Abstract
Phytonutrients retained palm olein (PRPOL) was prepared and blended into butterfat at different ratios. The physicochemical characteristics and the phytonutrient composition of blends, as well as its utilization in the preparation of functional chocolate spread were evaluated. The results showed that the redness, yellowness, slip melting point, free fatty acids, peroxide value, iodine value, unsaponifiable matter, diacylglycerol and monoacylglycerol increased while lightness, saponification value, and triacylglycerol significantly decreased upon incorporation of increased quantities of PRPOL into butterfat. The incorporation affected short chain, medium chain and long chain fatty acids content along with variation in the palmitic, stearic, oleic acids content of the blends as compared to butterfat alone. Improvement in carotenoids (6–27 fold), phytotosterols (3–15 fold), tocopherols and tocotrienols (4–17 fold), and squalene (1–6 fold) in blends was observed upon incorporation of PRPOL. Cholesterol level in the blends was reduced (10–50 %) as compared to the butterfat. The blends showed an intermediate solid fat content of PRPOL and butterfat. Moreover, radical scavenging activity of the blends increased with increase in PRPOL quantity. Prepared chocolate spreads showed similar fat, moisture, colour components (L*, a* and b*) and better emulsion stability. The hardness of the spreads was increased upon increasing quantity of PRPOL. The sensory evaluation showed that chocolate prepared by replacing butterfat with 20 % PRPOL had acceptable sensory attributes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Palm oil is one of the widely consumed vegetable oils in the world. The crude form of palm oil contains variety of natural phytonutrients including carotenoids, tocopherols, phytosterols, squalene, phenolics, and coenzyme Q10 (Goh et al. 1985). Red palm oil is the richest and cheapest source of natural carotenoids (Rukmini 1994). It consists of 500–700 ppm of carotenoids along with other natural phytonutrients at various levels (Sundram et al. 2003). The carotenoids of palm oil composed of approximately 30 % of α- carotene and 70 % of β-carotene. Both of these carotenes possess provitamin-A activity thereby helps in preventing night blindness, aids maintenance of tissues and promote cell growth. Similarly, tocopherols and tocotrienols are potent antioxidants that prevent membrane peroxidation of tissue lipids (Kamat et al. 1997). Additionaly, tocotrienols are efficient in reducing plasma cholesterol (Parker et al. 1993). Red palm oil contains 300 mg/kg of phytosterol including campesterol, stigmasterol and β-sitosterol; possess cholesterol-lowering properties (Sundram et al. 2003). Prasanth Kumar and Gopala Krishna (2014a) observed that crude palm olein, a liquid fraction of the crude palm oil contains more phytonutrients than the starting palm oil. The health benefits of palm oil phytonutrients have been reviewed by Sundram et al. (2003). The retention of these minor components in palm oil depends on the methods used for processing (Prasanth Kumar and Gopala Krishna 2014b). The commercial purification methods of palm oil consist of physical (steam) refining, bleaching and deodorization. All these steps operate at high temperature under vacuum. The complete degradation of carotenoids and partial removal of phytonutrients including tocopherols, tocotrienols and phytosterols by these methods have reported (Posada et al. 2007; Rossi et al. 2001).
Several methods have been developed to isolate/retain palm carotenoids and other phytonutrients from palm oil (Mayamol et al. 2007). Malaysian Palm Oil Promotion Council (MPOPC) has developed molecular distillation based process for the preparation of phytonutrients retained red palm olein from low acidic palm oil (Nagendran et al. 2000). The molecular distillation consists of several steps and operates with low acidic (<5 %) palm olein makes the process costly. Similarly, supercritical fluid extraction of low acidic phytonutrient enriched palm oil also developed (Lau et al. 2007). Recently Prasanth Kumar et al. (2013) have used enzymatic deacidification to retain phytonutrients in high acidic crude red palm olein.
Functional foods are in increasing demand and fast becoming a part of everyday life due to the growing awareness of consumers regarding human health on proper nutrition (El-Hadad et al. 2011). The functional foods can be consumed as a part of a daily diet to provide or maintain metabolic or physiologic effect to keep good mental and physical health, or to reduce the risk of diseases and to provide adequate nutritional effect (Roberfroid 2000). Vitamin A deficiency is one of the major nutritional problems in the developing countries (NNMB 1988–90; El-Hadad et al. 2011). Vitamin-A deficiency causes night blindness, xerophthalmia, damages the genitourinary system, respiratory system and gastrointestinal tract, and it has a role in immune response (Butt et al. 2006). The phytonutrients plays a role in the prevention of chronic diseases like cancer, arthritis, osteoporosis and coronary heart diseases (Wrick et al. 1993; Suzuki et al. 1993). Manorama et al. (1996) utilized red palm oil to combat vitamin A deficiency in school children. El-Hadad et al. (2011) prepared functional biscuits by replacing commercial shortening with red palm olein to enhance the level of carotenoids, tocotrienols and tocopherols. Al-Saqer et al. (2004) used red palm olein and red palm-shortening to prepare functional pan bread and sugar-snap cookies to provide higher amounts of vitamin E in the diet. El-Hadad et al. (2011) have used phytonutrients retained red palm olein prepared by molecular distillation to replace butterfat at 20 % level in chocolate spread.
Butterfat prepared from cow milk, or buffalo milk is one of the traditional dietetic sources of lipids in India. It is potentially used as cooking and frying medium and produced through the clarification of butter. Butter is oil in water emulsion obtained from milk and widely used as butter spread. Butter provides creamy mouth-feel, buttery aroma, palatability and desired texture to the food products. It lacks spreadability at refrigerated (10 °C) and room (21–25 °C) temperatures and these are the main disadvantages of butter as a spread (Danthine et al. 2014). Hence, several physical and chemical processes have been used to modify the properties of clarified butter (butterfat). Recently, chemical and enzymatic modifications of butterfat blended with canola oils were carried out to achieve spreadability at both room temperature and refrigerated temperature (Rodrigues and Gioielli 2003; Marangoni and Rousseau 1998; Rousseau et al. 1996). Apart from the poor spreadability, clarified butter contains 200–400 mg/100 g of cholesterol (Surendranath et al. 1996). Hence, consumption of butterfat and products rich in dairy fat contribute an appreciable amount of cholesterol intake. In addition, studies have reported a positive correlation between serum cholesterol level and dietary intake of cholesterol. The several methods have been adopted to reduce the levels of cholesterol in ghee (Kumar et al. 2010; El-Aziz et al. 2013). Rodrigues and Gioielli (2003) have blended butter oil with corn oil and modified chemical interesterification to get the improved physical and nutritional properties.
