Abstract
The availability of fruit like litchi has been limited by variability in yield, alternate bearing, seasonal differences and most commonly post harvest problems. The litchi fruit has a very short shelf-life during which red color turns brown which greatly affects the appeal to consumer although not the unique flavor. This review article focuses on the post harvest problems especially browning of litchi. The pericarp of litchi is also sensitive to desiccation and turns brown and brittle once moisture is reduced to half. A large number of approaches have been tried to solve this problem starting from hydro-cooling to gamma irradiation but single approach could not suffice for all. In modern era, the logical base of controlling browning is either to control the responsible enzyme or remove the undesirable product of enzyme catalyzed reaction. Thus enzyme technology with good postharvest practice can definitely solve this problem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litchi (Litchi chinensis Sonn.) belongs to the Sapindaceae family, and is native to subtropical areas of southern China. The various cultivars of litchi may vary widely not only in texture, size, shape, color due to cultivar differences but also in biochemical composition and their levels (Tables 1 and 2). Due to its specific climactic requirements, its production and varietal specification is confined to specific region (Table 3). In India, 497 mT of litchi is produced annually from 78,000 ha.
The litchi fruits are harvested from the tree through mechanical pluckers. The shape of fruit is round to oval having thin and leathery pericarp. The main characteristic of this fruit is its bright red color but intensity of color also depends upon cultivar and seasonal variations. Like wise, white aril has unique flavour and variations depends upon the cultivars. Trees with small seeded fruits or seedless fruits are prized because of the greater portion of the pulp.
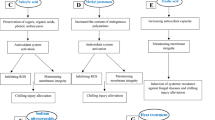
With a narrow genetic base, under given climactic conditions, fruits are available only for a very short period. On the other hand, post-harvest problems of fruits are one of the most pressing problems in the tropical countries like India. Each stage from harvest handling to public distribution has severe impact on quality (sensory/visual) and saleable quantity. Litchi fruit is highly perishable with a shelf life of 2–3 days at ambient temperature. Predicted post-harvest loss of this fruit may rise up to 50 % prior to its consumption (Jiang and Jiarui 1998). Pericarp browning, desiccation, chilling injury, micro-cracking, and post-harvest decay has been identified as the major constraints on expansion of litchi based farming or industries. Since appearance of fruit is deciding factor in consumer acceptance, the visual browning is of major concern. The pericarp browning initially occurs on protuberances and then extends over the entire surface of pericarp but mainly in epicarp and upper layers of mesocarp. Mesocarp cells are first ones to turn brown followed by epicarp and endocarp. Fruit moisture loss during storage, holding or transport typically reduces visual appeal, marketable/saleable value and sensory qualities also.
Being a non-climacteric fruit Litchi does not continue to ripen after harvest and is insensitive to exogenous ethylene. This fruit has to be harvested after attaining full maturity on tree. The color of the fruit is an important criterion to decide the harvesting stage. For distant market fruits are harvested when TSS (total soluble solids) attains 19° Brix and acidity 0.3 to 0.4 % (Chen et al. 2001). Fruits are collected in such a manner that they are not damaged from any sides because, if bruishes takes place, endogenous ethylene and carbon dioxide is liberated from injured site of pericarp and damaged tissues, which may affect the physiological and pathological state of neighbouring fruit clusters through initiating cascade of events leading to deteriorating enzyme activation. Damaged fruit can also encourage the establishment of pathogens which may spread to sound fruit, potentially causing severe losses. Generally, moisture loss from the damaged site may also increase by up to 30 % which also appears to substantially increase ethylene synthesis. Ethylene generated from the damaged site under biotic stress stimulates senescent processes and accelerates deterioration, causing increased respiration and loss of cell compartmentalization ultimately leading to unwanted browning reactions (Holcroft and Mitcham 1996).
It is believed that cell membranes are the primary sites of storage disorders but a complete biochemical pathway to elucidate the membrane injury mechanism has not yet been established. Membrane deterioration reduces its ability to act as a diffusion barrier, causing cell contents to leak, which translates into higher rates of electrolyte leakage. This makes electrolyte leakage assessments useful in quantifying cell damage.
Membrane injury under desiccation or chilling results in increased rates of ion leakage through micro-cracks, and the development of brown lesions or appearance of dark brown watery patches that increased fruit susceptibility to decay. Changes in membrane integrity is the primary effect that results when plant tissue such as fruit is exposed to stressful environments such as fluctuating temperatures or ethylene (Paliyath et al. 2008).
Pericarp browning
Litchi fruit has very short life of 2 to 3 days at ambient temperature. Pericarp browning of litchi is rapid and a major post-harvest problem, produce decay starts from third day of storage at >20 °C. Browning caused by temperature stress, decay and senescence is evident as typical dark and water-soaked areas on the pericarp whereas browning due to desiccation is differentiated by a pale-dry appearance of the pericarp. The minimum temperature recommendation varies depending upon the length of storage, pre-harvest sprays and post-harvest processing.
Pericarp pH is important in browning as reduced pH results in improved red colouration. Underhill and Critchley (1995) concluded that desiccation or moisture loss from pericarp tends to increase its pH (from 4.15 to 4.52 over 48 h period at 25 °C and 60 % RH) and suggested that at pH (>4) anthocyanin is converted to a colourless form and stimulates PPO activity which leads to independent browning in the epicarp to become more visible.
