the hydrolysates (Klompong et al. 2007; Liu et al. 2014; Villamil et al. 2017). In this sense, the objective of the present study was to produce protein hydrolysates from white shrimp and study their functionality and antioxidant activities.
Materials and methods
Materials
The raw material, Litopenaeus vannamei, was supplied by the Marine Aquaculture Station of the Federal University of Rio Grande, Rio Grande, Rio Grande do Sul, Brazil. The white shrimp was obtained from the culture tanks and then transported to the Fish Processing Plant of the Federal University of Rio Grande, in sealed boxes, submerged in flake ice. The processing began with the hygienization of the species in chlorinated water (5 ppm), followed by the process of removal of the cephalothorax and carapaces. The minced shrimp was then stored in polyethylene bags at − 18 °C. The enzymes Alcalase and Protamex were produced by Novozymes Latina Americana Ltda. ABTS (2,2′-azinobis (3-ethylbenzothiazoline sulfonic acid) and DPPH (2,2-Diphenyl-1-picrylhydrazyl) were purchased from Sigma Chemical Co. The other reagents used were analytical grade.
Obtaining white shrimp protein hydrolysates
Protein hydrolysates were obtained from white shrimp muscle proteins, which were homogenized in distilled water in the ratio 1:2 (w:v), and the mixture was heated at 90 °C for 20 min. The hydrolysis reaction was performed in a double-walled glass reactor, connected to a thermostated bath. The pH and temperature of the reaction were maintained according to optimum conditions of each enzyme, Alcalase (pH 8.0, 50 °C) and Protamex (pH 7.0, 50 °C). The pH of the hydrolysis reaction was kept constant with NaOH (1 N). The reaction started with the addition of the enzymes separately at a ratio of 1% to muscle mass and was stopped when it reached a degree of hydrolysis of 10 and 20%. After the reaction, the enzyme was inactivated by heating at 90 °C for 20 min in a thermostated bath, followed by cooling at room temperature. The samples were centrifuged at 14,000 g for 20 min at 4 °C to separate non-hydrolyzed feedstock. The supernatant was lyophilized and stored at − 80 °C for further analysis (Alemán et al. 2011; Liu et al. 2014; Ngo et al. 2014).
Degree of hydrolysis
The degree of hydrolysis (GH) was used to monitor the hydrolysis reaction, as the number of hydrolyzed peptide bonds in relation to the total number of peptide bonds of the protein, using the pH-sat method (Adler-Nissen 1986). The GH was determined as follows:
where B is the volume of the base consumed during the hydrolysis to maintain the pH constant (mL); NB is the normality of the base (mol L−1); α is the degree of dissociation; htot is the number of peptide bonds (mols equiv/kg) which for fish is 8.6 mol equiv/kg; MP is protein mass (g). The degree of dissociation α was calculated as follows:
where pH is the pH during hydrolysis and pK is the dissociation constant. The values of pK depend on the temperature of the reaction (K) and were calculated as follows:
Amino acid composition
The total amino acid composition of shrimp protein hydrolysate was measured by method described by White et al. (1986).
Functional properties
Protein solubility
The protein solubility of the protein hydrolysates with different DHs was determined at pH 2.0; 4.0, 7.0 and 10, according to the methodology proposed by Liu et al. (2014), with adaptations. Briefly, 800 mg of sample was dissolved in 80 mL of distilled water and the pH was adjusted using HCl and NaOH. The solution was stirred for 1 h at room temperature (25 ± 1) °C and then centrifuged at 4000 rpm for 10 min. After the centrifugation process, a filtration procedure was followed to separate the soluble fraction. The solubilized protein content was determined according to the method of Lowry et al. (1951).
Foam properties
The Foaming Capacity and foam stability properties of the hydrolysates were determined according to Sathe and Salunkhe (1981), with modifications. A mass of 2 g of the hydrolysate was dispersed in 200 mL of distilled water. The solution was adjusted to different pHs (2.0, 4.0, 7.0 and 10.0), and then homogenized in an Ultra-Turrax shaker at 10,000 rpm for 1 min under (25 ± 1) °C room temperature. The dispersions formed were transferred to a graduated 250 mL beaker. The foaming capacity was calculated as the % volume increase after stirring over the initial volume. The stability of the formed foam was obtained by standing (20 min) of the dispersion at room temperature at 25 °C and calculated as follows:
where A is the volume after shaking (mL) and B is the volume before shaking (mL).