Some of the edible oils such as coconut oil, sesame oil, mustard oil, groundnut oils, olive oil, etc. are being consumed in the virgin form. Similarly, the consumption of RPOL in virgin form is being prevented by the high free fatty acid content. Free fatty acids (FFA) are produced naturally in crude palm oil (CPO) and this gets increased by the action of powerful hydrolytic enzymes present on the exterior of the palm fruit and by microbial lipases most probably originating from yeast cells; and hydrolysis of oil during poor and lengthy storage (Abigor et al. 1985; Tan et al. 2009). Prasanth Kumar and Gopala Krishna (2014b) made an attempt to retain palm phytonutrients by enzymatic esterification of free fatty acids with added glycerol. The products obtained were different in the properties viz. slip melting point, partial glyceride composition, the TAG profile compared to starting RPOL. The study was focused to evaluate the incorporation of phytonutrients retained red palm olein into butterfat in different weight ratios by blending, evaluation of its on physicochemical and nutraceutical properties of chocolate spread.
Materials and methods
Commercial anhydrous butterfat was procured from the local supermarket, Mysore, Karnataka. Crude red palm oil was obtained from M/s Oil Palm India Ltd., Kottayam, Kerala. Lipozyme RM IM was obtained as kind gift from Novozymes India Ltd., Bangalore, India. Icing sugar, liquid glucose, skimmed milk, and cocoa powder werepurchased from local supermarkets in Mysore, Karnataka, India. Cholesterol, β-sitosterol, stigmasterol, stigmastanol, α-tocopherol, squaleneand 1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma Chemical Co. (St Louis, MO, USA) and standard
FAME mix from Supelco Inc. Bellefonte, PA, USA. All the solvents and chemicals used were of analytical reagent grade.
Preparation of phytonutrient retained palm olein (PRPOL)
Phytonutrients rich palm olein was prepared according to Prasanth Kumar et al. (2013). Crude red palm olein was prepared according to Kumar and Krishna (2014b). Crude palm oil (5 kg) was heated at 65 °C to get the complete dissolution of crystals. Then the melted palm oil was kept in a chamber adjusted with the temperature of 25 °C and the slow crystallization process was carried out for 24 h. The crystallized solid material was separated by vacuum filtration. The obtained products were liquid crude red palm olein and solid crude red palm stearin. The liquid red palm olein was being used for the preparation of phytonutrient retained red palm olein. The enzymatic deacidification of CRPOL was carried out by using immobilized Rhyzomucormeihei lipase (Lipozyme RM IM) with added glycerol in a solvent free system. The reaction conditions including the molar ratio of glycerol to FFA, enzyme concentration, incubation period and temperature were followed according to the previously standardized conditions (Kumar et al. 2013). The reaction was carried out with 100 g portion of CRPOL in 500 ml Soxhlet flask and added glycerol in 1:2 molar ratios to FFA (as palmitic acid). Lipozyme RM IM (5 %, w/w) was used for the reaction and was carried out at 63 °C with a rotating speed of 150 rpm under vacuum. The reaction was carried out for 12 h and the reaction was stopped by removing the enzyme by filtration. The same enzyme was washed with hexane, dried and reused for the subsequent batches of deacidification. The obtained products were stored at 10 °C till the analysis and the product preparation.
Preparation of blends
The blends of PRPOL with butterfat were prepared at different ratios (0:100, 20:80, 30:70, 40:60, 50:50, and 100:0). The fat samples were melted at 65 °C and weighed at required quantities in Soxhlet flask (500 ml capacity). The blending was carried out in Rotavapor(BuchiLabortechnik, Switzerland) at 65 °C with 150 rpm under 5 mmHg vacuum for the period of 15 min to ensure complete dissolution of fat crystals. The blends were cooled to room temperature, transferred to pet jars and stored in 4 °C until further usage.
Physicochemical characteristics of blends
Blends of nutraceuticals retained palm fat and butterfat were characterised by evaluating the colourusing a Hunter Lab Labscan XE spectrophotometer (Hunter Associates Laboratory Inc, Restone Virginia). The 15 ml Sample (15 ml) was placed in a sample cup and was used for transmittance colour measurements in liquid media. The colour of samples was obtained by using a 2/°C (2° observer/illuminant C). The results were expressed as L*, a*, b* respectively indicating lightness (0–100), green–red components and blue–yellow components. The slip melting point (SMP) of different fractions was analysed according to AOCS Official Methods Cc 3-25 (AOCS 1997). Samples were taken in an open capillary tube to the height of 1 cm. The samples were tempered at 10 °C for 16 h. the tubes were heated slowly in a temperature controlled water bath until the fat column rise due to hydrostatic pressure. The temperature at which the rising of fat column occurs is expressed as the SMP using average of four replicates.
The chemical characteristics include free fatty acids (FFA) (AOCS Ca 5a-40), saponification value (SV) (AOCS Cd 3-25), iodine value (IV) (AOCS Cd 1d-92), peroxide value (PV) (AOCS Ca 8-53) and unsaponifiable matter (AOCS Ca 6a-40) were analysed according to the standard methods and practices recommended by the AOCS (AOCS 2003). The experiments were carried out in quadruplicate sets and the results were expressed as average with standard deviation.
Glyceride (TAG, DAG, MAG) composition of the blends
TAG, DAG, and MAG contents of the blends were estimated by using the standard column chromatographic method. A glass column (i.d. 1.8 cm; length, 30 cm, Borosil Glass Works Ltd., Mumbai, India) was used in which a silica (100–120 mesh size) bed was prepared from slurry of silica in petroleum ether. MAG, DAG, and TAG were eluted with the standard solvent system and the quantity of each fraction was determined gravimetrically after evaporating the solvent as per AOCS Cd 11c-93 (AOCS 2003).