Water loss or dehydration causes rapid loss of membrane integrity which leads to interactions of substrate with various enzymes such as peroxidase (POD), lipoxygenase (LOX), polyphenol oxidase (PPO), anthocyanase and phenylalanine ammonia lyase (PAL). The major browning takes place upon bringing the enzymes (e.g. PPO) in close contact with the proposed substrate (e.g. –epicatechin) to initiate browning reaction (Sun et al. 2006). It is worth mention that the substrates of PPO are not completely characterized. Peroxidase activity coupled with ascorbic acid oxidation also enhances anthocyanin degradation. On the other hand, non enzymatic browning involves hydrolytic reaction produce aglycone moiety or substituted chalcone. All these enzymatic reactions ultimately lead to the formation of polymeric brown pigments involving colored o-quinones as browning precursors (Wang et al. 2010).
Cyanidin-3-glucoside, the major anthocyanin representing 91.9 % of total anthocyanin, is present in vacuole and separated from the cytosolic PPO enzyme due to compartmentalization. The level of this anthocyanin tends to decline with membrane leakage during fruit storage (Zhang et al. 2000, 2005). Litchi anthocyanins are also not directly oxidized by PPO but are first hydrolyzed by anthocyanase to anthocyanidins which are more vulnerable to degradation. These are further degraded to produce substrates like catechol for PPO and/or peroxidase action (Jiang et al. 2004). Ruenroengklin et al. (2009) purified anthocyanins and PPO and studied their reactions in presence of catechol, gallic acid and (−) epicatechin to explain unususual observation that litchi (Litchi chinensis Sonn.) browning does not result directly from PPO or POD. A coupled enzymatic system of anthocyanase and PPO or POD is responsible for degradation based enzymatic browning. Liu et al. (2010) recently documented that litchi PPO directly oxidize (−) epicatechin and the oxidative products of (−)-epicatechin in turn catalyze litchi anthocyanin degradation and ultimately leads to browning. Such results have presented the complicative process of browning. Decreased content of epicatechins or anthocyanins and increased level of lipofuscin like substances may be responsible for higher browning index of litchi pericarp during extended storage (Yang et al. 2011).
An improved scheme was proposed by Reichel et al. (2012) for litchi pericarp browning: (1) PPO-mediated oxidation of abundant (−) epicatechin (1.4–2.0 g/hg), resulting in dark brown pigments and (2) micro-crack induced formation of light brown surface scurf, supposedly with additional involvement of POD. The distinction of actions taken by these two enzymes is yet very tough to elucidate.
Post-harvest biochemistry of fruit pericarp have thrown light on membrane associated signal cascades where the key enzyme is phospholipase D (PLD) and it was originally proposed to be important in phospholipid catabolism, initiating a lipolytic cascade in membrane deterioration during senescence and stress. Increase in PLD activity results in a drastic degradation of phospholipids and a distinct accumulation of polyamines. Recent studies in plants indicate that PLD action plays an important role in trans-membrane signalling and cellular regulation under abiotic stress like dehydration. Study on the catabolism of phospholipids initiated by PLD in fruit developmental processes showed that PLD expression and activities are intimately linked to fruit development, ripening and desiccation-browning.
Control of pericarp browning
The pericarp browning, chilling injury and decay associated with post-harvest can be controlled by a number of ways and has been attempted by the use of controlled atmosphere storage (CAS) for color, modified atmosphere packaging (MAP) for quality, sulphur-based treatments and other chemical treatments (Table 4).
Fumigation method
Commercially, sulfur fumigation is being used to address major post-harvest problems in litchi fruits. The initial drawback associated with fumigation was the bleaching effect of sulfur and this was overcome by dipping fruits in acidic solution which helped in the restoration of red color (Zauberman et al. 1991). This red color restoration was due to the stabilization of anthocyanins in flavylium chlorinated cationic form. Other undesirable effects of sulfur fumigation include altered lower pH and flavor of fruit along with more obvious sulfur content in aril. Mature fruits are likely to have more sulfur in aril due to micro-cracking and pericarp degradation. Build-up of SO2 residue in aril has been shown by time lapses from fumigation to harvesting and then afterwards at high temperature storage. This control has been threatened by consumer preferences and regulatory issues involved in food items with minimal pesticide residue. So, alternatives like dipping of fruits in metabisulfite at different concentrations with acid have been explored which increased anthocyanin content with decreased POD/PPO activities. The arils have no content of sulfur whereas pericarp have limited sulfur residue (Liang et al. 2012).
