Abstract
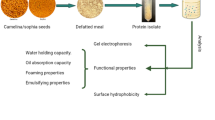
The aim of this work was to compare functional properties including solubility, emulsifying and foaming properties of native and thermally treated adzuki, soy and pea protein isolates prepared under the same conditions. These functional properties were tested at four pH values: pH 3.0, pH 5.0, pH 7.0 and pH 8.0. The lowest solubility at all pH values were obtained for isolate of adzuki whereas isolates of soybean had the highest values at almost all pHs. Thermal treatment reduced solubility of soy and pea isolates at all pH values, whereas solubility of adzuki isolate was unchanged, except at pH 8. Native isolate of adzuki had the best emulsifying properties at pH 7.0 whereas at the other pH values some of native pea and soybean protein isolates were superior. After thermal treatment, depending on tested pH and selected variety all of three species could be a good emulsifier. Native soy protein isolates formed the most stable foams at all pHs. Thermal treatment significantly improved foaming properties of adzuki isolate, whereas reduced foaming capacity of soy and pea isolates, but could improve foam stability of these isolates at specific pH. Appropriate selection of legume seed as well as variety could have great importance in achievement of desirable functional properties of final products. All three tested species could find specific application in wide range of food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For a long time legumes have been recognized as a valuable and low cost source of high quality protein products such as flour, concentrates and isolates. Nevertheless, the application on an industrial scale has only soybean proteins and to lesser extent pea protein products, in part due to insufficient information relating to functional properties of proteins of other legumes.
Adzuki bean (Vigna angularis), like other legumes, is an excellent source of high quality proteins, fiber and several other nutrients. After soybean, adzuki is the second most popular legume in Japan and is commonly used as paste or boiled and sweetened whole beans, and in desserts, snacks, and confectionery items. In European countries, it is only a part of vegetarian diet as a low-fat alternative to meat and other animal-based proteins.
Adzuki seeds contain about 25 % protein (on a dry basis), almost half of which is globulin (Meng et al. 2002). The major storage protein of adzuki bean has been referred to 7S globulin or vicilin-like protein (Tjahjadi et al. 1988). This globulin accounts for about 80 % of the total globulin fraction of adzuki bean whereas 11S globulin (legumin) makes up only about 10 % (Meng and Ma 2002a). 7S globulin is glycoprotein that consists of two types of subunits, α- and β1- subunits with molecular weight of 55 kDa, and 35 kDa, respectively (Chen et al. 1984). Vicilin-like proteins undergo proteolytic modification or “nicking” and this processing creates a high degree of heterogeneity in the subunit population (Fukuda et al. 2007). The second most abundant protein, legumin consists of two types of subunits, acidic and basic with a molecular mass of 40 and 20 kDa, respectively (Meng and Ma 2002a, b). In current literature, data about functional properties and possible use of protein isolates of adzuki are insufficient. There are data reported on the foaming properties of adzuki protein concentrate (Chau et al. 1997), solubility, surface hydrophobicity and thermal stability of purified 7S globulin of adzuki (Fukuda et al. 2007, 2008) and thermal, emulsifying and gelling properties of globulin extracts of adzuki proteins (Meng et al. 2002; Meng and Ma 2001, 2002a, b).
In contrast to adzuki, functional properties including solubility, emulsifying, foaming and gelling properties of soy protein isolates (Sorgentini et al. 1995; Yan et al. 2012; Deak et al. 2007; Hou and Zhao 2011) and pea protein isolates (Karaca et al. 2011; Aluko et al. 2009; Barac et al. 2010; Boye et al. 2010a, b; Adebiyi and Aluko 2011; Taherian et al. 2011) are well documented. However, opposite results were obtained concerning functional properties of soy and pea protein isolates. Some researches (Fernandez-Quintela et al. 1997; Sun and Arntfield 2010) obtained better functionality of soy protein isolates, whereas others (Sumner et al. 1981; Sosulski and McCurdy 1987; Aluko et al. 2009) indicated to better properties for pea isolates. Variations in the results among different studies could be due to differences in the protein purity of the samples studied, method of protein isolation as well as the specific conditions used for the tests (Boye et al. 2010a). Furthermore, significantly different functionalities among isolates prepared from the same plant species were observed. Several studies (Pesic et al. 2005; Khatib et al. 2002; Maninder et al. 2007; Barac et al. 2010, 2011) attributed this to different ratio for the major proteins, which is in turn influenced by genotype characteristics, environmental conditions and processing conditions (Periago et al. 1998; Fuhrmeister and Meuser 2003; Dua et al. 2009; Taherian et al. 2011).