Preparation of fatty acid methyl esters and Fatty acid analysis of blends by GC
Fatty acid methyl esters (FAME) of the oil samples were prepared by transesterification, according to AOCS Ce 1b-89, using methanolic KOH as catalyst (AOCS 2003). Analysis was carried out using a gas chromatograph (model-GC-20A, Shimadzu Corporation, Japan) equipped with FID detector and a glass capillary column (30 m × 0.25 mm), coated with poly (90 % biscyanopropyl/10 % cyanopropylphenyl) siloxane with a film thickness of 0.2 µm (SP-2380) (Supelco Analytical, Bellefonte, Pennsylvania, USA). The column oven temperature was programmed as follows: 60 °C for 5 min, raised at 10 °C/min to 180 °C, held for 15 min, injector temperature 220 °C and detector temperature 230 °C. A reference standard FAME mix (Supelco Inc., Bellefonte, PA, USA) was analyzed under the same operating conditions to determine peak identity. FAME were expressed as relative area percent.
Solid fat content and thermal behavior of nutraceutical retained palm red palm olein and butterfat blends
Differential scanning calorimetry (DSC 8000, PerkinElmer DSC 8000) was used to obtain the thermograms of melting and crystallization. An empty aluminum pan was used as a reference, and each sample was accurately weighed (8 ± 0.1 mg) for DSC analysis. The sample was heated to 80 °C and held for 10 min. Thereafter, the temperature was decreased at 10 °C/min to −60 °C. After holding for 10 min at −60 °C, the melting curve was obtained by heating the samples to 80 °C at 5 °C/min (Lee and Foglia 2000). The solid fat content (SFC, %) was obtained melting thermograms by Universal Analysis 2000 (TA Instruments Inc.). Each DSC thermogram was divided at different temperatures (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, and 55 °C) and the total crystallization energy (J/g) was calculated into percentage (%) at each temperature for SFC (%).
Phytonutrients composition of phytonutrients retained red palm olein and butterfat blends
Tocopherols and tocotrienols determination
Determination of tocopherol composition by HPLC (Shimadzu) system consisted of LC-10A pump, injector fitted with 20 µl loop and FLD detector was used for the analysis of tocopherols and tocotrienols. The analysis was achieved by normal phase HPLC separation on silica column. The mobile phase was hexane: isopropyl alcohol (99.5:0.5, v/v) at the flow rate of 1 ml/min and were detected by fluorescence at the excitation and emission wavelengths of 290 and 330 nm respectively. The tocopherols were quantified based on peak areas with an external standards α-tocopherol and the tocotrienols were expressed as α-tocopherol equivalent as suggested by AOCS Ce 8-86 (AOCS 2003).
Carotenoids determination
Carotene content was determined by diluting 1 g of melted palm oil at 65 °C to 10 ml using hexane and from this 1 ml aliquot further diluted to 10 mL with hexane and measured absorbance at 446 nm using a UV–Vis spectrophotometer (Shimadzu UV-1601 (Shimadzu Corporation, Kyoto, Japan) followed by calculation using the diffusion coefficient of 383 and expressed as mg/kg oil (Chandrasekaram et al. 2009).
Sterols determination
Sterol content and composition of the oil samples was determined by HPLC according to Sánchez‐Machado et al. (2004). A sample of 5 g was weighed in an Erlenmeyer flask to which KOH was added and saponified by refluxing and stirring for 1 h with constant shaking, after cooling to ambient temperature, the mixture was transferred to a separatory funnel to extract the non-saponifiable fraction with 6 × 50 ml portions of petroleum ether, pooled, washed with 10 % ethanol to remove the alkali. Then the washed petroleum ether fraction was dried with anhydrous sodium sulphate and evaporated to dryness in a rotavapor at 50 °C. The residue was dissolved with mobile phase (30:70 v/v, methanol: acetonitrile) and filtered through 0.5 µm membrane (Millipore, Bedford, MA, USA) and used for HPLC analysis.
Sterols was analysed using HPLC with UV detection (HPLC–UV). Which was comprised of a LC-20A pump (Shimadzu, Tokyo, Japan), a 20 μl detection loop and a UV–vis diode-array detector. Temperature was kept constant at 30 ± 0.1 °C with a column heater. Separation was performed in a Kromasil C8 5 μm column (250 × 4.6 mm i.d.; Supelco Inc., Bellefonte, PA, USA), with 30:70 (v/v) methanol: acetonitrile at 1.2 ml/min as mobile phase. Detection wavelength was 205 nm (Sánchez‐Machado et al. 2004). Twenty microliters of the extract was injected into the HPLC column.
Squalene
Squalene in the samples was estimated by HPLC according to Nenadis and Tsimidou (2002). The samples (0.02 g) was dissolved in 1 mL of hexane, and 20 μL of the mixture was injected into an HPLC (10A VP, Shimadzu Corporation, Kyoto, Japan) equipped with a UV detector (SPD-10AV VP, Shimadzu). The analysis was achieved by isocratic separation on C18 column, 10 µm, u-Bondapack, (4.6 × 300 mm; Millipore, Milford, MA) with a mobile phase of acetone: acetonitrile (40/60, v/v) and at a flow rate of 1 ml/min. The analysis was performed at 25 °C with a wavelength of 208 nm. Identification was confirmed through spiking with a squalene standard solution and was quantified using the standard squalene.
Antioxidant activity DPPH radical scavenging assay
The stable 1,1-diphenyl-2- picrylhydrazyl radical (DPPH) was used for determination of free radical-scavenging activity of the crude extracts. Different concentrations (50, 100, 250, 500, and 750 ppm) of palm oil samples were added at an equal volume (4 ml) to 10−4 M toluenic solution of DPPH (5.0 × 10−4 M). The mixture was incubated at room temperature for 30 min in the dark and then the absorbance was read at 517 nm against blank. Radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula, % radical scavenging activity = {(Acontrol − Asample)/Acontrol} × 100. Where, Acontrol is the absorbance of the control without extract, and Asample is the absorbance of reaction mixture. The DPPH activity (IC50 value) was calculated using a plot of percent radical-scavenging activity against concentration (mg/ml) to determine the concentration of extract necessary to reduce DPPH by 50 % (Shimada et al. 1992).