Emulsifying properties
The emulsifying properties of protein hydrolysates with different DHs, emulsifying activity index (EAI, m2 g−1) and emulsion stability index (ESI, min) were determined according to the turdibimetric method (Pearce and Kinsella 1978). A volume of 120 mL, at a concentration of 2 mg mL−1, was homogenized with 40 mL of soybean oil, on ultra-turrax shaker at 10,000 rpm for 1 min at room temperature (25 ± 1) °C. After stirring, the pH of the solution was adjusted to pH 2.0; 4.0, 7.0 and 10 with the aid of HCl (2 M) and NaOH (2 M). An aliquot of 50 μL of solution was diluted in 5 mL of 0.1% (w/v) sodium dodecyl sulfate (SDS). The absorbance of the diluted emulsion was measured at 500 nm at reaction times of 0 and 10 min. The emulsifying activity index (EAI, m2 g−1) and emulsion stability index (ESI, min) were determined as follows:
where A is absorbance at 500 nm and c is the protein concentration (g mL−1), c is the protein concentration (g mL−1), A0 and A10 are the absorbance of the emulsions diluted at 0 and 10 min.
Antioxidants properties
DPPH radical scavenging activity
The DPPH radical scavenging activity by the hydrolysates with different DHs was evaluated by the method of Shimada et al. (1992) and Centenaro et al. (2011), with modifications. Samples of 500 μL of hydrolysates with different concentrations (2.5, 5.0 and 7.5 mg mL−1) were added to 500 μL of 0.1 mmol L−1 DPPH in 95% ethanol. The mixture was homogenized in vortex, and kept standing in the absence of light for 30 min. After standing, the absorbance of the solution was measured at 517 nm. The sequestration capacity of the DPPH free radical was calculated as follows:
where Acontrol is the absorbance without sample and Asample is the absorbance with sample.
Reducing power
The reducing power of the protein hydrolysates was evaluated according to the method described by Zhang et al. (2008). A 250 μL volume of the hydrolysate (2.5, 5.0 and 7.5 mg mL−1) was mixed with 250 μL of 0.02 mol L−1 phosphate buffer (pH 6.6) and 2 mL of 1% potassium ferricyanide (w/v). The mixture was incubated at 50 °C for 20 min, and 2 mL of 10% trichloroacetic acid was added, followed by 2 s vortexing. A 500 μL aliquot was withdrawn and added with 400 μL of distilled water and 100 μL of 0.1% ferric chloride. After 10 min of reaction, the absorbance of the resulting solution was measured at 700 nm in a spectrophotometer, and an increase in the absorbance of the solution indicates that there was an increase in the reducing power.
Capture of the ABTS+
The ability to capture the ABTS cationic radical by hydrolysates was measured according to the method described by Chi et al. (2015). The solution containing the cationic radical was prepared from the stock solution of 7 mM ABTS with 2.45 mM potassium persulfate (5 mL:88 μL), kept standing in the absence of light, for 16 h at room temperature. The ABTS+ solution was diluted with ethanol until it reached a measured absorbance of 0.700 ± 0.05 at 734 nm. 600 μL of ABTS+ solution was mixed with 200 μl of sample at different concentrations (2.5, 5.0 and 7.5 mg mL−1). After 10 min of standing, the absorbance was measured at 734 nm. The capture of the ABTS+ was calculated as follows:
where Acontrol is the absorbance without sample and Asample is the absorbance with sample.
Statistical analysis
Data processing was be performed according to Statistica software for Windows 7.0, in the analysis of variance (ANOVA) and Tukey means comparison test at the 5% level of significance (p < 0.05).
Results and discussion
Amino acid composition
The protein hydrolysis process allows the cleavage of the proteins in amino acids and peptides with low molecular weight that have a high quality of amino acids and can be used as nutraceutical or functional foods (Chalamaiah et al. 2012). The amino acid compositions of the protein hydrolysates obtained in the study are presented in Table 1. In general, glutamic acid was the most abundant amino acid for all samples, making shrimp hydrolysates a great additive for use in food composition as flavor enhancers (Witono et al. 2016).