The influence of processing on functional properties of plant protein isolates is well documented. Boye et al. (2010b) reviewed the influence of processing techniques including alkaline extraction/isoelectric precipitation, acid extraction, water extraction, micellization, ultrafiltration and enzymatic modification on yield, characteristics and functionality of pea, bean and chickpea protein isolates. Also, Barac et al. (2004) reviewed functionality of soy protein isolates and methods for its improvement. Both groups of authors suggested that besides other factors (e.g. amino acid composition, protein structure and conformation, their hydrophobicity, hydrophobicity/hydrophilicity ratio, the interactions that occur betwen proteins and other food components) processing conditions such as temperature, pH, pressure, solvent to starting material ratio, addition of enzymes etc. affected functionality of legume protein isolates.
Concerning that numerous factors influence functional properties of plant protein isolates, comparative study of these properties of isolates prepared and tested under the same conditions could better explain differences among them. So, the aim of this work was to investigate functional properties including solubility, emulsifying and foaming properties of native and thermally treated adzuki, soybean and pea protein isolates prepared under the same conditions.
Material and methods
Preparation of protein isolates
In the present study isolates were prepared from adzuki, soybean and pea. Adzuki seeds were purchased from local market. The soybean (Glycine max., L.) seeds from three genotypes, Olga, Bosa and Domestic black, as well as pea (Pisum sativum, L.) seeds were gifts from Jugoinspekt d.o (Begrade, Serbia). To avoid differences that may be caused by the method of preparation, all isolates were prepared by isoelectric precipitation using the same procedure. Two types of isolates were prepared, native and thermally treated. Briefly, legume seeds were ground in a kitchen type mill (Philips, Holland) and the resulting flour (~ 100 mesh) was treated with n-hexane (flour: n-hexane, 1/20; w/w) to remove the fat. The defatted flour was then dispersed in distilled water (1/10), stirred using magnetic stirrer for 15 min. Then, the pH of suspension was adjusted to pH 9.0 with 1 N NaOH, stirred at room temperature for 60 min and centrifuged (4,000×g for 10 min). Supernatant was separated, and the insoluble part was re-extracted for 30 min at pH 9.0 and centrifuged again. Supernatants were merged and then precipitated at pH 4.5 with 1 N HCl, stored for 2 h at +4 °C and centrifuged (4,000×g for 15 min). The insoluble fraction was re-dissolved at pH 9.0 for 30 min, precipitated at pH 4.5 and centrifuged under the same conditions. Insoluble fraction (protein isolate) was collected, re-suspended in distilled water adjusted to pH 7.0 with 1 N NaOH and lyophilized. Thermally treated isolates were prepared according to the same procedure, but before lyophilization, neutralized suspensions were heated at 90 °C for 3 min, immediately cooled in ice bath and then lyophilized.
Protein solubility
Protein solubility was determined according to the method of Wu et al. (1998). Four samples of 20 mg of each isolate were dispersed in 20 ml of milliQ water and stirred for 30 min to obtain uniform dispersions. Each suspension was adjusted with 1 M NaOH or 1 M HCl to pH 3.0; 5.0; 7.0 and 8.0, stirred for 1 h and centrifuged for 15 min at 17,000×g (Sigma, Germany). Protein content in supernatant was measured by using method of Bradford (1976). Total protein content was determined by extracting 20 mg of isolate in 20 ml of 0.5 M NaOH. Solubility was expressed as a percentage of total protein content.
Sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS–PAGE) of soluble proteins
Soluble proteins of adzuki, pea and soy isolates at each investigated pH values were analyzed by SDS-PAGE under reducing conditions following procedure of Fling and Gregerson (1986). Runs were carried out with 5 % (w/vol) stacking and 12.5 % (w/vol) resolving polyacrylamide gels. SDS-PAGE was performed with electrophoresis unit LKB-2001-100 in conjunction with power supply LKB-Macrodrive 5 and LKB-MultiTemp as a cooling unit (LKB, Upsala, Sweden). Supernatants, prepared according to the previously described method for protein solubility determination, were diluted with sample buffer (0.055 M Tris–HCl, pH 6.8, 2 % (w/vol) SDS, 7 % (vol/vol) glycerol, 4.3 % (vol/vol) β-mercaptoethanol, 0.0025 % (w/vol) bromophenol blue). Supernatant to buffer ratio was 1:3 (vol/vol). Prior to electrophoresis, samples were heated at 90 °C for 5 min and cooled at the room temperature and 25 μl of each diluted supernatant was loaded per well. The gels were run at 30 mA per gel for 6 h to completion. Gels were fixed and stained with 0.23 % (wt/vol) Coomassie Blue R-250 [dissolved in 3.9 % (w/vol) trichloroacetic acid (TCA), 6 % (vol/vol) acetic acid, and 17 % (vol/vol) methanol] for 45 min and destained with 8 % acetic acid and 18 % (vol/vol) ethanol. The gels were scanned and analyzed using SigmaGel software (Jandel Sci, USA). Molecular weights of the polypeptides were estimated by using low molecular weight calibration kit (Pharmacia, Sweden). Molecular weight markers included: phosphorylase B (94.0 kDa), bovine albumin (67.0 kDa), ovalbumin (43.0 kDa), carbonic anhydrase (30.0 kDa), soybean trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa). Each SDS-PAGE analysis was done at least in duplicate.
Emulsifying properties
Emulsifying properties of isolate were assayed as described by Pearce and Kinsella (1978). Pure sunflower oil (15 ml) and 45 ml 0.1 % protein suspension of isolate prepared in water were homogenized in mechanical homogenizer for 1 min at the highest settings (7,500 rpm). Fifty-micro liter portions of emulsions were pipetted from the bottom of the container at 0 and 10 min after homogenization. Each sample was diluted with 10 ml 0.1 % SDS solution. Absorbances of these diluted emulsions were measured at 500 nm. Emulsifying activity index (EAI) and emulsifying stability index (ESI) were calculated using values for absorbance at 0 (A 0 ) and 10 min (A 10 ) after emulsion formation. EAI and ESI were measured on two different days. Each day two different emulsions of the same sample were produced and three aliquots of each emulsion were taken for further analysis. Emulsifying properties were expressed as EAI and ESI according to following equations:
where: T = 2.303 A 0 /l; A 0 = absorbance measured immediately after emulsion formation; F-dilution factor = 200, l = path length of the cuvette, C = weight of protein/unit volume (g/ml) of aqueous phase before emulsion formation; Φ = oil volume fraction of the emulsion (calculated by drying the emulsion);
where: Δt = 10 min and ΔA = A 0 − A 10 .
Foaming properties
Foaming properties were expressed as foaming capacity (FC) and foam stability (FS) as described by Barac et al. (2011, 2012). Foaming was attained by bubbling a stream of air (6 dm3 min−1) for 15 s through a Waters filter holder (Waters, USA) placed at the bottom of a 250 ml graduated column containing 30 ml 0.1 % protein solution in water adjusted to pH 3.0, 5.0, 7.0 and 8.0. Total foam volume was taken at 0 min for both parameters and at 3 min for foam stability. Foaming properties were expressed as:
Statistical analysis
The statistical analyses of the data were performed with a software Statistica version 5.0 (StatSoft Co., Tulsa, OK). Results were expressed as the mean values ± standard deviation of three separate determinations. The data were subjected to analysis of variance (ANOVA) and Duncan’s multiple range tests to determine significant differences (p < 0.01) between mean values within each group.
Results and discussion
Protein solubility
Protein solubility of both types of isolates (native and thermally treated) prepared from adzuki, soybean and pea are shown in Table 1. Native isolates prepared from each of three legumes showed typical U-shape of solubility-pH profiles with minimum values obtained near isoelectric point (pH 5.0) and maximum values detected at pH 8.0. In general, the lowest solubility at all pH values was obtained for isolate of adzuki, whereas isolates of soy bean were shown the highest solubility at almost all pHs. Depending on pH value, solubility of native adzuki isolate was ranged from 3.28 % (pH 5.0) to 69.66 % (pH 8.0) and at lower pH values (pH 3.0; pH 5.0) this parameter was approximately two times lower than the solubility of soybean and pea isolates. At higher pH values, especially at pH 8.0 these differences were less pronounced, but still significant (p < 0.01). These results are inconsistent with the results of Meng and Ma (2002a). They have been reported that pure globulin fraction of adzuki had better solubility than soybean isolates. But, they compared solubility of pure globulin fraction of adzuki with commercial soy protein isolate and this could be the main reason for disagreement with our results. Namely, it is well known that processing history (beside other factors), which is unknown for commercial isolate, may cause significant differences even among isolates prepared from the same species (Taherian et al. 2011). Nevertheless, lower solubility of adzuki isolate obtained in this study compared to isolates of pea and soy beans prepared under the same procedure is unexpected knowing the content, composition and properties of the dominant proteins of soybean, adzuki and pea. Vicilin-like proteins are usually considered as more soluble proteins than legumin-like (11S) proteins. In addition, vicilin-like protein represents over 80 % of all adzuki globulins, whereas in soy and pea protein isolates makes about 30–40 % (Pesic et al. 2005; Barac et al. 2006, 2010). Due to these facts better solubility of adzuki isolate than soybean or pea isolates should be expected. However, it is well known that proteins from legumes tend to form pH-induced aggregates (Yuan et al. 2002). Therefore, the lower solubility of native adzuki isolates compared to pea or soybean proteins isolates could be attributed to greater tendency of native adzuki proteins for aggregation into less soluble complexes than pea and soybean proteins.