Chocolate spread composition and formulation
Palm nutraceuticals enriched food spread was prepared according to El-Hadad et al. (2011) with some modification. The composition of food spread includes icing sugar (23 %), liquid glucose (23 g), fat (30 %), skimmed milk (15 %) and cocoa powder (9 g). The spread formulation was maintained without the aid of the food additives such as potassium sorbate (shelf-life enhancer), lecithin (emulsifier) and glycerol mono stearate (emulsifier). The sugar and milk was mixed and heated on electric heater at 100 °C. The fat phase consists of previously prepared blends of butterfat and phytonutrient retained red palm olein in varying combination, which was melted at 63 °C and had been kept aside. The cocoa powder was added into the fat phase and mixed at a low speed for 2 min using a hand blender. Then mixture containing sugar and milk was added and mixed low speed 1 for 2 min. The chocolate spreads were immediately transferred into glass jars.
Properties of chocolate spread
Emulsion stability
Emulsion stability of the spread was evaluated according to Hutton and Campbell (1977). The spread (50 g) was taken in centrifuge tubes and incubated in a water bath maintained at 30 °C for 30 min. The tubes were centrifuged at 3000 rpm for 30 min. The amount of water separated out was measured and calculated using the formula emulsion stability (ES) = {(ml of water in the spread − ml of water separated) × 100/ml of water in the spread} (Yasumatsu et al. 1972).
Hardness determination
Hardness of spread samples were measured at 23 °C using a TX-XT2 texture analyzer (Stable Microsystems Ltd., London, United Kingdom). A 45° conical probe penetrated into the sample at 1 mm/s to a depth of 5 mm from the sample surface and same was withdrawn at the same speed. During penetration, the force increased up until the point of maximum penetration depth. The penetration force (g) was reported as hardness. Triplicate readings for each sample were obtained.
Colour analysis
Blends of nutraceuticals retained palm fat and butterfat were characterised by evaluating the colour using a Hunter Lab Labscan XE spectrophotometer (Hunter Associates Laboratory Inc., Restone Virginia). The 15 ml of the sample were placed in a sample cup and was used for transmittance colour measurements in liquid media. The colour of samples was obtained by using a 2/°C (2° observer/illuminant C). The results are expressed as L*, a* and b* values.
Sensory evaluation
The sensory analyses of the food spread were evaluated by trained panellists. Parameters such as colour, taste, spreadability, texture, flavour, consistency and overall acceptance were evaluated. The samples were kept for 1 h at room temperature (25 °C) before the analysis. The samples were provided in white plastic cups along with bread slices. The 9 hedonic scales were used to categorise the sample. The scale 1 shows extremely dislike, and 9 shows extremely like on the hedonic scale (Kramer and Twigg 1973).
Fat and moisture content
Fat content and moisture content of the spread were evaluated according to AOAC methods (AOAC 2005).
Statistics
Data are expressed as the mean ± standard deviation of triplicate analyses. Analysis of variance (ANOVA) and least significant difference (LSD) tests were conducted to identify differences among means using SAS Statistical Program Computer Package version 6.12 (SAS, Cary, NC, USA). Statistical significance have been declared at p < 0.05.
Results and discussion
Physicochemical properties
Table 1 shows the physicochemical properties of butterfat, PRPOL and their blends. The results of the lightness, redness and yellow colour components of the native and blended samples showed that butterfat was brighter than PRPOL. Hence, the lightness of blends was reduced upon incorporation of PRPOL into blends. The red colour components were higher in PRPOL, which was contributed by the retention of carotenoids in PRPOL. The butterfat showed lower redness, and it was increased upon incorporation of PRPOL. The yellow colour component was higher in butterfat as compared to PRPOL. Hence, the blends showed increase in yellow components upon the increase in level of butterfat in blends. PRPOL can be used as natural colorant in different food products.
The slip melting point (SMP) of butterfat and PRPOL was 34 and 39 °C respectively. The measured melting points of blends were within 34.5–36.5 °C. SMP of the blends increased with the increase in the content of PRPOL in the blends. SMP of all blends was below 37 °C (Table 1). However, 9:1, 8:2, 7:3 and 6:4 blends were the suitable substrate ratio due to their desirable melting range (32–36 °C). Shin et al. (2009) have reported that the fat with melting ranges between 32 and 38 °C was suitable for the application in spreadable butter based fat of margarine.
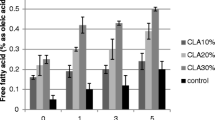
FFA of native oils and blends are presented in Table 1. FFA of PRPOL showed slightly higher FFA than the butterfat. Blends showed the FFA content according to the relative composition of the native oils in the blends. Hence, the increase in FFA was observed in blends with increase in incorporation level of PRPOL. The blending of PRPOL with butterfat provided blends with lower FFA than PRPOL which indicates blends were more desirable than PRPOL in respect to FFA content. Prasanth Kumar et al. (2009) have showed the blending of lower FFA oil with higher FFA oil provides blends containing intermediate FFA
Peroxide value (PV) is one of the quality indices of oils and fats. The saturated fatty acid rich oils usually contain very less or no PV even during storage. The PV of butterfat indicates no peroxides and hence has good stability. In this study, PRPOL has low levels of PV as it mostly constitutes by the initial PV of the CRPOL, which was used for the preparation of PRPOL. However, the blending of PRPOL with butterfat caused a reduction in PV as compared to the PV of PRPOL. PV slightly increased with increase in PRPOL in blends. The increase in PV of the blends was according to the corresponding weight percentage of the PRPOL (Table 1). There was no effect on the PV of the blends during the blending process, and the PV in blends was dependent on the quality of the blending components.
The iodine value (IV) of native oils and its blends indicated the degree of unsaturation is presented in Table 1. The IV of butterfat and PRPOL showed the significant variation in the degree of unsaturation of the substrate. The blends showed an increase in IV with increase in incorporation of PRPOL in butterfat.