Cost effective conventional pre-treatments
Pre-cooling is the simplest way to protect the sensitive fruit from field heat and initiation of membrane damage. Different types of cooling methods like hydro and air cooling have been adopted by the farmers upon scientific advice. It has been proven that hydro-cooling is more effective than room cooling in decreasing the core temperature of the fruit. Alternatives like hot water sprays and steam have been explored at the farms for variable duration which not only helps in reducing the pH but also uniformly distributes the acids on the pericarp. Initially longer duration have been tried but the longer steaming process have affected the edible aril portion of fruit so this technology was commercially unsuccessful. Later on, Kaiser et al. (1995) exposed fruit to steam at 95 °C for 2 s to damage the cuticle and solubilize cellular and organelle membranes. Two seconds of steam followed by hydro-cooling and a 4 min dip at acidic pH and treatment with an anti-transpirant resulted in firm fruit with good taste and colour after 28 days storage at 1 °C. Olesen et al. (2004) after comparison with other studies (Lichter et al. 2000) suggested that hot water spray was equally effective as the hot water dip but a hot water spray was about half as effective as a hot benomyl dip. Taiwanese litchi cultivars were found more heat tolerant upon vapor heat treatment at 45 °C core temperature for 42 min maintained the quality of Tai So and Wai Chee litchi cultivars at 5 °C for 4 weeks, retaining the appearance and increasing disease control (Jacobi et al. 1993). Steam treatments were also tried in combination with anti-transpirant. It was hypothesized that complexing of anthocyanins at the pericarp may increase its stability and red color retention. Hydro-cooling reduced the browning percentage but resulted in a higher percentage of rot. Litchi fruit cv. McLean’s Red dipped in hot water at 55 °C for 2 min, packed in bi-axially oriented polypropylene (BOPP) showed an increase in CO2 composition around the fruit with a decrease in weight loss and fruit firmness. The loss in fruit firmness was associated with hot water treatment (Sivakumar and Korsten 2006b). Similarly, hot water dipping combined with acid dips have substantially protected the red color of the fruit during storage at ambient temperature (Fang et al. 2013). On the other hand, hydro-cooling of 30 min reduced the temperature of pericarp by 6 °C. It has also delayed the increase in electrolyte leakage and polyphenol oxidase/peroxidase activity in pericarp (Liang et al. 2013). The air currents could be more effective in controlling pericarp browning in litchi. The tolerance to high or low air temperature depends on pericarp thickness, wax deposits and cuticular layer (Bryant 2012).
Acid treatment and pH maintenance
Red pericarp colour can be temporarily restored in desiccated brown fruit by the application of acid dips (Underhill and Critchley 1995). Duvenhage (1993) compared treatment of 8 % HCl alone against combinations of acid and sulphur treatments and found that the acid dip showed some success but caused some browning and decline in flavour in certain cultivars. A dip in calcium nitrate at 1 % for 5 min followed by 4 % HCl for 3 min gave results comparable with sulphur and acid treatments. Zheng and Tian (2006) suggested that application of oxalic acid (1–4 mM) can effectively control the pericarp browning of litchi fruit during post-harvest storage. Low pH treatment caused more peel injury. Combinatorial treatment of fumigation and low pH could facilitate an increased diffusion of sulfur from pericarp to aril consequently causing more health hazard (Lemmer and Kruger 2000). Likewise, EDTA and HR (hexyl-resorcinol) in BOPP packaging were found effective in controlling browning but orange pink dominated at place of pinkish-red color of pericarp (Sivakumar and Korsten 2006a, b). Dipping in hot water (98 °C) for 30 s, followed by treatment with minimal 10 % concentration of oxalic acid had enhanced the effectiveness of browning inhibition and resulted in the retention of pericarp redness in cv. Hong Huay (Saengnil et al. 2006) but with brown edible aril (Lichter et al. 2000).
Antioxidants treatment
The various mechanisms of litchi pericarp browning are mainly attributed to the oxidation process of phenolics, the degradation of anthocyanins by the enzymes PPO or POD or anthocyanase. Some chemical treatments which inhibit their enzymatic activities have been shown promising in reducing desiccation browning. The PPO activity is inhibited by antioxidants such as ascorbic acid or isoascorbic and L-cysteine or N-acetyl cysteine (Liu et al. 2006). A 5 min post-harvest dip in citric acid (100 mM) and glutathione (10 mM) reduced PPO activity by 80 % compared to the control, and significantly reduced browning (Jiang and Fu 1998). In solution, it was shown that the degradation of anthocyanin by PPO occurs only after all ascorbic acid is consumed (Jiang et al. 2004). Exogenous application of anthocyanins can prevent enzymatic browning of pericarp through their action as iron chelation and electron donating capacity (Duan et al. 2007). The initial levels of ascorbic acid in pericarp would therefore be expected to influence the development of browning. The intrinsic anti-oxidative potential of pericarp is also necessary for prevention of browning and loss of disease resistance. Yi et al. (2010) showed the antagonistic effect of vacuum infiltrated ATP (adenosine triphosphate) and DNP (di-nitro phenol) on free radical reducing power, scavenging capacity and phenolic content in Peronophythora litchii-inoculated fruits and, in non-inoculated non-infiltrated (without ATP) fruits the level of enzymatic and non-enzymatic system was poor. Pericarp browning was negatively correlated with phenolic content, antioxidant capacity and shelf life whereas it was positively correlated with weight loss and malondialdehyde content (Barman et al. 2014).