Comparing solubility of native soybean isolates prepared from different genotypes at investigated range of pH values, no significant (p > 0.01) differences among them were observed. The exceptions were solubility of isolates of Olga and Domestic black at pH 5.0, and isolate of Olga at pH 8, which were significantly different from the other soy isolates. In the case of native pea protein isolates, these differences were more pronounced and significant at all pH values. Barac et al. (2010, 2012) attributed these variations of solubility to protein composition of pea isolate and different nature of complexes formed during processing of isolate (during isoelectric precipitation) or during solubilization of isolate at specific pH.
The U-shape of solubility-pH dependence is maintained even after thermal treatment. Nevertheless, comparing the data from Table 1, it is obvious that thermal treatment differently affected solubility of isolates of investigated species. Generally, thermal treatment reduced solubility of soy and pea isolates at all pH values, whereas the solubility of adzuki protein isolates, except at pH 8.0, was unaffected. At pH 8.0, solubility of thermally treated isolate of adzuki was 9.02 % higher than solubility of the native isolate (Table 1). The observed increase at pH 8.0 could be result of higher hydrophilicity of complexes formed during solubilisation of thermally treated isolates than those formed during solubilisation of native ones at the that pH.
Even that, solubility of thermally treated adzuki isolate was lower than solubility of thermally treated soy bean and pea isolates. Comparing solubility of heat treated soy bean and pea isolates, obtained results indicated that selection of genotype strongly influence this parameter at all pHs.
Soluble protein composition
Soluble protein profiles of native adzuki, soybean and pea isolates at investigated pH values separated on SDS-PAGE under reducing conditions are provided on Figs. 1 and 2. SDS-patterns of all of three types of native isolates (Fig. 1) at pH 8.0 were similar to those obtained by numerous authors (Meng and Ma 2002a; Griga 2002; Barac et al. 2006, 2010). SDS-patterns of soluble proteins of native adzuki isolate were pH dependent, especially at low pH values. In qualitative sense, SDS-patterns of soluble proteins of native adzuki isolate at pH 7.0 and 8.0 as well as native isolate of two other species were very similar. At pH 5.0, almost all of adzuki, soy and pea proteins were insoluble which was in a good agreement with the results presented in Table 1. At this pH, low molecular weight fractions, which were moved with leading front (Fig. 1), were the major soluble fractions of all native isolates. At pH 3.0 the soluble fractions of adzuki protein isolate are made up of small amount of the most abundant 52 kDa-subunit of vicilin, whereas soluble fractions of soy and pea isolates were composed of almost all major proteins. This could explain approximately two times lower solubility of adzuki protein isolate than two other ones.
SDS-PAGE analysis of soluble proteins of native isolates prepared from adzuki (commercial seed), soybean (Bosa, Olga and Domestic black) and pea (Calvedon, Provansalac). Abbreviations: α,α’, β-subunits of β-conglycinin; A and B –acidic and basic subunits of glycinin; V-subunits of vicilin, L-subunits of legumin; cv- convicilin; M.W.- molecular weight standards; A-adzuki; B-Bosa; C-Calvedon; D-Domestic black; P-Provansalac; O-Olga
Figure 2 shows soluble protein composition of thermally treated isolates at pH 8.0 and 3.0. Soluble protein compositions of thermally treated samples were very similar at pH 8.0 and pH 7.0, whereas the SDS-patterns of these proteins at pH 5.0 were very similar to their native SDS-patterns. For that reason, only SDS-patterns of thermally treated isolates at pH 8.0 and pH 3.0 were presented. Heating plant proteins above 80 °C causes dissociation of their quaternary structures, denatures their subunits, and promotes the formation of more or less soluble protein aggregates via electrostatic, hydrophobic and disulfide interchange mechanisms (Barac et al. 2004). Thermal treatment enhanced solubility of major adzuki proteins, whereas reduced solubility of soy protein isolates at pH 8.0 as evident on SDS-PAGE patterns (Fig. 2). On SDS- patterns of treated adzuki isolate at pH 3.0 and 8.0 (Fig. 2), it could be observed high molecular weight (HMW) complexes on the top of the resolving gel which could not be noticed on SDS-patterns of native isolate. This indicated that heat treatment induced denaturation of adzuki proteins and their re-association into complexes with improved hydrophilicity at pH 8.0. The obtained results are in agreement with the results of Meng and Ma (2002a). They reported that high molecular weight complexes were formed in thermally treated solution of adzuki globulins.