Saponification value (SV) indicates the average molecular weight of the oils and fats. The oils with short chain fatty acid show higher SV and directly proportional to the short chain fatty acids on the glycerol backbone. As compared to PRPOL, the butterfat contains higher SV due to the presence of comparatively high amounts of short and medium chain fatty acids in butterfat (Table 1). Hence, the SV of blends have shown a reduction in SV upon incorporation of PRPOL, and it was according to the corresponding weight ratios of blending components.
The unsaponifiable matters (USM) of oils include non-saponifiable compounds such as, tocopherols, tocotrienols, carotenoids and phytosterols. The higher value of USM always indicates the higher the phytonutrients. The PRPOL showed higher USM than the butterfat indicating presence of higher amounts of phytonutrients in it (Table 1). The level of USM in blends was significantly affected by the corresponding weight percentage of blending components. Even though, butterfat contains USM, it is produced from animal source. Therefore, the USM contains the detrimental compounds like cholesterol in foods. Hence, replacing butterfat with the use of PRPOL may enhance the level of USM as well as limit the presence of cholesterol in blends.
Glyceride (TAG, DAG and MAG) composition
The TAG was the major portion of the glycerides of native and blended samples which was highest (95.2 %) in butterfat and lowest (59.2 %) in PRPOL (Table 1). The result has showed that the level of TAG in blends gradually decreased with elevating PRPOL incorporation into butterfat. The partial glycerides include DAG and MAG which shows emulsification property in food products. Here, the level of DAG in butterfat and PRPOL were 3.7 and 36.2 % respectively while the MAG content was 3.7 and 4.1 % for butterfat and PRPOL respectively. Further, the content of DAG in blends was ranged from 7.0 to 20 % and that was contributed by the incorporation of 10-50 % of PRPOL into butterfat. The MAG content was found to be 1.2, 1.6, 1.9, 2.2, and 2.5 % for the blends of 90:10, 80:20, 70:30, 60:40, and 50:50 respectively. The elevated content of DAG and MAG in blends depended on the elevated incorporation of PRPOL to butterfat. These obtained contents of DAG and MAG in the blends may be sufficient to provide emulsification properties during the formulation of food products (e.g., food spreads). Hence, incorporation of PRPOL in butterfat will help to reduce the use of additional emulsifier such as, lecithin, glycerol monostearate, etc. and thereby it will help to reduce the cost of food formulation.
Fatty acid composition
Table 2 shows the fatty acid composition of PRPOL, butterfat and its blends. The butterfat showed a wide range of fatty acids from short chain fatty acids to long chain fatty acids including odd and even chain fatty acids. The contents of short and medium-chain fatty acids in butterfat decreased, and long-chain fatty acids increased upon increase in level of incorporation of PRPOL. The saturated long chain fatty acids including palmitic acid, and stearic acid showed variation in the content in blends upon the incorporation of PRPOL. Palmitic acid increased from 32.8 to 36.9 %, the stearic acid content reduced from 12.6 to 8.4 % with 50 % of replacement of butterfat with PRPOL. The oleic acid content increased in blends upon the incorporation of PRPOL that ranged from 26.8 to 33.4 % in blends. Similarly, the linoleic acid content was observed in the range of 2.5–6.6 %. The total fatty acids together constituted 67 % of saturated fatty acids (SFA) in butterfat. The PRPOL showed lower SFA content and higher monounsaturated and polyunsaturated fatty acids contents than butterfat. Hence, the incorporation of PRPOL reduced the content of SFA and increased the content of MUFA and PUFA in blends compared to butterfat.
Solid fat content (SFC)
SFC of butterfat, PRPOL and blends are presented in supplementary figure 1. The butterfat had shown a higher SFC than the PRPOL at 0–25 °C and similar SFC at 30 °C. PRPOL showed higher SFC than butterfat at 30–50 °C. SFC of blends showed the intermediate SFC content of butterfat and PRPOL. Hence, the addition of PRPOL to butterfat showed a decrease in SFC than butterfat. SFC of 40 and 50 % PRPOL incorporated blends showed slightly lower SFC than the PRPOL and butterfat. As per Timms (1985) the SFC of the fat blends may vary compared to the weighted average of corresponding components fats and sometimes it may be lower than the SFC of component fats. All the blends showed SFC almost close to butterfat at 25–40 °C. The slightly higher solid fat content of PRPOL above 25 °C may help to improve the hardness of the products. The SFC of less than 32 % at 10 °C are desirable for the spreadability of fats at refrigeration temperature (Lida and Ali 1998). Hence, the higher SFC (>32 %) of blends at 10 °C may be desirable to provide spreadability at higher than refrigeration temperature.
Thermal behaviours of the butterfat and PRPOL blends
Supplementary figure 2 shows the endothermic crystallization of butterfat and PRPOL blends. The results show that the blends were crystallized in two major endothermic steps. The onset temperature for the crystallization peak 1 was 17.8 °C, which varied upon incorporation of PRPOL. The 50:50 combination of the blend showed an onset temperature of 24.0 °C for the peak1. This data indicates the variation in the solid fat content of the blends. Hence, the onset temperature of the butterfat shifted from 17.8 to 24.0 °C upon incorporation of PRPOL. The incorporation of PRPOL has enhanced the solid fat content of the butterfat blends. Hence, crystallization process accelerated with increased incorporation of PRPOL. The onset temperature of peak1 of all the samples showed a positive linear correlation (r 2 = 0.99) with increased concentration with PRPOL. The onset temperature for the peak2 was 13.2 °C for butterfat, and it further reduced to 11.4 °C upon incorporation of PRPOL.
The melting profile of butterfat and PRPOL blends at various temperature ranges are presented in Supplementary figure 3. The melting of blends started at different levels. A distinct melting peak (peak1) was observed upon incorporation of PRPOL. The peak1 and peak2 were found within the melting region of 10–20 °C. Whereas, the peak3 was found after 20 °C with an onset temperature of around 20 °C at a broad temperature range 20 °C up to 36.9 °C. Furthermore, small peaks were observed beyond 37 °C which indicates higher melting point glycerides in the blends with the higher content of PRPOL. The melting thermogram of the blends indicates that the blends contain glycerides of lower melting to higher melting ranges.