Controlled and modified atmosphere
The modified atmosphere packaging has the advantage of low cost and easy implementation. Apart from control of browning, this strategy also controls the disease incidence and cross-contamination during transport and storage. Litchi fruit cv. Huaizhi stored at 1 °C under controlled atmosphere (3–5 % CO2 and 3–5 % O2) at 90 % RH showed good browning control while retaining the fruit quality up to 30 days (Lin et al. 1988). The low density polyethylene (LDPE) of 37.5 μ thickness was used for storage of chemical treated litchi which had shelf life of 21 days under low temperature (Semeerbabu et al. 2007), Whereas, Pesis et al. (2002) found that litchi packed in micro-perforated had less decay but poorer taste than sealed packaged fruit. Duan et al. (2004) suggested that litchi cv. Huaizhi stored in pure O2 (100 % O2 and 0 % CO2) for 6 days at 28 °C showed significantly reduced pericarp browning. It was evident from their investigations that the fruit kept in pure O2 maintained high levels of ATP, ADP (adenosine diphosphate) and energy charge and this may contribute to maintenance of membrane integrity which reduced decompartmentation of enzymes and substrates. The pure O2 (100 %) also inhibited the activities of PPO and anthocyanase which are involved in enzymatic browning mechanism. Therefore, pure O2 atmosphere helps to prevent the degradation of anthocyanin by preventing hydrolysis of sugar moieties from anthocyanin to anthocyanidin and the degradation of anthocyanidin by PPO to brown polymers. Temperature intensity and its duration also have a profound influence on energy charge. Fruit stored for 30 days at 3–5 °C had much lower ATP content and adenylate energy charge (AEC) level than fruit stored for 10 and 20 days (Liu et al. 2011). Application of high O2 storage (70 % O2 + 0 % CO2) for 1 week followed by 5 % O2 + 5 % CO2 storage at 3 °C and 95 % RH for 14, 24 and 48 days showed significant reduction of decay while browning increased after 14 days in cv. Heiye. According to Tian et al. (2005) the anthocyanidin contents in the pericarp were observed to decrease slowly when compared to control, The ethanol content responsible for the off-flavors were reduced when the fruits were exposed to 70 % O2 for 1 week followed by 5 % O2 + 5 % CO2 at 5 °C. Control atmospheric (CA) conditions were also more effective in reducing total phenol content and decreasing fruit decay in comparison with MAP treatment. Litchi fruit stored for 10 days at 3–5 °C followed by the shelf-time of 12 h at 25 °C had lower activities of lipase, PLD and LOX, and also lower levels of membrane permeability, than with fruit stored at the same conditions for 20 and 30 days (Liu et al. 2011).
Active and passive packaging
Packaging combined with refrigeration can reduce moisture loss by maintaining a high level of RH at the fruit surface and avoiding exposure to air currents. Active packaging involves a water barrier covering a paper capillary network, with an internal layer of fabric permeable to water vapour but not free water. Somboonkaew and Terry (2010, 2011) chemically treated Litchi fruit cv. Mauritius and then packed in laminated polyethylene bags perforations (micro) showed decreased weight loss, higher anthocyanins with evolution of CO2 and ethylene but less organic acids. PropaFresh™ PFAM (polypropylene film integrated with anti-mist) packages significantly reduced the sugar transformation for maintenance of its levels and volatiles like acetaldehyde and ethanol are produced. BOPP-3 had the best potential to control decay, retain the colour and the overall litchi fruit quality during a marketing chain of 20 days (Sivakumar and Korsten 2006a). Shrink wrap packaging is in constant contact with the fruit, eliminating the difference in temperature between fruit and packaging whereas the modified atmosphere packages made from BOPP and PVC were used for enhancing shelf-life of litchi fruits for 9 days at 20 °C. The three factor interactions of packaging, temperature and time were found to enhance fruit quality and shelf-life (Mangaraj et al. 2012).
Ethylene biosynthesis associated chemicals
During storage of litchi, level of ethylene from wounded sites increased whereas polyamine content decreased with increased browning of pericarp. The more promising effect of 1-MCP (300 nL L−1) on ‘McLean’s Red’ than ‘Mauritius’. 1-MCP (300 nL L−1) significantly reduced the PPO and POD activities, retained membrane integrity, anthocyanin content and prevented the decline of pericarp colour values (Reuck et al. 2009). Sivakumar and Korsten (2010) investigated that the 1-MCP pre-treatment with controlled atmosphere (3 % O2 and 7 % CO2) at 2 °C for 21 days were more effective in preventing pericarp browning along with limiting oxidation enzymes activity and maintaining anthocyanin content. However, the effectiveness of this treatment has to be tested with different cultivars, late seasonal fruit, and the time delay between harvesting and packing operations. Postharvest treatments of polyamines for their effect on browning and found that spermine was the most effective of those tested in reducing browning. Polyamines not only inhibit ethylene production but also stabilize membrane system and provide protection against peroxidation. It was reported that polyamines at 1 mM concentration can increase the shelf-life under refrigeration. These chemicals were also used in combination with fungicide to control post harvest decay. Despite some success, the difficulties involved in registering chemicals for use on minor crops may limit the adoption of these types of treatments (Jiang and Chen 1995).
Food grade coatings
Edible coating reduces the moisture transfer, oxidation, metabolic processes and create modified atmosphere between fruit (endogenous) and storage atmosphere. Edible coatings also diminishes the physical/mechanical impacts and microbial growth to some extent and maintain the sensory quality, ultimately prolonging their shelf-life. The respiration rate, sarcocarp temperature, the activity of PPO and weight loss of litchi with chitosan coating was lower than the uncoated litchi. The storage time of coated litchi was 5 days longer than the uncoated. The coating material of chitosan prepared in acetic acid forms double-sides film on litchi’s pericarp; one was more uniform and closely packed like a barrier, the other was rougher and better transport (Lin et al. 2011). Several coatings have shown some degree of success in controlling desiccation browning. A 0.1 % thiabendazole dip followed by the application of a 1 % chitosan coating delayed the peak in PPO activity and reduced browning in litchi (Zhang and Quantick 1997). Ducamp-Collin et al. (2008) combined chemical and surface coating treatment on litchi cultivars and significant change in PPO/POD activities with respect to cultivar, surface coating and in combination with applied chemical was observed. The optimal performance of chitosan mixed with acids may be explained by stable acidification of the epicarp during soaking and to minimize the fruit’s buffering capacity. Humidifying fruit prior to chitosan treatment favoured acid (pH 0.8) impregnation at a given storage temperature (Caro and Joas 2005). The peeled fruit have also been coated with chitosan to retard the weight loss and the decline in sensory quality (Dong et al. 2004). The surface coating (tradename : ProLong) coatings (sucrose esters at 1.5 or 2.5 %) also slightly delayed the peak in PPO activity and showed some success in reducing browning in litchi fruit stored at 4 °C (Zhang and Quantick 1997). Polysaccharide coatings has been evaluated and shown to delay browning to some extent but were not considered commercially viable. Acidification of peel and peel coating with polysaccharide coating (tradename : Semperfresh) can retard dehydration and discoloration (Joas et al. 2005; Kaewchana et al. 2006; Rattanapanone et al. 2007) by decreasing PPO activity and maintaining anthocyanins in colored form. Coatings used commercially on other fruits have often proven unsuitable for litchi, either due to cracking of the wax (Underhill and Simons 1993) or an alkaline pH (Tongdee et al. 1998). Despite some success, coatings on this fruit are not commonly used in commercial operations.