The results suggested that a lower degree of hydrolysis provides a higher concentration of HAA and PCAA amino acids. The hydrolysates were characterized by high levels of hydrophobic amino acids (HAA) in addition to aspartic acid and glutamic acid (NCAA). These amino acids can act as proton donors or electron lipid radicals scavenger factors that determine antioxidant activity of protein hydrolysates (Jain and Anal 2017). Additionally, the presence of all essential amino acids (EAA) in the hydrolysates with the maximum percentage of 35.47% (A20) indicates their good nutritional quality, as well as their antioxidant bioactivity.
All samples showed high concentrations of glutamic acid, aspartic acid, arginine, glycine, lysine, proline. The results found for the major amino acids diverge from the results of Simpson et al. (1998) who reported that the most abundant amino acids in shrimp muscle hydrolysates were glycine, proline, arginine and valine. This was in agreement with the observations by Chalamaiah et al. (2012) who reported that many hydrolysates derived from marine proteins have been reported to exhibit variations in their composition of amino acids, which depend not only on the raw material but also on the enzyme type and hydrolysis conditions.
Functional properties of hydrolysates
Solubility
Figure 1 shows the protein solubility (%) of the shrimp protein hydrolysates obtained from Alcalase 2.4 L and Protamex with DH 10 and 20%. Minimum solubility values were presented at pH 4.0 and maximum values at pH 2. The change in solubility can be attributed to the net load of the amino acid residues after the hydrolysis process, which increases as the pH moves away from the isoelectric point, promoting the aggregation of hydrophobic interactions (Taheri et al. 2013).
The protein hydrolysate obtained from the enzyme Protamex, with DH of 20% presented higher protein solubility in comparison with the other hydrolysates. A positive relationship was observed between the degree of hydrolysis and the solubility of the hydrolysate, indicating that the degradation of the proteins in smaller peptides results in a marked increase in solubility due to the reduction of the molecular weight of the structure, the unfolding of the peptide chains, and release of soluble aggregates (Liu et al. 2014, Ghribi et al. 2015). The low solubility of the hydrolysates with lower degree of hydrolysis may be attributed to the structure of the rigid macromolecule with subunits bound by several intermolecular and intramolecular disulfide bonds and hydrophobic interactions (Paraman et al. 2007).
Foam properties
Table 2 shows foam capacity and foam stability of the hydrolysates produced with Alcalase 2.4 L and Protamex with DH 10 and 20%. The results show an increase in foam capacity of hydrolysates of 10% DH, indicating that high molecular weight peptides are more susceptible to foaming, since they have the ability to form a cohesive interfacial film capable of enveloping and retaining air (Villamil et al. 2017). Our results showed a similar tendency with the results of Salem et al. (2017) where the foam properties of octopus (Octopus vulgaris) protein hydrolysate increased with the increase in DH.
The 10% DH hydrolysates obtained by Protamex demonstrated higher foam capacity (%) than the 10% DH hydrolysates obtained with Alcalase, indicating that the enzyme breaking specificity releases amino acid residues with better foaming properties. For FS, the lowest values were found at pH 4.0. According to Liu et al. (2014) minimum values at pH 4 are due to proximity to the isoelectric point of the proteins involved.
Emulsifying properties
Table 2 shows the emulsifying properties of the hydrolysates for emulsifying activity index (EAI, m2 g−1) and emulsion stability index (ESI, min).
For both hydrolysates, the highest values of emulsification were observed at pH 10. According to Taheri et al. (2013), alkaline pH promotes enhanced emulsifying properties due to the unfolding of the polypeptides because of the negative charges in this pH range. The repulsion resulting from this change allows greater orientation at the interface providing the exposure of hydrophilic and hydrophobic peptide residues which promote important interactions in the emulsion properties.
The hydrolysate with 10% DH from the Alcalase enzyme presented higher emulsion formation capacity. Witono et al. (2016) suggests an inverse relationship between the extent of the hydrolysis and the emulsifying properties.
Similar trend was observed in the studies of collagen hydrolysates from Spanish mackerel and eel protein hydrolysate, where the researchers presented a negative correlation between size of the peptide and emulsion formation (Chi et al. 2014; Baharuddin et al. 2016).