The presence of HMW complexes was not observed on electrophoretic patterns of other thermally treated isolates. Thermal treatment showed different effects on pea protein isolates, enhancing solubility of Provansalac, but reducing solubility of Calvedon at pH 8.0 compared to native isolates. At pH 3.0, both types of thermally treated pea isolates showed reduced solubility.
Emulsifying properties
Suitability of a protein isolate as an emulsifier depends on the rate at which proteins diffuse into the interface and on the deformability of its conformation under the influence of interfacial tension (surface denaturation). A protein with ideal qualities as an emulsifier for an oil-in-water emulsion would have a relatively low molecular weight, a balanced amino acid composition in terms of charged, polar and non polar residues, good water solubility, well-developed surface hydrophobicity, and a relatively stable conformation (Belitz et al. 2009). Emulsifying properties of native and thermally treated isolates of adzuki, soy and pea were expressed as two parameters, EAI and ESI (Table 2). In general, significant differences in EAI and ESI values were found among native isolates of different species, as well as among emulsifying properties of the isolates prepared from different genotypes within the same species. Also, emulsifying properties of all native isolates were pH dependent. Depending on pH, EAI and ESI values of adzuki isolate ranged from 25.93 to 60.70 m2/g and from 30.04 to 101.41 min., soy bean protein isolates ranged from 10.76 to 57.63 m2/g and from 15.95 to 66.32 min. and pea isolates ranged from 12.90 to 46.50 m2/g and 29.60 to 92.81 min., respectively. The best emulsifying properties showed adzuki isolate at pH 7.0, which is in a good agreement with results reported by Meng and Ma (2002a). Pea protein isolate of Provansalac formed the emulsions with the highest ESI at pH 7.0 and pH 8.0, whereas Calvedon protein isolate formed emulsion with the highest ESI at pH 5.0.
The EAI index of soy and pea isolates had the pH-dependence similar to those observed for their solubility. The lowest mean values of EAI were registered at pH 5.0 near isoelectric point of soy and pea proteins, whereas emulsifying activity significantly increased below and above this value. This indicated that solubility was one of the major factors that influenced their emulsifying activity, and was in agreement with previous reports of Fuhrmeister and Meuser (2003), Pesic et al. (2005) and Barac et al. (2010), which showed a positive relationship between protein solubility and emulsifying activity of pea and soybean protein products. On the other hand, adzuki isolate deviated from this trend. Namely, regardless to different solubility at pH 3.0 and 5.0 (Table 1) and different soluble protein compositions (Fig. 1), less pronounced differences were found between EAI of emulsion prepared at these pH values. In addition, native adzuki isolate had higher EAI at pH 7.0 than at pH 8.0 although the solubility was lower at pH 7.0 than at pH 8.0 and soluble protein compositions were similar at these pH values (Fig. 1). These facts pointed out that other factors (such as deformability of protein and/or protein complexes formed at appropriate pH values) rather than solubility were of crucial importance for emulsion activity of adzuki isolates.