Irradiation control
It is well known that differential response of a produce to irradiation depends not only upon the dosage but also on its cultivar and storage. Generally, this strategy is well suited to short tem storage at low temperature. Ilangantileke et al. (1993) and Kumar et al. (2012) documented that irradiation up to 0.5–1.0 KGy dose in combination with low temperature storage or dips treatment allowed significant retention of anthocyanins like cyanidin-3-O-rutinoside/glucoside and maintained the market quality for a larger duration (30–45 days) by reducing loss of red color of the pericarp and eliminating microbial load. The effect was persistent for short term duration but it failed to retain the overall fruit quality and safety during long term cold storage (>16 days). Fruits packed in vitafilms were irradiated with higher irradiation dosage but the fruit quality tend to decrease after storage (Sivakumar and Korsten 2010). Due to psychological perception of consumer, health and environment hazards, and food safety regulatory issues, this methodology have been restricted in many countries.
Use of biotic agents
Application of antagonists to control post-harvest management is more likely to be efficacious than in the field, because the storage environment around the fruit can be managed more easily and chemical control of post-harvest problem produces many challenges. Martínez-Castellanos et al. (2011) sprayed Lactobacillus plantarum on ripe litchis and then stored at 10 °C with 75 % RH. This inoculation have not only maintained the red colour with maximum retention of total anthocyanins but also increased the phenolics in the rind portion. Among the various combination treatments of packaging and antimicrobial substances B. subtilis + BOPP-1 had the best potential to control decay, retain the colour and the overall litchi fruit quality during a marketing chain of 20 days (Sivakumar et al. 2008).
Enzyme based strategy
The benefits of improved colour through these methods may be outweighed by disadvantages such as reduced fruit quality, health issues and environmental concerns. Recently, there has been a surge of interest in using enzyme inhibitors as a biochemical control. Various specific and non-specific inhibitors have been employed and their effectiveness has been investigated in various fruits (Choi et al. 2005; Sun et al. 2011; Wang et al. 2011). The membrane integrity of litchi cultivar feizixiao broken down most seriously than other cultivars because of its increased PLD activity (Sun et al. 2012).
Phosholipase D has been suggested a key enzyme in mediating membrane deterioration. Phospholipase D and its catalyzed products initiate the oxylipin pathway and other cascade which signals the production of browning pigments. Production of phosphatidic acids is a biomarker for initiating membrane damage. Previous studies have shown that phosphatidic acid produced under stress can stimulate free radical production through the activation of NADPH oxidase (Li et al. 2009). An increase in sub-micromolar level of calcium activates the enzyme directly and/or promotes membrane association of PLD to interact with substrate available in the membrane. PLD stimulation by low pH in the cytosol has been reported to be linked with fruit ripening and senescence. The reduced ATPase activities of vacuolar and plasma membrane sites (H+-ATPase) are negatively regulated during stress or senescence so as to build up the level of calcium ions in cytosol and promotion of PLD activity. The overall membrane structure, its rigidification and tailoring of fatty acid substituents for providing substrates for PLD action may have profound effect on pericarp (Paliyath et al. 2008).
PLD is a membrane bound enzyme and belongs to the class of phospho-hydrolases. It is generally found in mitochondrial membranes, microsomal membranes and cytosol. In healthy unstressed fruits, this enzyme is found to a considerable level in the cytosol. As ripening starts, PLD molecules were observed in membrane network and after attainment of full maturity PLD molecules were found to be in the cell wall space. This may represent a stage where permeability is compromised and these enzyme molecules leaked out of the cell or possibly were transported by exocytosis. This enzyme has much broad substrate specificity based on acyl chains and can also hydrolyze variety of head groups. The cytosolic and microsomal PLD activities and their kinetic constants fluctuate with stages of fruit development. Thus, enzyme association–dissociation kinetics appears to be a major mode of enzyme regulation.
Since the activity of PLD appears to be the key step in regulating the sequence of enzyme reactions and the flow of metabolites through the catabolic pathway, this indirectly implies that controlling the PLD is critical for enhancing shelf-life and quality preservation of fruits and vegetables. Inhibition of its activity by various treatments can possibly check the degradation of phospholipids in the membrane resulting in the inhibition of browning reaction and improving the quality of fruit. The stage of application is critical since PLD inhibition has very little effect once the membrane deterioration is accelerated. The combinatorial approach of PLD inhibition and the use of antioxidants would not only reduce the phospholipid degradation as well as downstream oxidative processes. Addition of certain substances to limit the production of phosphatidic acids for example trans-phosphatidylation is the siphoning-off reaction for phosphatidic acids or inactivating PLD can be used a tool to control pericarp browning and membrane degradation (Paliyath et al. 2008).