The pH affects emulsifying properties by altering the hydrophobicity of the protein surface and the protective layer surrounding the lipid globules (Taheri et al. 2013). Minimum values of emulsifying capacity were observed at pH 4.0, probably because this pH is close to the isoelectric point of fish proteins, where some molecules would be precipitated or with reduced loads, resulting in a reduction of their emulsifying properties.
Antioxidant properties of hydrolysates
DPPH radical scavenging activity
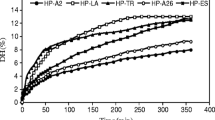
Figure 2 shows the results of the DPPH radical scavenging activity by the protein hydrolysates of white shrimp. The results indicate that all the samples presented antioxidant activity against radical sequestration, indicating the protein hydrolysates of shrimp as electron donors, capable of stabilizing the DPPH free radical (Chi et al. 2015; Salem et al. 2017). The results showed that an increase in the concentration of the samples provides a higher antioxidant activity, corroborating the results found by Zhang et al. (2008) and Jemil et al. (2014).
DPPH• scavenging activity (%) of shrimp protein hydrolysates obtained from Alcalase 2.4 L and Protamex with different DHs. (a–c) in the same hydrolysates indicate a significant difference between indicate a significant difference between concentrations (p < 0.05). (A–D) in the same concentration indicate significant difference between hydrolysates (p < 0.05). A10: Alcalase DH 10%, A20: Alcalase DH 20%, P10: Protamex DH 10%, P20: Protamex DH 20%
Among the different hydrolysates, the samples with lower DH presented a greater sequestration capacity of the DPPH radical in all the tested concentrations, when compared with the samples of greater DH. This behavior is associated with peptide length and composition of protein hydrolysates (Jemil et al. 2014). According to Shavandi et al. (2017), the capturing ability of the DPPH radical depends on the enzyme used in the hydrolysis process and the protein concentration tested.
Reducing power
Figure 3 shows the reducing power of shrimp protein hydrolysates produced with Alcalase 2.4 L and Protamex with DH 10 and 20%. The reducing power of a compound can be used to measure its antioxidant potential and has been studied in different researches (Zhang et al. 2008; Oliveira et al. 2014).
Reducing Power expressed as absorbance (700 nm) of shrimp protein hydrolysates obtained from Alcalase 2.4 L and Protamex with different DHs. (a–c) in the same hydrolysates indicate a significant difference between indicate a significant difference between concentrations (p < 0.05). (A–D) in the same concentration indicate significant difference between hydrolysates (p < 0.05). A10: Alcalase DH 10%, A20: Alcalase DH 20%, P10: Protamex DH 10%, P20: Protamex DH 20%
The higher the absorbance value the greater the reducing power of the samples. The presence of reducing compounds leads to the reduction of the Fe3+/ferricyanide complex in the ferrous form (Fe2+) through the electron donation, with the concentration of Fe2+ being monitored at 700 nm (Jemil et al. 2014). The 10% DH hydrolysates obtained with the Alcalase enzyme were the ones with the highest reducing power. According Piotrowicz and Mellado (2015) the enzyme type influences the reducing power of the hydrolysates, because for the same degree of hydrolysis it was verified a significant difference.
The hydrolysates with the lowest degree of hydrolysis showed a higher reduction power when compared to hydrolysates with a higher degree of hydrolysis, indicating that the reducing power of the hydrolysates is associated to the degree of extension of the hydrolysis (Klompong et al. 2007).
ABTS•+ scavenging activity (%)
The method of the ABTS radical capture is a method used to measure the antioxidant activity of hydrophilic and lipophilic compounds (Centenaro et al. 2014). Figure 4 shows the ability to capture the ABTS free radical of the protein hydrolysates with different DH. The results showed that a higher degree of hydrolysis (20%) provided a higher capture effect of the ABTS radical for samples, diverging from the results found for the analyses of reducing power and the sequestration capacity of the DPPH radical. According to Alemán et al. (2011), the capacity of a radical exerted by hydrolysates is not only related to the size of the chain, but also to the specificity of breaking of the enzyme used in the hydrolysis process.