Phytonutrient composition of butterfat, phytonutrients retained palm olein, and its blends
Carotenoids are the speciality of palm oil, and it imparts a red colour to the oil. Table 3 shows the carotenoid content of native and blended samples. The data shows that the butterfat has the lowest and PRPOL has the highest amounts of carotenoids content. Hence, the incorporation of PRPOL enhances the level of carotenoids in blends as compared to butterfat alone. The level of carotenoids has shown a positive correlation (r 2 = 0.91) with the red colour component (a*) of blends. PRPOL contributed red colour to the blends. Therefore, synthetic colouring agent can be replaced with the use of PRPOL in various food preparations.
Phytosterols are one of the considerable nutraceutical found in the unsaponifiable fractions of palm oil. Table 3 shows the individual phytosterol composition of native and blended fat samples. Kumar et al. (2010) have reported that the cholesterol content of butterfat, which is around 3190 mg/kg. The result shows that the sterol part of butterfat comprises only cholesterol (2550 mg/kg). Shin, Akoh, and Lee (2009) have reported that only cholesterol was present in butterfat and no other sterols. Verleyen et al. (2002) have reported that the major phytosterols present in palm oil are campesterol, sitosterol, and stigmasterol which exhibit beneficial effects on health. The beneficial phytosterols (other than cholesterol) content increased with increased level of RPOL in blends. Phytosterols of blends showed β-sitosterol + campesterol was highest among other sterols. Stigmasterol content shows that it increased to 0.7–10.0 folds in blends as compared to butterfat. The overall composition of sterols of blends revealed that there is a considerable decrease in cholesterol content and increase in amounts of other phytosterols in the blends upon incorporation of PRPOL.
The presence of tocopherols and tocotrienols provide antioxidant, hypocholesterolemic, anti-thrombotic, anti-atherogenic and anti-inflammatory properties to foods (Lewis 2001). The tocopherol content in butterfat was 2.8 mg/kg (Table 3) which is in agreement with the earlier report (Shin et al. 2009). The tocopherol composition of PRPOL revealedthat it was rich in tocopherols (129 mg/kg) and tocotrienols (836 mg/kg). Tocotrienols are the powerful natural antioxidants than tocopherols and the availability of tocotrienols in vegetable oils are scarce except some of the edible oils such as palm oil, rice bran oils and oils from bran sources (wheat bran), etc. The α-tocotrienol, γ-tocotrienol and δ-tocotrienol together constituted the tocotrienol composition of PRPOL. Hence, incorporation of PRPOL into butterfat enhanced the level of tocotrienols as compared to butterfat. The results showedthat blending of butterfat with the PRPOL is helpful to incorporate significant amounts of tocopherols and tocotrienols in blends and the use of these blends may provide a health beneficial effect upon consumption.
Squalene present in the palm oil being removed during steam refining and its level in the palm fatty acid distillate (PFAD) has been reported (Gapor et al. 2002). In this study, evaluated the content of squalene in butterfat (31 mg/kg), PRPOL (315 mg/kg) and blends (53–187 mg/kg) (Table 3). The incorporation of PRPOL to butterfat has shown an increase in the squalene content of the blends with higher levels of PRPOL in blends. The squalene protects the skin from detrimental environmental effect and possesses antioxidant activity, blood cholesterol lowering activity, anticancer and antitumor activities (Nandi et al. 2007; Loganathan et al. 2008; Newmark 1997). Hence, the use of blends with the higher levels of PRPOL may provide better health benefits of squalene to consumers.
DPPH radical scavenging activity
The DPPH radical was used to evaluate the free radical scavenging properties of the native and blended samples. The DPPH radical scavenging activity (RSA) analyzed and expressed as IC50 values. The samples with the lower IC50 values indicate stronger radical scavenging activity. PRPOL, after 30 min of reaction with DPPH, exhibited greater radical scavenging activity (IC50 = 21.8 mg/ml) than the butterfat (76.0 mg/ml). The blends showed the RSA in the descending order was 10 % PRPOL + 90 % butterfat > 20 % PRPOL + 80 % butterfat > 30 % PRPOL + 70 % butterfat > 40 % PRPOL + 60 % butterfat > 50 % PRPOL + 50 % butterfat with the IC50 values of 48.9, 54.3, 59.2, 65.2, and 70.6 mg/ml respectively (Table 3). The variation in the radical scavenging activity among the blends indicates that the incorporation of phytonutrients and the enhanced RSA depends on the elevated levels of PRPOL into the blends. The results show that PRPOL has stronger RSA than butterfat. Hence, incorporation of PRPOL to butterfat and its use as food spread may enhance free radicals quenching efficiency of the spreads.
Characteristics of Nutra-spread
The properties of phytonutrients incorporated chocolate spread are shown in Table 4. The results show that there is not much difference in the colour lightness, redness and yellow colour components of spreads (Table 4). The butterfat spread showed slightly higher brightness than the spreads containing the PRPOL. The low brightness value of the spreads indicates that the colour features of the spread as dark due to the ingredient used, cocoa powder, which is dark in colour. The red and yellow colour components showed a slight increase with the increased content of PRPOL in spreads. But, the results showed that the redness colour components of the spreads did not show high correlation (r 2 = 0.62) with the carotenoids contents (Tables 3, 4). The content of carotenoids was correlated well with the red colour components of the blends. The darkness of the chocolate spreads might be preventing the reflectance of the red and yellow colour in the spreads. Hence, all the spreads showed almost similar colour pattern.
Fat and Moisture content
Fat is one of the major ingredients, which provides phytonutrients to the spreads. The fat content was similar in all the spreads and did not show any significant differences (Table 4). The fat content of the spread was almost similar to the corresponding level of fat used in the spread formulation.
The aqueous phase in the spread formulation constituted the moisture content of the spread. Similarly, the skimmed milk contributed moisture content in the spreads. As compared to the amount of skimmed milk in the spread formulation, the obtained moisture content was slightly higher and the moisture content may also be contributed by the use of liquid glucose as an ingredient, which contains some percentage of moisture in it.