Conclusion
The usage of sulfur fumigation to preserve the red color of litchi fruit has certainly many drawbacks. To rule out these drawbacks, numerous methods and protocols have been attempted and adopted. Each method or process protocol has certain limit of controlling the pericarp browning so a method must be devised to control the cause of pericarp browning. Additionally, such method should have minimal effect on taste and aroma also. Since this is an enzymatic browning so control must be on the responsible enzyme, PLD. The complexity of interwoven network of substrate channelling through other enzymes like PPO and POD are posing some challenges for critical elucidation of the role of PLD in storage life of fresh fruit and vegetables. High output research are already underway to dissect the biochemical mechanisms involved in pericarp browning but controlling pericarp browning, whatsoever means, would definitely promote litchi based farm industry.
References
Barman K, Siddiqui MW, Patel VB, Prasad M (2014) Nitric oxide reduces pericarp browning and preserves bioactive antioxidants in litchi. Sci Hortic 171:71–77
Bryant PH (2012) A model of postharvest moisture loss under air currents to reduce pericarp browning of litchi (Litchi chinensis Sonn.). Postharvest Biol Technol 73:8–13
Caro Y, Joas J (2005) Postharvest control of litchi pericarp browning (cv. Kwai Mi) by combined treatments of chitosan and organic acids II. Effect of the initial water content of pericarp. Postharvest Biology and Technology 38:137–144
Chen W, Wu Z, Ji Z, Su M (2001) Postharvest research and handling of litchi in China - A review. Acta Horticulturae Proceedings of the First International Symposium on Litchi and Longan, Guangzhou, China, 16–19 June 2000, 558: 321–329
Choi YJ, Tomas-Barberan FA, Saltveit ME (2005) Wound-induced phenolic accumulation and browning in lettuce (Lactuca sativa L.) leaf tissue is reduced by exposure to n-alcohols. Postharvest Biol Technol 37:47–55
Dong H, Cheng L, Tan J, Zheng K, Jiang Y (2004) Effects of chitosan coating on quality and shelf life of peeled litchi fruit. Journal of Food Engineering 64:355–358
Duan X, Jiang Y, Su X, Liu H, Li Y, Zhang Z, Zheng Y, Jiang W (2004) Role of pure oxygen treatment in browning of litchi fruit after harvest. Plant Sci 167:665–668
Duan X, Jiang Y, Su X, Zhang Z, Shi J (2007) Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem 101:1365–1371
Ducamp-Collin MN, Ramarson H, Lebrun M, Self G, Reynes M (2008) Effect of citric acid and chitosan on maintaining red colouration of litchi fruit pericarp. Postharvest Biology and Technology 49:241–246
Duvenhage JA (1993) Control of post-harvest decay and browning of litchi fruit by sodium metabisulphite and low pH dips. S.A. Litchi Growers’ Assoc. Yearbook 5:31–32
Fang F, Zhang Z, Zhang X, Wu Z, Yin H, Png X (2013) Reduction in activity/gene expression of anthocyanin degradation enzymes in lychee pericarp is responsible for the color protection of the fruit by heat and acid treatment. Journal of Integrative Agriculture 12(9):1694–1702
Gautam S, Mishra BB, Hajare SN, Kumar S, Saxena S, More VS, Wadhawan S, Bandyopadhyay N, Sharma A (2013) Process for retaining pericarp color and extending shelf life of litchi. BARC Newsletter Founder day special issue 245–250
Holcroft DM, Mitcham EJ (1996) Postharvest physiology and handling of litchi (Litchi chinensis Sonn.). Postharvest Biology and Technology 9:265–281
Ilangantileke SG, Noomhorn A, Upadhyay IP, Srinivas Rao M (1993) Effect of irradiation and storage temperature on the shelf life and quality of Thai litchi. In: Champ BR, Highley E, Johnson GI (Eds) Postharvest Handling of Tropical Fruits, ACIAR Conference Proceedings, Chang Mai, Thailand 50: 352–354
Jacobi KK, Wong LS, Janet EG (1993) Lychee (Litchi chinensis Sonn.) fruit quality following vapour heat treatment and cold storage. Postharvest Biol Technol 3:111–119
Jiang YM, Chen F (1995) A study on polyamine change and browning of fruit during cold storage of litchi (Litchi chinensis Sonn.). Postharvest Biology and Technology 5:245–250
Jiang Y, Fu J (1998) Inhibition of polyphenol oxidase and the browning control of litchi fruit by glutathione and citric acid. Food Chem 62(1):49–52
Jiang Y, Duan X, Joyce D, Zhang Z, Li J (2004) Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem 88:443–446
Joas J, Caro Y, Ducamp MN, Reynes M (2005) Postharvest control of pericarp browning of litchi fruit (Litchi chinensis Sonn cv Kwaiimi) by treatment with chitosan and organic acids I. Effect of pH and pericarp dehydration. Postharvest Biology and Technology 38:128–136
Kaewchana R, Techavuthiporn C, Kanlayanarat S (2006) Sucrose fatty acid coating retards pericarp browning of litchi cv Hong Huay. Acta Hort 712:579–583