ABTS•+ scavenging activity (%) of shrimp protein hydrolysates obtained from Alcalase 2.4 L and Protamex with different DHs. (a–c) In the same hydrolysates indicate a significant difference between indicate a significant difference between concentrations (p < 0.05). (A–D) In the same concentration indicate significant difference between hydrolysates (p < 0.05). A10: Alcalase DH 10%, A20: Alcalase DH 20%, P10: Protamex DH 10%, P20: Protamex DH 20%
In several studies involving protein hydrolysates and their antioxidant activity, some researchers reported that the free radical capture capacity improved as the degree of protein hydrolysis increased (Thiansilakul et al. 2007), while some researchers reported the opposite (Klompong et al. 2007).
Conclusion
The study investigated the functional and antioxidant properties of minced white shrimp protein hydrolysates from Alcalase and Protamex enzymes for 10 and 20% DH. The functional and bioactive properties of the protein hydrolysates were influenced by the extent of the hydrolysis. White shrimp protein hydrolysates appear as compounds with good functional and antioxidant properties, and can be added in food products aiming at increasing the functionalities and extending their useful life due to the considerable antioxidant power presented by the hydrolysates.
References
Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science Publishers, London
Alemán A, Pérez-Santín E, Bordenave-Juchereau S, Arnaudin I, Gómez-Guillén MC, Montero P (2011) Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res Int 44:1044–1051. https://doi.org/10.1016/j.foodres.2011.03.010
Baharuddin NA, Halim NRA, Sarbon NM (2016) Effect of degree of hydrolysis (DH) on the functional properties and angiotensin I-converting enzyme (ACE) inhibitory activity of eel (Monopterus sp.) protein hydrolysate. Int Food Res J 23:1424–1431
Centenaro GS, Salas-Mellado M, Prentice-Hernández C (2011) Antioxidant activity of protein hydrolysates of fish and chicken bones. Adv J Food Sci Technol 3:280–288
Centenaro GS, Salas-Mellado M, Pires C, Batista I, Nunes ML, Prentice C (2014) Fractionation of protein hydrolysates of fish and chicken using membrane ultrafiltration: investigation of antioxidant activity. Appl Biochem Biotechnol 172:2877–2893. https://doi.org/10.1007/s12010-014-0732-6
Chalamaiah M, Dinesh Kumar B, Hemalatha R, Jyothirmayi T (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135:3020–3038. https://doi.org/10.1016/j.foodchem.2012.06.100
Cheung IWY, Li-Chan ECY (2014) Application of taste sensing system for characterisation of enzymatic hydrolysates from shrimp processing by-products. Food Chem 145:1076–1085. https://doi.org/10.1016/j.foodchem.2013.09.004
Chi C, Cao Z, Wang B, Hu F, Li Z, Zhang B (2014) Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules 19:11211–11230. https://doi.org/10.3390/molecules190811211
Chi CF, Hu FY, Wang B, Li T, Ding GF (2015) Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J Funct Foods 15:301–313. https://doi.org/10.1016/j.jff.2015.03.045
Dey SS, Dora KC (2014) Optimization of the production of shrimp waste protein hydrolysate using microbial proteases adopting response surface methodology. J Food Sci Technol 51:16–24. https://doi.org/10.1007/s13197-011-0455-4
FAO (2014) The state of world fisheries and aquaculture. Food and Agriculture Oraganization of the United Nations, Rome
Ghribi A, Gafsi IM, Sila A, Blecker C, Danthine S, Attia H, Bougatef A, Besbes S (2015) Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem 187:322–330. https://doi.org/10.1016/j.foodchem.2015.04.109
Gunasekaran J, Kannuchamy N, Kannaiyan S, Chakraborti R, Gudipatti V (2015) Protein hydrolysates from shrimp (Metapenaeus dobsoni) head waste: optimization of extraction conditions by response surface methodology. J Aquat Food Prod Technol 24:429–442. https://doi.org/10.1080/10498850.2013.787134
Jain S, Anal K (2017) Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J Food Sci Technol 54:1062–1072. https://doi.org/10.1007/s13197-017-2530-y
Jemil I, Jridi M, Nasri R, Ktari N, Salem RBSB, Mehiri M, Hajji M, Nasri M (2014) Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem 49:963–972. https://doi.org/10.1016/j.procbio.2014.03.004