Thermal treatment had inconsistent impact on the emulsifying properties of investigated isolates. The emulsion activity of adzuki isolate at pH 3.0, pH 5.0 and 8.0 improved upon thermal treatment for 36.51 % to 63.59 % (Table 2). On the other hand, stability of emulsions prepared with this isolate at pH 5.0 and pH 7.0 values was lower than stability of those prepared with native one. Inconsistent effect of heat treatment could be observed also in the case of emulsifying properties of soy and pea isolates. For example, EAI of soy isolate prepared from Domestic black was improved at pH 3.0, pH 7.0 and pH 8.0 and ESI value only at pH 8.0. In contrast to this, thermal treatment improved EAI of isolate of Bosa only at pH 7.0, whereas stability of this emulsion was 41.34 % (pH 3.0) to 116.62 % (pH 5.0) higher than that prepared with native material. Comparing all of thermally treated isolates, it could be observed that the best emulsifying stability showed isolate of Calvedon at pH 3, adzuki isolate at pH 5 and soy bean isolates at pH 7 (Domestic black) and pH 8 (Olga). On the other hand, the most stable emulsion formed isolate of Provansalac at pH 3, isolate of Bosa at pH 5, isolate of Calvedon at pH 7 and isolates of Calvedon and adzuki at pH 8.
It is worth to mention that the solubility of native and thermally treated isolates of Calvedon was lower at pH 5.0 than those of Provansalac. But, emulsifying properties of the native protein isolate of Calvedon were better compare to native isolate of Provansalac and opposite was obtained for the thermally treated pea isolates (Table 2). These results indicated that the properties of complexes formed during solubilisation and thermal treatment of pea isolates were crucial factors for their emulsifying properties when the protein solubility is low.
These results suggesting that appropriate selection of legume seed, variety as well as heat treatment should be taken into account when the production of protein isolates was considered for application as emulsifiers in specific food products.
Foaming properties
Foaming properties of adzuki protein isolate has been investigated but in literature opposite trends were presented. Earlier work of Chau et al. (1997) showed that foams prepared from protein concentrates of adzuki were superior to those prepared from soybean. In contrast to this, the work of Meng and Ma (2002a) suggested that commercial soy isolate had better foaming capacity than adzuki globulins. According to our results, comparison of foaming properties of adzuki isolate and isolates from soy and pea greatly depended on pH value at which the foam was formed. At pH 3.0, native adzuki isolate had lower foam capacity (266.7 %, Table 3.) than all investigated isolates of soybean (400 %–900.0 %) and pea (316.8 %–333.8 %). At the other pH values (pH 5.0; 7.0 and 8.0), foam capacity of native isolates could be very similar depending on genotype from which isolate was prepared. Among all investigated isolates, native soy protein isolates formed the most stable foams at all pHs.
Foam stability of native adzuki isolate especially at lower pH values (pH 3.0 and pH 5.0), was low in comparison to soybean and pea isolates. At these pH values, adzuki isolate formed extremely unstable foams which completely disappeared after 3 min. Thermal treatment significantly changed foaming properties of prepared isolates. So, thermal treatment could be a very useful method for improving foaming properties of adzuki isolate and could make it more comparable to soy isolates. This treatment significantly (p < 0.01) improved foam capacity of adzuki isolate at all pH values, except at pH 5.0, as well as greatly improved foam stability of adzuki isolate, especially at pH range 3–7. The improvement in foaming capacity has been associated with higher concentration of soluble and flexible protein molecules that enable to reduction of surface tension and with their rapid adsorption and denaturation at the air/water interface during bubbling (Makri et al. 2005). On the other hand, stability of foams depends on the strength of the protein film and its permeability for gases. Since there was no significant differences between solubility of proteins of native and thermally treated adzuki isolate at specific pH (pH 3.0, 5.0 and 7.0, Table 1.), improved foam capacity of treated isolate at these pH values was consequence of increased flexibility and easier reorganization of thermally treated proteins and/or their complexes at the air/water interface. Furthermore, such flexible and denatured molecules and complexes more effectively interacted at the interface and formed stronger and more cohesive films then native one (Belitz et al 2009).
As in the case of emulsifying properties, thermal treatment had different effect on foaming properties of soy and pea isolates. Thermally-treated isolates prepared from these species had reduced foaming capacities at almost all investigated pH values. The exceptions were isolate of Bosa, which had improved FC values at pH 7.0 and pH 8.0, and isolate of Calvedon at pH 3.0.