Kaiser C, Levin J, Wolstenholme BN (1995) Vapour, heat and low pH dips improve litchi (Litchi chinensis Sonn.) pericarp colour retention. J SAfr Soc Hort Sci 4:6–12
Kumar S, Mishra BB, Saxena S, Bandyopadhyay N, More V, Wadhawan S, Hajare SN, Gautam S, Sharma A (2012) Inhibition of pericarp browning and shelf life extension of litchi by combination dip treatment and radiation processing. Food Chem 131:1223–1232
Lemmer D, Kruger FJ (2000) Factors influencing SO2- Residues on commercially fumigated HLH Mauritius and McLean’s Red litchi fruit. S.A. Litchi Growers’ Assoc. Yearbook 11:42–46
Li M, Hong Y, Wang X (2009) Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim Biophys Acta 1791:927–935
Liang YS, Chen NL, Ke LS (2012) Influence of dipping in sodium metabisulfite on pericarp browning of litchi cv. Yu Her Pau (Feizixiao). Post harvest Biology and Technology 68:72–77
Liang YS, Wongmetha O, Wu PS, Ke LS (2013) Influence of hydrocooling on browning and quality of litchi cultivar Feizixiao during storage. International Journal of Refrigeration 36:1173–1179
Lichter A, Dvir O, Rot I, Akerman M, Regev R, Wiesblum A, Fallik E, Zauberman G, Fuchs Y (2000) Hot water brushing : an alternative method to SO2 fumigation for color retention of litchi fruit. Post harvest Biology and Technology 18:235–244
Lin ZF, Li SS, Zhang DL, Liu SX, Li YB, Lin GZ, Chen MD (1988) The changes of oxidation and peroxidation in postharvest litchi fruit. Acta Botanica Sinica 30:383–387
Lin B, Du Y, Liang X, Wang X, Yang J (2011) Effect of chitosan coating on respiratory behavior and quality of stored litchi under ambient temperature. Journal of Food Engineering 102:94–99
Liu H, Shi J, Song L, Jiang Y, You Y (2006) Browning control and quality maintenance of litchi fruit treated with combination of N-acetyl cysteine and isoascorbic acid. Journal of Food Technology 4(2):147–151
Liu L, Cao S, Xu Y, Zhang M, Xiao G, Deng Q, Xie B (2010) Oxidation of (−)-epicatechin is a precursor of litchi pericarp enzymatic browning. Food Chem 118:508–511
Liu H, Song L, You Y, Li Y, Duan X, Jiang Y, Joyce DC, Ashraf M, Lu W (2011) Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biology and Technology 60:24–30
Liu T, Wang H, Kuang J, Sun C, Shi J, Duan X, Qu H, Jiang Y (2014) Short term anerobic pure oxygen and refrigerated storage conditions affect the energy status and selective gene expression in litchi fruit. LWT - Food Sci Technol. doi:10.1016/J.LWT.2014.09.003
Mangaraj S, Goswami TK, Giri SK, Tripathi MK (2012) Permselective MA packaging of litchi (cv. Shahi) for preserving quality and extension of shelf-life. Post Harvest Biology and Technology 71:1–12
Martínez-Castellanos G, Pelayo-Zaldívar C, Perez-Flores LJ, López-Luna A, Gimeno M, Bárzana E, Shirai K (2011) Postharvest litchi (Litchi chinensis Sonn.) quality preservation by Lactobacillus plantarum. Postharvest Biology and Technology 59:172–178
Neog M, Saikia L (2010) Control of post-harvest pericarp browning of litchi (Litchi chinensis. Sonn) J Food Sci Technol 47(1):100–104
Olesen T, Nacey L, Wiltshire N, O’Brien S (2004) Hot water treatments for the control of rots on harvested litchi (Litchi chinensis Sonn.) fruit. Postharvest Biology and Technology 32:135–146
Paliyath G, Tiwari K, Yuan H, Whitaker BD (2008) Structural deterioration in produce: phospholipase D, membrane deterioration and senescence. In: Paliyath G, Murr DP, Handa AK, Lurie S (eds) Postharvest biology and technology of fruits, vegetables and flowers, Istth edn. Wiley-Blackwell, Iowa, pp 195–239
Pesis E, Dvir O, Feygenberg O, Arie RB, Ackerman M, Lichter A (2002) Production of acetaldehyde and ethanol during maturation and modified atmosphere storage of litchi fruit. Postharvest Biology and Technology 26:157–165
Rattanapanone N, Plotto A, Baldwin E (2007) Effect of edible coatings and other surface treatments on pericarp color of thai litchi cultivars. Proc Fla State Hort Soc 120:222–227
Reichel M, Triani R, Wellhöfer J, Sruamsiri P, Carle R, Neidhart S (2012) Vital Characteristics of Litchi (Litchi chinensis Sonn.) Pericarp that define postharvest concepts for Thai cultivars. Food Bioprocess Technol. doi:10.1007/s11947-011-0762-9
Reuck KD, Sivakumar D, Korsten L (2009) Integrated application of 1-methylcyclopropene and modified atmosphere packaging to improve quality retention of litchi cultivars during storage. Postharvest Biology and Technology 52:71–77
Ruenroengklin N, Sun J, Shi J, Xue SJ, Jiang Y (2009) Role of endogenous and exogenous phenolics in litchi anthocyanin degradation caused by polyphenol oxidase. Food Chem 115:1253–1256
Saengnil K, Lueangprasert K, Uthaibutra J (2006) Control of enzymatic browning of harvested ‘Hong Huay’ litchi fruit with hot water and oxalic acid dips. Sci Asia 32:345–350