Colour characteristics
The prepared spreads were dark in colour due to the use of cocoa powder in the formulation. Hence, all the spreads showed a lower brightness having almost similar units of colour for red and yellow colour components and the use of blends with different levels PRPOL did not make any difference in the red and yellow colour components of the spreads and the values obtained were comparatively low (Table 4). Hence, the ratio of red to yellow colour components have shown near to 1 indicating that there is no variation in colour. The colour of the spreads was dark. Therefore, the red and yellow colour components of the spreads were not significant.
Emulsion stability and hardness
The emulsion stability of all the spreads was evaluated after 15 days of storage at room temperature and the emulsion stability for all the spreads were found to be 1 (Table 4). The results showed that all the blends have excellent emulsion properties. Hence, chocolate spreads with excellent emulsion stability were achieved without the aid of other additional emulsifying agents in the spread formulation.
The hardness1 for all the spread samples has showed slight increase in trends with the increased incorporation of PRPOL (Table 4). The higher hardness may be due to the higher solid fat content at the temperature above 30 °C by PRPOL. The hardness2 was comparatively low as compared to hardness1 and then also the hardness was increased according to the increased level of PRPOL.
Sensory properties
The organoleptic properties of chocolate spreads are presented in the Fig. 1. The properties such as colour, spreadability, texture, flavour, taste and overall acceptability of all the samples showed a good score. These parameters remain similar, even after the use of blends with different levels of PRPOL. The other properties include flavour and taste has shown decreases in scores with the increased level of PRPOL. The decrease in score may be due to the strong raw flavour of PRPOL, which could not be masked by the use of cocoa powder. The taste of the spread was also dependent on the level of PRPOL and 20 % level showed a taste similar to that of butterfat and indicated that the 20 % of PRPOL was ideal for the replacement of butterfat and also to enhance the phytonutrient status of the blend. These results agreed with the results reported by El-Hadad et al. (2011). A mild deodorization process to PRPOL may be helpful to improve the organoleptic properties. The variation in the flavour and taste affected the overall acceptability of the spreads. As per the acceptability scores the spreads formulated with 20 % incorporation of PRPOL into butterfat was suitable for delivering all its health improving properties.
Conclusion
PRPOL and butterfat blends were evaluated for physicochemical and phytonutrients characteristics. The blends showed variation in physicochemical characteristics upon incorporation of PRPOL into butterfat. The phytonutrients viz tocopherols, tocotrienols, phytosterols, carotenoids, and squalene increased, and cholesterol content was decreased along with better radical scavenging activity upon the incorporation of PRPOL into butterfat. The prepared chocolate spreads showed better emulsion stability and many physicochemical characteristics. The sensory profile showed up to 20 % replacement of PRPOL was ideal for formulating chocolate spread, and its level can be increased by applying mild deodorization process to reduce its raw flavor. The higher level of phytonutrients of PRPOL incorporated chocolate spread as compared to butterfat indicates that it can be used as functional chocolate spreads with improved health benefits.
References
Abigor DR, Opute FI, Opoku AR, Osagie AU (1985) Partial purification and some properties of the lipase present in oil palm (Elaeisguineensis) mesocarp. J Sci Food Agri 36(7):599–606
Al-Saqer JM, Sidhu JS, Al-Hooti SN, Al-Amiri HA, Al-Othman A, Al-Haji L, Ahmed N, Mansour IB, Minal J (2004) Developing functional foods using red palm olein. IV. Tocopherols and tocotrienols. Food Chem 85(4):579–583
AOAC (2005) Official methods of analysis, AOAC international, 18th edn, Revised 2007, Toluene distillation method (Moisture in animal feed) 925.04, Roese-Gotlieb Method (Fat in cream) 920.111, Soxhlet method (Fat in cocoa products) 963.15
AOCS (2003) Official methods and recommended practices of the American Oils Chemists’ Society, 5th edn. American Oils Chemists’ Society (Part 1, A-C), Champaign
Butt MS, Rasool J, Sharif K (2006) Note. Preparation and characterisation of cake rusks by using red palm oil fortified shortening. Food Sci Technol Int 12(1):85–90
Chandrasekaram K, Ng MH, Choo YM, Chuah CH (2009) Effect of storage temperature on the stability of phytonutrients in palm concentrates. Am J Appl Sci 6:529–533
Danthine S, Lefébure E, Trinh HN, Blecker C (2014) Effect of palm oil enzymatic interesterification on physicochemical and structural properties of mixed fat blends. JAOCS 91(9):1477–1487
El-Aziz MA, Mahran GA, Asker AA, Sayed AF, El-Hadad SS (2013) Blending of butter oil with refined palm oil: impact on physicochemical properties and oxidative stability. Int J Dairy Sci 8(2):36–47
El-Hadad NN, Youssef MM, El-Aal MHA, Abou-Gharbia HH (2011) Utilisation of red palm olein in formulating functional chocolate spread. Food Chem 124(1):285–290
Gapor MT, Mohamad S, Rosnah MS, Hazrina AR (2002) Process for recovery of squalene from palm oil products. MPOB Information Series, MPOB
Goh SH, Choo YM, Ong SH (1985) Minor constituents of palm oil. JAOCS 62(2):237–240
Hutton CW, Campbell AM (1977) Functional properties of a soy concentrate and a soy isolate in simple systems. J Food Sci 42(2):454–456
Kamat JP, Sarma HD, Devasagayam TPA, Nesaretnam K, Basiron Y (1997) Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol cellular Biochem 170:131–138
Kramer A, Twigg BA (1973) Quality control for the food industry, vol 2—Applications (No. Ed. 3). The AVI Pub. Comp. Inc., Westpoint