Klompong V, Benjakul S, Kantachote D, Shahidi F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 102:1317–1327. https://doi.org/10.1016/j.foodchem.2006.07.016
Liu Y, Li X, Chen Z, Yu J, Wang F, Wang J (2014) Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem 151:459–465. https://doi.org/10.1016/j.foodchem.2013.11.089
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Martini NDN, Nursyam H, Fadjar M (2015) The effects of different intensive culture systems on white shrimp (Litopenaeus vannamei) muscle protein pattern. J Life Sci Biomed 5:97–101
Ngo DH, Ryu B, Kim SK (2014) Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem 143:246–255. https://doi.org/10.1016/j.foodchem.2013.07.06
Oliveira CF, Coletto D, Correa APF, Daroit DJ, Toniolo R, Cladera-Olivera F, Brandelli A (2014) Antioxidant activity and inhibition of meat lipid oxidation by soy protein hydrolysates obtained with a microbial protease. Int Food Res J 21:775–781
Paraman I, Hettiarachchy NS, Schaefer C (2007) Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chem 84:593–599. https://doi.org/10.1094/CCHEM-84-6-0593
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723. https://doi.org/10.1021/jf60217a041
Piotrowicz IBB, Mellado MMS (2015) Antioxidant hydrolysates production from Argentine anchovy (Engraulis anchoita) with different enzymes. Int Food Res J 22:1203–1211
Salem RBS, Bkhairia I, Abdelhedi O, Nasri M (2017) Octopus vulgaris protein hydrolysates: characterization, antioxidant and functional properties. J Food Sci Technol 54:1442–1454. https://doi.org/10.1007/s13197-017-2567-y
Sathe SK, Salunkhe DK (1981) Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity, and gelation properties. J Food Sci 46:71–81
Shavandi A, Hu Z, Teh S, Zhao J, Carne A, Bekhit A, Bekhit AED (2017) Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent. Food Chem 227:194–201. https://doi.org/10.1016/j.foodchem.2017.01.099
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948. https://doi.org/10.1021/jf00018a005
Simpson BK, Nayeri G, Yaylayan V, Ashie INA (1998) Enzymatic hydrolysis of shrimp meat. Food Chem 61:131–138. https://doi.org/10.1016/S0308-8146(97)00131-3
Souza DM, Borges VD, Furtado P, Romano LA, Wasielesky W, Monserrat JM, Oliveira GL (2016) Antioxidant enzyme activities and immunological system analysis of Litopenaeus vannamei reared in biofloc technology (BFT) at different water temperatures. Aquaculture 451:436–443. https://doi.org/10.1016/j.aquaculture.2015.10.006
Taheri A, Anvar SAA, Ahari H, Fogliano V (2013) Comparison the functional properties of protein hydrolysates from poultry byproducts and rainbow trout (Onchorhynchus mykiss) viscera. Iran J Fish Sci 12:154–169
Thiansilakul Y, Benjakul S, Shahidi F (2007) Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem 103:1385–1394. https://doi.org/10.1016/j.foodchem.2006.10.055
Villamil O, Váquiro H, Solanilla JF (2017) Fish viscera protein hydrolysates: production, potential applications and functional and bioactive properties. Food Chem 224:160–171. https://doi.org/10.1016/j.foodchem.2016.12.057
White JA, Hart RJ, Fry JC (1986) An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J Autom Chem 8:170–177. https://doi.org/10.1155/S1463924686000330
Witono Y, Taruna Y, Windrati WS, Azkiyah L, Sari TN (2016) ‘Wader’ (Rasbora jacobsoni) protein hydrolysates: production, biochemical, and functional properties. Agric Agric Sci Procedia 9:482–492. https://doi.org/10.1016/j.aaspro.2016.02.167
Zhang SB, Wang Z, Xu SY (2008) Antioxidant and antithrombotic activities of rapeseed peptides. J Am Oil Chem Soc 85:521–527. https://doi.org/10.1007/s11746-008-1217-y
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq) for their financial support and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarship. They also thank the Marine Aquaculture Station of the Federal University of Rio Grande for the donation of the raw material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Latorres, J.M., Rios, D.G., Saggiomo, G. et al. Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei). J Food Sci Technol 55, 721–729 (2018). https://doi.org/10.1007/s13197-017-2983-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2983-z