Significant differences (p < 0.01) were especially pronounced in the case of foam stability. Heat treatment reduced foam stability of soy isolate prepared from Olga at pH 3.0, 7.0 and 8.0, but increased at pH 5.0, whereas Dometic black isolate showed decreased foam stability at all pH values. In contrast to this, thermal treatment had no significant influence on foam stability of isolate of Bosa at pH 3.0, 7.0 and 8.0 whereas at pH 5.0 stability of foam was decreased. Foam prepared with thermally treated pea protein isolates also showed different stability with respect to both, pH and genotypes. Foam obtained with Provansalac pea protein isolate had significantly (p < 0.01) improved stability at all pH values especially at pH 5.0, whereas foam formed with Calvedon pea protein isolate at pH 5.0 and 7.0 was extremely unstable. Therefore, heat-treated isolates of Provansalac and Adzuki could have better application as foam-stabilising ingredients than those prepared from Calvedon or soybean varieties. The isolate of Olga variety could be usefull as foam-stabilising additive in food products with low pH values (pH 3–5) whereas isolate of Bosa variety could find application in products with higer pH values (pH 7–8).
Conclusion
Results of this investigation showed that functional properties of isolates prepared from different species depended on several factors such as: choice of species and varieties, preparing conditions and pH at which specific parameters were tested. The native isolates of soybean had the highest solubility at almost all pHs whereas the lowest solubility were obtained for adzuki isolates. Native isolate of adzuki had the best emulsifying properties (EAI and ESI) at pH 7.0 whereas at pH 5.0 pea protein isolate of Calvedon was superior. Native soy protein isolates formed the most stable foams at all pHs. Thermal treatment reduced solubility of soy and pea isolates at all pH values, whereas,except at pH 8.0, had no significant influence on adzuki isolate. Furthermore, thermal treatment significantly improved foaming properties of adzuki isolate, whereas reduced foaming capacity of soy and pea isolates. But, this treatment could improve foam stability of these isolates at specific pH. Depending on tested pH and selected variety thermally treated isolates of all of three species could be a good emulsifier. Therefore, selection of adequate species and variety for application in food industry as emulsifying or foaming ingredients depends on which desirable functional properties should be achieved in final products. All of three tested species and their varieties could find application in wide range of food products, but their proper selection and preparing conditions could be of great importance.
References
Adebiyi AP, Aluko RE (2011) Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem 128:902–908
Aluko RE, Mofolasayo OA, Watts BM (2009) Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J Agric Food Chem 57:9793–9800
Barac M, Stanojevic S, Jovanović S, Pesic M (2004) Soy protein modification- a rewiev. Acta Period Technol 35:3–17
Barac MB, Jovanovic ST, Stanojevic SP, Pesic MB (2006) Effect of limited hydrolysis on traditional soy protein concentrate. Sensors 6:1087–1101
Barac M, Cabrilo S, Pesic M, Stanojevic S, Ristic N (2010) Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int J Mol Sci 11:4973–4990
Barac M, Cabrilo S, Pesic M, Stanojevic S, Pavlicevic M, Macej O, Ristic N (2011) Functional properties of pea (Pisum sativum, L) protein isolates modified with chymosin. Int J Mol Sci 12:8372–8387
Barac M, Cabrilo S, Stanojevic S, Pesic M, Pavlicevic M, Zlatkovic B, Jankovic M (2012) Functional properties of protein hydrolysates from pea (Pisum sativum, L) seeds. Int J Food Sci Technol 47:1457–1467
Belitz HD, Grosch W, Schieberle P (2009) Food chemistry, 4th edn. Springer, Berlin (Chapter 2)
Boye JI, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed SH (2010a) Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int 43:537–546
Boye J, Zare F, Pletch A (2010b) Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int 43:414–431
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chau C-F, Cheung PC-K, Wong Y-S (1997) Functional properties of protein concentrates from three Chinese indigenous legume seeds. J Agric Food Chem 45:2500–2503
Chen THH, Gusta LV, Tjahjadi C, Breene WM (1984) Electrophoretic characterization of adzuki bean (Vigna angularis) seed proteins. J Agric Food Chem 32:396–399
Deak NA, Murphy PA, Johnson LA (2007) Characterization of fractionated soy proteins produced by a new simplified procedure. J Am Oil Chem Soc 84:137–149
Dua S, Mahajan A, Sandal M, Gagan J (2009) Physico-chemical properties of defatted pea seed meal proteins with emphasis on salts and pH. J Food Sci Technol 46:251–254