Semeerbabu MT, Kudachikar VB, Baskaran R, Ushadevi A, Matche RS, Ramana KVR (2007) Effects of post-harvest treatments on shelf life and quality of litchi fruit stored under modified atmosphere at low temperature. Journal of Food Sci Technol 44(1):106–109
Sharma M, Jacob JK, Subramanian J, Paliyath G (2010) Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.) Scientia Hort 125:239–247
Sivakumar D, Korsten L (2006a) Influence of modified atmosphere packaging and postharvest treatments on quality retention of litchi cv. Mauritius. Postharvest Biology and Technology 41:135–142
Sivakumar D, Korsten L (2006b) Evaluation of the integrated application of two types of modified atmosphere packaging and hot water treatments on quality retention in the litchi cultivar McLean’s Red. J Hort Sci Biotechnol 81:639–644
Sivakumar D, Korsten L (2010) Fruit quality and physiological responses of litchi cultivar McLean’s Red to 1-methylcyclopropene pre-treatment and controlled atmosphere storage conditions. LWT–Food Sci Tech 43:942–948
Sivakumar D, Arrebola E, Korsten L (2008) Postharvest decay control and quality retention in litchi (cv. McLean’s Red) by combined application of modified atmosphere packaging and antimicrobial agents. Crop Prot 27:1208–1214
Somboonkaew N, Terry LA (2010) Physiological and biochemical profiles of imported litchi fruit under modified atmosphere packaging. Postharvest Biology and Technology 56:246–253
Somboonkaew N, Terry LA (2011) Influence of temperature and packaging on physiological and chemical profiles of imported litchi fruit. Food Res Int 44:1962–1969
Sun J, Jiang Y, Wei X, Shi J, You Y, Liu H, Kakuda Y, Zhao M (2006) Identification of (−) epicatechin as the direct substrate for polyphenol oxidase isolated from litchi pericarp. Food Res Int 39:864–870
Sun J, You X, Li L, Peng H, Su W, Li C, He Q, Liao F (2011) Effects of a phospholipase D inhibitor on postharvest enzymatic browning and oxidative stress of litchi fruit. Postharvest Biology and Technology 62:288–294
Sun J, Li C, Prasad KN, You X, Li L, Liao F, Peng H, He X, Li Z, Zhang Y (2012) Membrane deterioration, enzymatic browning and oxidative stress in fresh fruits of three litchi cultivars during six day storage. Sci Hortic 148:97–103
Tian SP, Li BQ, Xu Y (2005) Effects of O2 and CO2 concentrations on physiology and quality of litchi fruit in storage. Food Chem 91:659–663
Tongdee SC, Sarpetch C, Roe DJ, Suwanagul A, Neamprem S (1998) Effect of heat-acid treatment on quality of litchi fruit. South African Litchi Growers AssociationYear book 9:44–46
Underhill SJR, Critchley C (1995) Cellular localisation of polyphenol oxidase and peroxidase activity in Litchi chinensis Son. pericarp. Australian Journal of Plant Physiology 22:627–632
Underhill SJR, Simons DH (1993) Litchi (Litchi chinensis Sonn.) pericarp desiccation and importance of postharvest micro-cracking. Sci Hortic 54:287–294
Wang JB, Wang XS, Jin ZQ (2010) Enzymatic browning of postharvest litchi : a review. In: Dongliang Q et al. (eds) Trees in Sapindaceae family, Proc 3rd IS on Longan, Litchi and other fruits. Acta Hort 863: 613–617
Wang HC, Hu ZQ, Wang Y, Chen HB, Huang XM (2011) Phenolic compounds and the antioxidant activities in litchi pericarp: Difference among cultivars. Sci Hortic 129:784–789
Yang S, Sun J, Feng L, Yang E, Chen Y, Dong X, Su X, Jiang Y (2011) Lipofuscin-Like substance involved in pericarp browning of postharvest litchi fruit during storage. The open food science Journal 5:47–50
Yi C, Jiang YM, Shi J, Qu HX, Xue SJ, Duan XW, Shi JY, Prasad KN (2010) ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process. Food Chem 118:42–47
Zauberman G, Ronen R, Akerman M, Weksler A, Rot I, Fuchs Y (1991) Postharvest retention of red colour of litchi fruit pericarp. Sci Hort 47:89–97
Zhang D, Quantick PC (1997) Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit. Postharvest Biology and Technology 12:195–202
Zhang D, Quantick PC, Grigor JM (2000) Changes in phenolic compounds in Litchi (Litchi chinensis Sonn.) fruit during postharvest storage. Post Harvest Biology and Technology 19:165–172
Zhang Z, Huber DJ, Qu H, Yun Z, Wang H, Huang Z, Huang H, Jiang Y (2015) Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. doi:10.1016/J.FoodChem.2014.09.001
Zhang Z, Pang X, Xuewu D, Ji Z, Jiang Y (2005) Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem 90:47–52
Zheng X, Tian S (2006) Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem 96:519–523
Acknowledgments
This review was sponsored by institutional grant by ICAR-CIPHET, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhushan, B., Pal, A., Narwal, R. et al. Combinatorial approaches for controlling pericarp browning in Litchi (Litchi chinensis) fruit. J Food Sci Technol 52, 5418–5426 (2015). https://doi.org/10.1007/s13197-015-1712-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1712-8