Kumar M, Sharma V, Lal D, Kumar A, Seth R (2010) A comparison of the physico-chemical properties of low-cholesterol ghee with standard ghee from cow and buffalo creams. Int J Dairy Technol 63(2):252–255
Lau HLN, Choo YM, Ma AN, Chuah CH (2007) Production of refined carotene-rich palm oil from palm mesocarp (Elaeisguineensis) using supercritical carbon dioxide. J Food Lipids 14(4):396–410
Lee KT, Foglia TA (2000) Synthesis, purification, and characterization of structured lipids produced from chicken fat. J Amer Oil Chem Society 77(10):1027-1034
Lewis J (2001) Process for the production of tocotrienol. US Patent, 6(838,104)
Lida HMDN, Ali ARM (1998) Physico-chemical characteristics of palm-based oil blends for the production of reduced fat spreads. JAOCS 75(11):1625–1631
Loganathan R, Selvaduray KR, Radhakrishnan A, Nesaretnam K (2008) Palm oil: rich in health promoting phytonutrients. Palm Oil Dev 50:16–26
Manorama R, Brahmam GNV, Rukmini C (1996) Red palm oil as a source of β-carotene for combating vitamin A deficiency. Plant Foods Hum Nutr 49:75–82
Marangoni AG, Rousseau D (1998) Chemical and enzymatic modification of butterfat and butterfat-canola oil blends. Food Res Int 31(8):595–599
Mayamol PN, Balachandran C, Samuel T, Sundaresan A, Arumughan C (2007) Process technology for the production of micronutrient rich red palm olein. JAOCS 84(6):587–596
Nagendran B, Unnithan UR, Choo YM, Sundram K (2000) Characteristics of red palm oil, a carotene-and vitamin E–rich refined oil for food uses. Food Nutr Bull 21(2):189–194
Nandi S, Gangopadhyay S, Ghosh S (2007) Lipase catalyzed synthesis of neutral glycerides rich in micronutrients from rice bran oil fatty acid distillate. J Oleo Scie 57(11):599–603
National Nutrition Monitoring Bureau (NNMB) (1988–90) Indian Council of Medical Research, Repeat survey
Nenadis N, Tsimidou M (2002) Determination of squalene in olive oil using fractional crystallization for sample preparation. JAOCS 79:257–259
Newmark HL (1997) Squalene, olive oil, and cancer risk: a review and hypothesis. Cancer Epidemiol Biomark Prev 6(12):1101–1103
Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ (1993) Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem 268:11230–11238
Posada LR, Shi J, Kakuda Y, Xue SJ (2007) Extraction of tocotrienols from palm fatty acid distillates using molecular distillation. Sep Purif Technol 57(2):220–229
Prasanth Kumar PK, Gopala Krishna AG (2014a) Impact of different deacidification methods on quality characteristics and composition of olein and stearin in crude red palm oil. J Oleo Sci 63:1209–1221
Prasanth Kumar PK, Gopala Krishna AG (2014b) Physico-chemical characteristics and nutraceutical distribution of crude palm oil and its fractions. Grasas Aceites 65:e018
Prasanth Kumar PK, Bhatnagar AS, Hemavathy J, Gopala Krishna AG (2009) Changes in physico-chemical characteristics of some vegetable oils upon blending with coconut oil. J Lipid Sci Technol 41(4):136–142
Prasanth Kumar PK, Jeyarani T, Asha MR, Maya Prakash, Gopala Krishna AG (2013) A process for preparation of palm fat containing natural palm oil nutraceuticals and having emulsifier property useful for preparation of food spreads without any added emulsifier. Indian patent DEL 2056/13 dated 31-st August 2013
Roberfroid MB (2000) Defining functional foods. Funct Foods 9
Rodrigues JN, Gioielli LA (2003) Chemical interesterification of milkfat and milkfat-corn oil blends. Food Res Int 36(2):149–159
Rossi M, Gianazza M, Alamprese C, Stanga F (2001) The effect of bleaching and physical refining on colour and minor components of palm oil. JAOCS 78:1051–1055
Rousseau D, Forestière K, Hill AR, Marangoni AG (1996) Restructing butterfat through blending and chemical interesterification. 1. Melting component and triacylglycerol modifications. JAOCS 73:963–972
Rukmini C (1994) Red palm oil to combat vitamin A deficiency in developing countries. Food Nutr Bull United Nations Univ 15:126
Sánchez-Machado DI, Lopez-Hernandez J, Paseiro-Losada P, Lopez-Cervantes J (2004) HPLC method for the quantification of sterols in edible seaweeds. Biomed Chromatogr 18(3):183–190
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Shin JA, Akoh CC, Lee KT (2009) Production and physicochemical properties of functional-butterfat through enzymatic interesterification in a continuous reactor. J Agric Food Chem 57(3):888–900
Sundram K, Sambanthamurthi R, Tan YA (2003) Palm fruit chemistry and nutrition. Asia Pacific J Clin Nutr 12(3):355–362
SurendraNath B, Usha MA, Rama Murthy MK (1996) Effect of deep-frying on cholesterol oxidation in ghee. J Food Sci Technol 33(5):425–426
Suzuki YJ, Tsuchiya M, Wassall SR (1993) Structural and dynamic membrane properties of a-tocopherol and a-tocotrienol: implications to the molecular mechanism of their antioxidant potency. Biochemistry 32:10692–10699
Tan CH, Ghazali HM, Kuntom A, Tan CP, Ariffin AA (2009) Extraction and physicochemical properties of low free fatty acid crude palm oil. Food Chem 113(2):645–650
Timms RE (1985) Physical properties of oils and mixtures of oils. JAOCS 62(2):241–249
Verleyen T, Sosinska U, Ioannidou S, Verhé R, Dewettinck K, Huyghebaert A, De Greyt W (2002) Influence of the vegetable oil refining process on free and esterified sterols. JAOCS 79(10):947–953
Wrick K, Friedman LJ, Brewda JK, Caroll JJ (1993) Consumer viewpoint on designer foods. Food Technol 47:94–104
Yasumatsu K, Sawada K, Moritaka S, Misaki M, Toda J, Wada T, Ishii K (1972) Whipping and emulsifying properties of soybean products. Agric Biol Chem 36(5):719–727
Acknowledgements
The authors are thankful to Prof. Ram Rajasekharan, Director CSIR-CFTRI for providing infrastructural facilities. First author is grateful to CSIR, New Delhi for the award of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prasanth Kumar, P.K., Jeyarani, T. & Gopala Krishna, A.G. Physicochemical characteristics of phytonutrient retained red palm olein and butter-fat blends and its utilization for formulating chocolate spread. J Food Sci Technol 53, 3060–3072 (2016). https://doi.org/10.1007/s13197-016-2279-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2279-8