Fernandez-Quintela A, Macarulla MT, Del Barrio AS, Martinez JA (1997) Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum Nutr 51:331–342
Fling SP, Gregerson DS (1986) Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris-buffer system without urea. Anal Biochem 155:83–88
Fuhrmeister H, Meuser F (2003) Impact of processing on functional properties of protein products from wrinkled peas. J Food Eng 56:119–129
Fukuda T, Prak K, Fujioka M, Maruyama N, Utsumi S (2007) Physicochemical properties of native Adzuki bean (Vigna angularis) 7S globulin and the molecular cloning of its cDNA isoforms. J Agric Food Chem 55:3667–3674
Fukuda T, Maruyama N, Ramlan M, Salleh M, Mikami B, Utsumi S (2008) Characterization and crystallography of recombinant 7S globulins of Adzuki bean and structure − function relationships with 7S globulins of various crops. J Agric Food Chem 56:4145–4153
Griga M (2002) Morphology and anatomy of Pisum sativum somatic embryos. Biol Plant 45:173–182
Hou Y, Zhao XH (2011) Limited hydrolysis of two soybean protein products with trypsin or neutrase and the impacts on their solubility, gelation and fat absorption capacity. Biotechnology 10:190–196
Karaca AC, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44:2742–2750
Khatib KA, Herald TJ, Aramouni FM, MacRitchie F, Schapaugh WT (2002) Characterization and functional properties of soy β-conglycinin and glycinin of selected genotypes. J Food Sci 67:2923–2929
Makri E, Papalamprou E, Doxastakis G (2005) Study of functional properties of seed storage proteins from indigenous European legume crops (lupin, pea, broad bean) in admixture with polysaccharides. Food Hydrocoll 19:583–594
Maninder K, Sandhu KS, Singh N (2007) Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars. Food Chem 104:259–267
Meng G-T, Ma C-Y (2001) Thermal properties of Phaseolus angularis (red bean) globulin. Food Chem 73:453–460
Meng G-T, Ma C-Y (2002a) Characterization of globulin from Phaseolus angularis (red bean). Int J Food Sci Technol 37:687–695
Meng G-T, Ma C-Y (2002b) Thermal gelation of globulin from Phaseolus angularis (red bean). Food Res Int 35:377–385
Meng G-T, Ching K-M, Ma C-Y (2002) Thermal aggregation of globulin from an indigenous Chinese legume, Phaseolus angularis (red bean). Food Chem 79:93–103
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26:716–723
Periago MJ, Vidal ML, Ros G, Rincón F, Martínez C, López G, Rodrigo J, Martínez I (1998) Influence of enzymatic treatment on the nutritional and functional properties of pea flour. Food Chem 63:71–78
Pesic MB, Vucelic-Radovic BV, Barac MB, Stanojevic SP (2005) The influence of genotypic variation in protein composition on emulsifying properties of soy proteins. J Am Oil Chemists Soc 82:667–672
Sorgentini DA, Wagner JR, Anon MC (1995) Effects of thermal treatment of soy protein isolate on the characteristics and structure-function relationship of soluble and insoluble ractions. J Agric Food Chem 43:2471–2479
Sosulski FW, McCurdy AR (1987) Functionality of flours, protein fractions and isolates from field peas and bean. J Food Sci 52:1010–1014
Sumner AK, Nielsen MA, Youngs CG (1981) Production and evaluation of pea protein isolate. J Food Sci 46:364–372
Sun XD, Arntfield SD (2010) Gelation properties of salt-extracted pea protein induced by heat treatment. Food Res Int 43:509–515
Taherian AR, Mondor M, Labranche J, Drolet H, Ippersiel D, Lamarche F (2011) Comparative study of functional properties of commercial and membrane processed yellow pea protein isolates. Food Res Int 44:2505–2514
Tjahjadi C, Lin S, Breene WM (1988) Isolation and Characterization of Adzuki bean (Vigna angularis cv Takara) proteins. J Food Sci 53:1438–1443
Wu W, Hettiarachy N, Qi M (1998) Hydrophobicity, solubility and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J Am Oil Chem Soc 75:845–850
Yan B, Ren J, Zhao M, Luo D, Gu L (2012) Effects of limited enzymatic hydrolysis with pepsin and high-pressure homogenization on the functional properties of soybean protein isolate. LWT Food Sci Technol 46:453–459
Yuan Y, Velev O, Chen K, Campbell BE, Kaler EW, Lenhoff AM (2002) Effect of pH and Ca2 + -Induced associations of soybean proteins. J Agric Food Chem 50:4953–4958
Acknowledgments
This investigation was supported by the Ministry of Science and Developmental of the Republic of Serbia (Grants no. 31069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barac, M.B., Pesic, M.B., Stanojevic, S.P. et al. Comparative study of the functional properties of three legume seed isolates: adzuki, pea and soy bean. J Food Sci Technol 52, 2779–2787 (2015). https://doi.org/10.1007/s13197-014-1298-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1298-6